Tough and Elastic α-Tricalcium Phosphate Cement Composites with Degradable PEG-Based Cross-Linker
Abstract
:1. Introduction
2. Materials and Methods
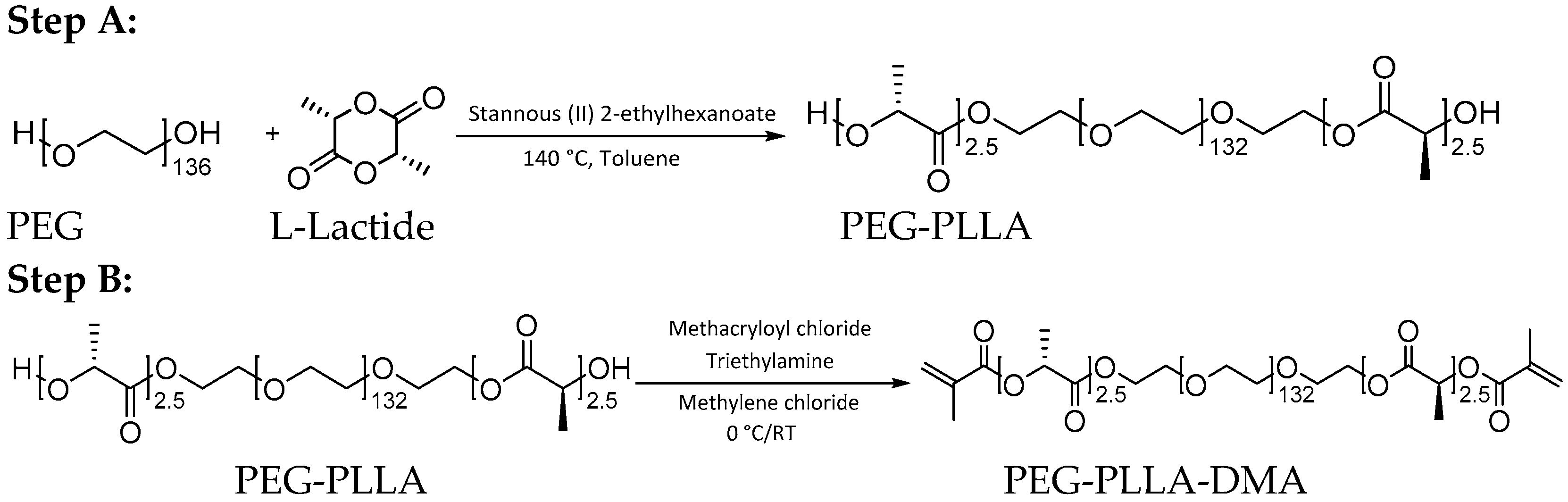
2.1. Cross-Linker Synthesis and Characterization
2.1.1. Cross-Linker Synthesis
2.1.2. 1H Nuclear Magnetic Resonance Spectroscopy
2.1.3. Gel Permeation Chromatography in N,N-Dimethylformamide
2.1.4. Matrix Assisted Laser Desorption-Ionization Time-of-Flight Mass Spectrometry
2.1.5. Fourier Transform Infrared-Spectroscopy
2.2. Pure Hydrogel Production and Characterization
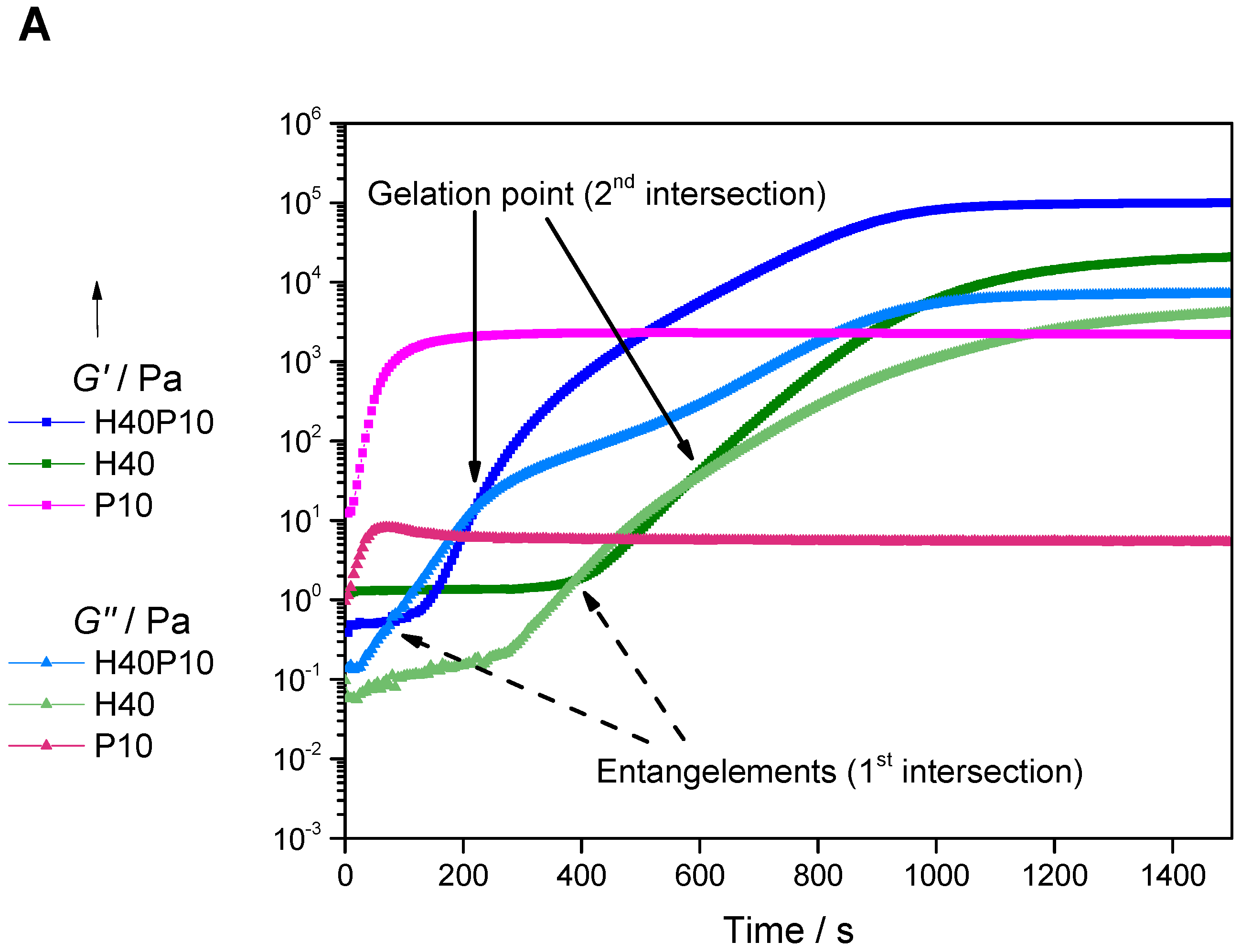
2.2.1. Determination of Gelation Point via Rheological Analysis
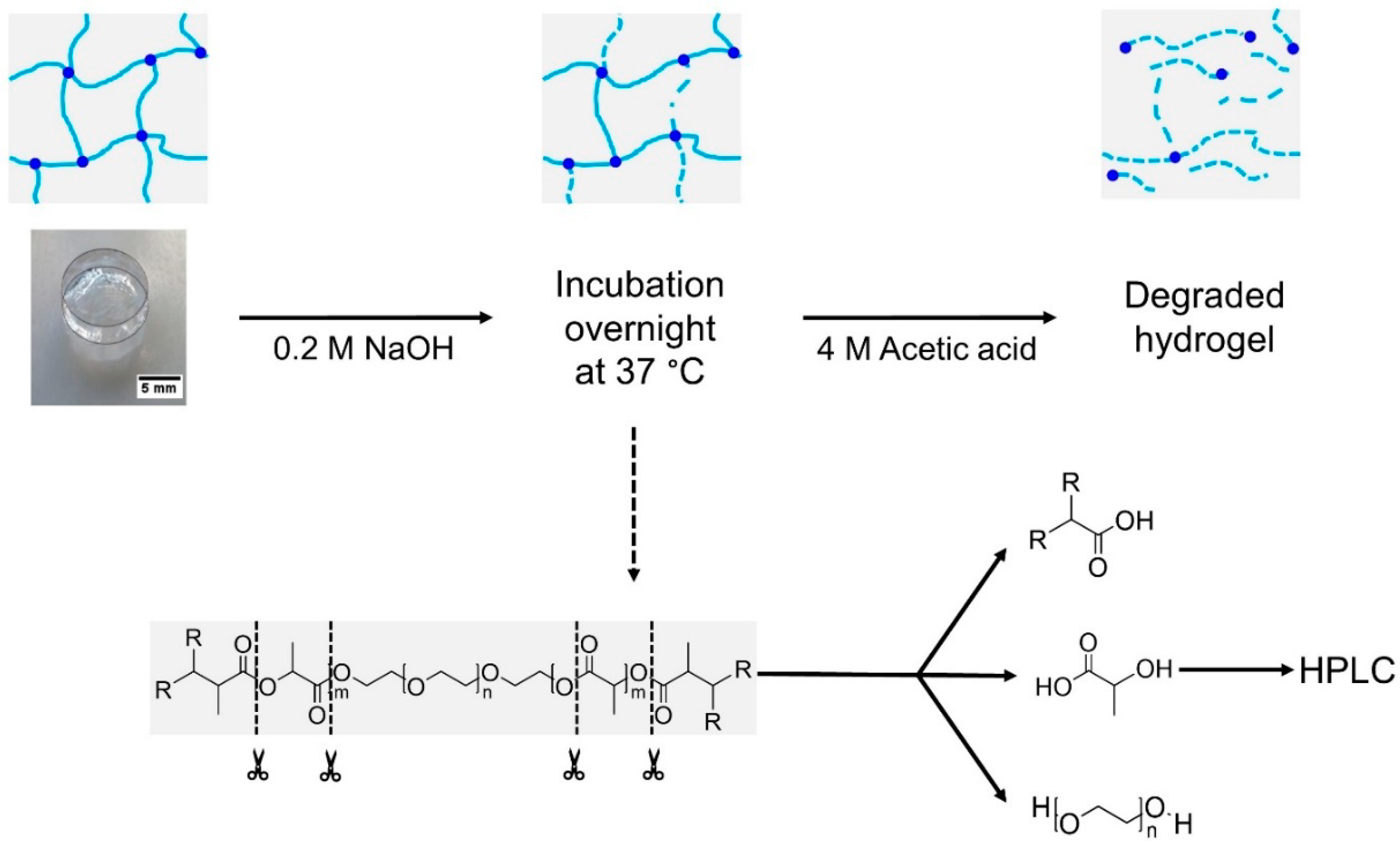
2.2.2. Degradation and Swelling Behavior
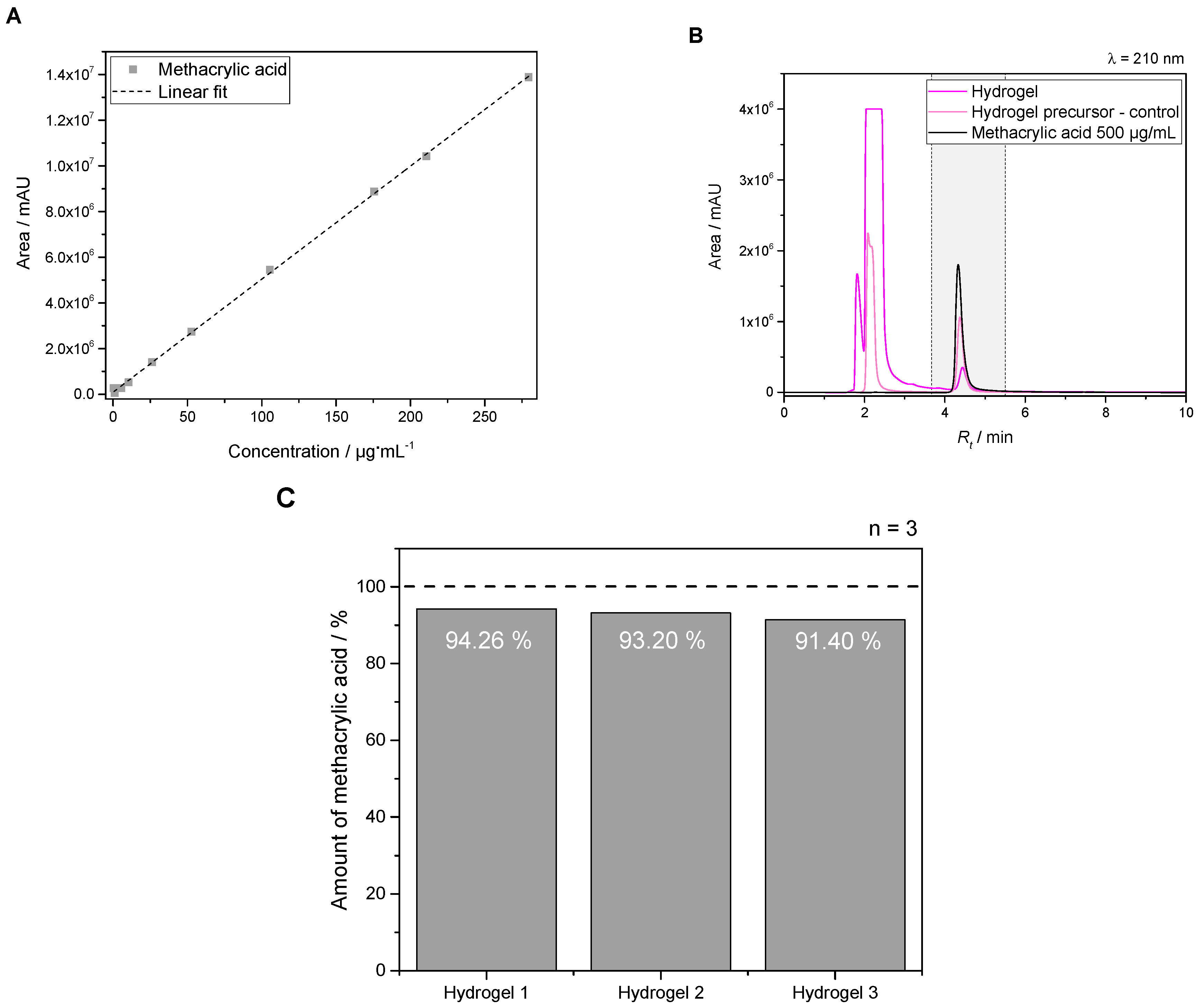
2.2.3. Degree of Cross-Linking for Synthesized Hydrogel Precursor via Determination of Methacrylic Acid
2.3. Cement Composite Formation and Characterization
2.3.1. Synthesis of α-Tricalcium Phosphate
2.3.2. Production of Inorganic Reference and Dual Set Composites
2.3.3. Initial Setting Time Determination via Gillmore Needle Test
2.3.4. Phase Composition and Conversion via X-ray Diffraction and Rietveld Refinement
2.3.5. Surface Analysis via Scanning Electron Microscopy
2.3.6. Mechanical Characterization—Four-Point Bending and Compression Test
2.3.7. Statistical Analysis
3. Results
3.1. Cross-Linker and Hydrogel Characterization
3.1.1. Cross-Linker Characterization
3.1.2. Hydrogel Characterization
3.1.3. Degradation Behavior of Hydrogels in PBS
3.1.4. Determination of Methacrylate Units Involved in Gelation for PEG-PLLA-DMA–Hydrogels
3.2. Inorganic Reference and Composite Characterization
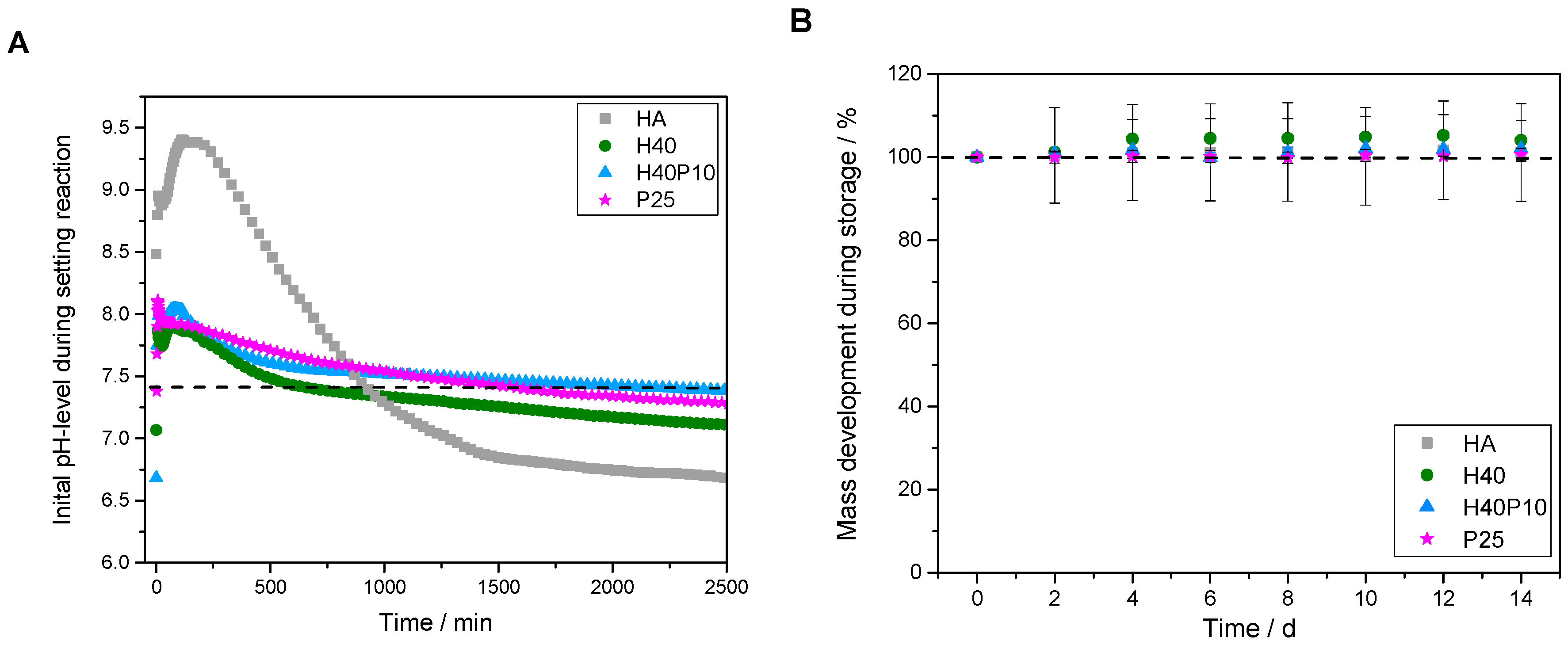
3.2.1. Determination of Initial Setting Time
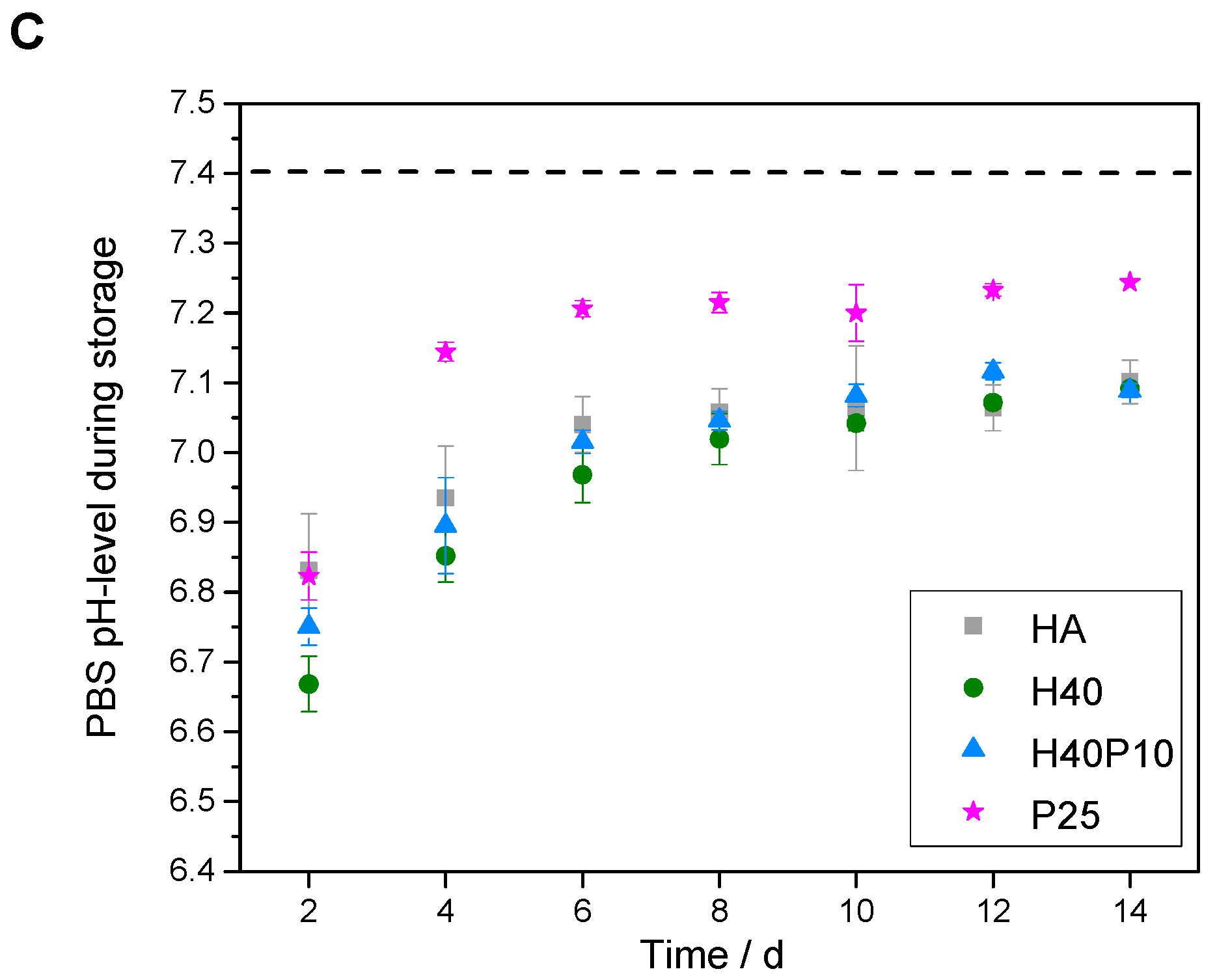
3.2.2. Analysis of Parameters for Biological and Material Technological Relevance
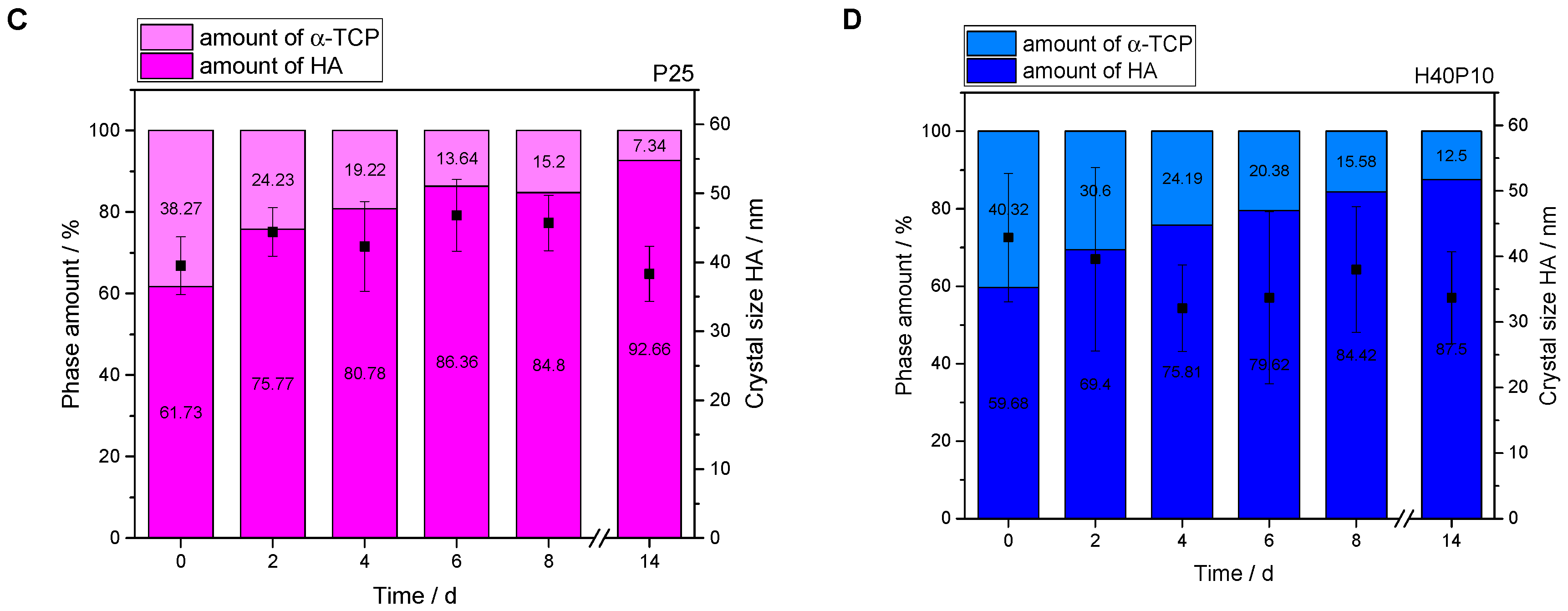
3.2.3. XRD Measurements and Rietveld Refinement
3.2.4. Microscopical Surface Characterization
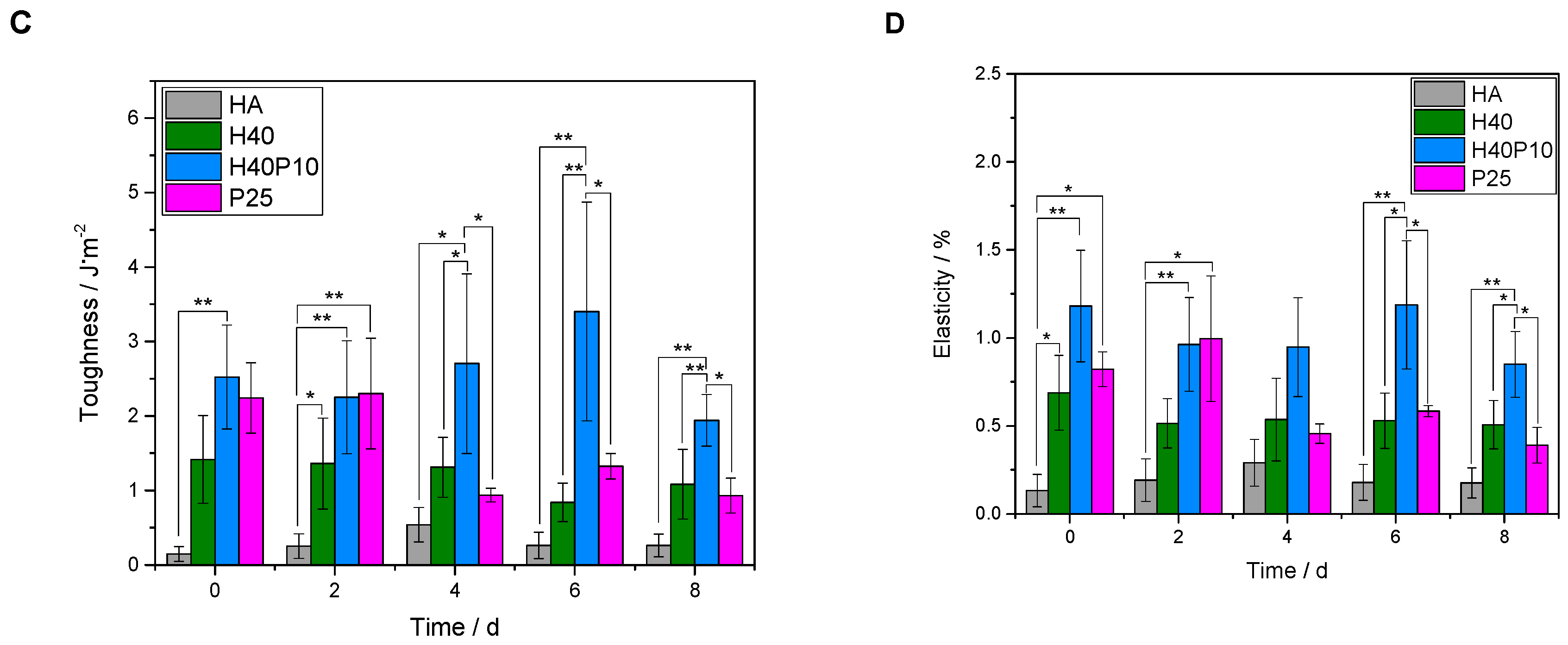
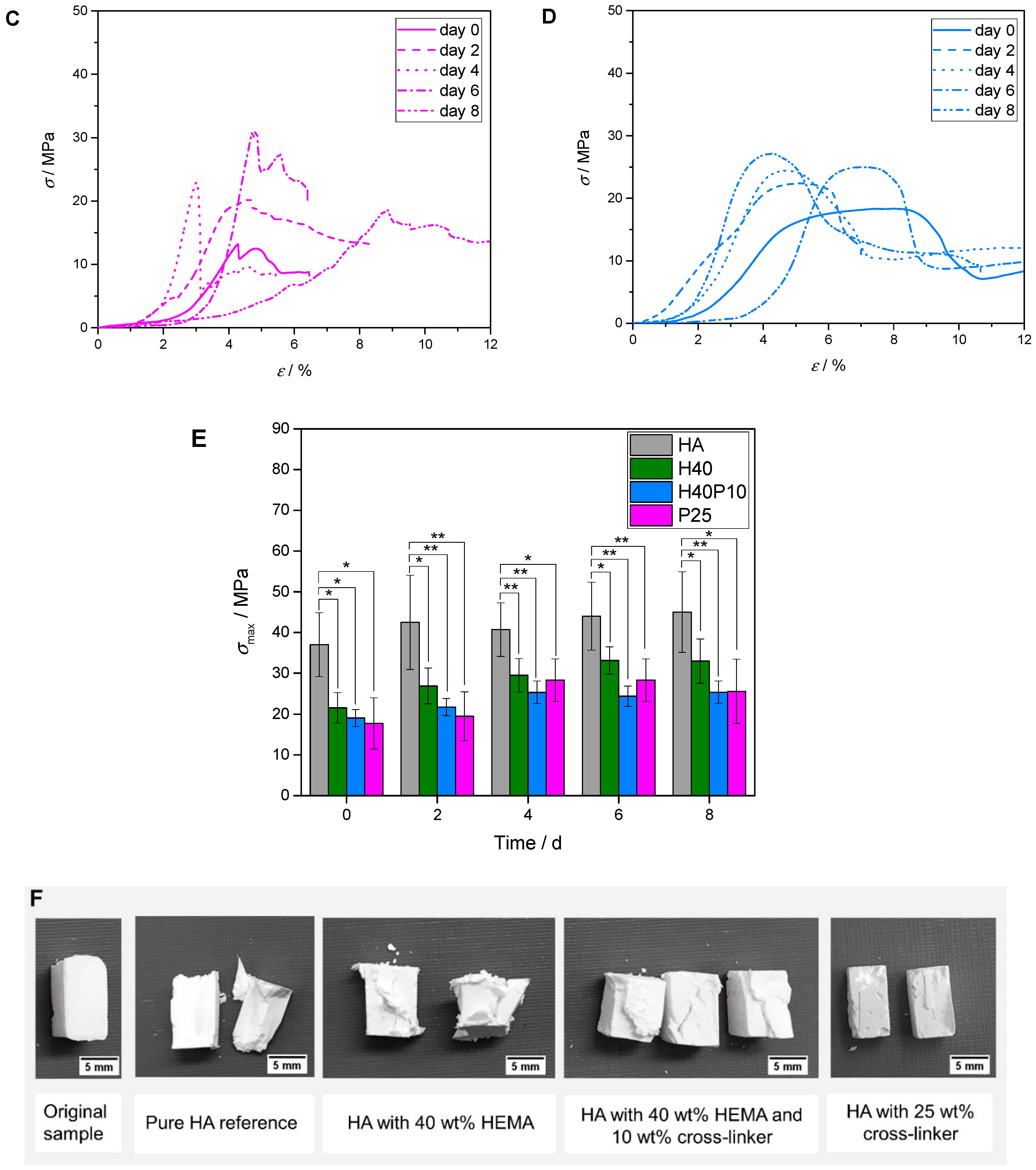
3.2.5. Bending Strength
3.2.6. Compressive Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hing, K.A. Bone repair in the twenty–first century: Biology, chemistry or engineering? Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2004, 362, 2821–2850. [Google Scholar] [CrossRef] [PubMed]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Weiner, S.; Wagner, H.D. The material bone: Structure-mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Barinov, S.; Komlev, V. Calcium phosphate bone cements. Inorg. Mater. 2011, 47, 1470–1485. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Espanol, M.; Montufar, E.B.; Perez, R.A.; Mestres, G. New processing approaches in calcium phosphate cements and their applications in regenerative medicine. Acta Biomater. 2010, 6, 2863–2873. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite–polymer biocomposites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar] [CrossRef]
- Bohner, M. Calcium orthophosphates in medicine: From ceramics to calcium phosphate cements. Injury 2000, 31, D37–D47. [Google Scholar] [CrossRef]
- Geffers, M.; Groll, J.; Gbureck, U. Reinforcement Strategies for Load-Bearing Calcium Phosphate Biocements. Materials 2015, 8, 2700–2717. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, L.A.; Carrodeguas, R.G.; Boschi, A.O.; de Arruda, A.C. Dual-Setting Calcium Phosphate Cement Modified with Ammonium Polyacrylate. Artif. Organs 2003, 27, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, C.; Liu, Y.; Zhang, S. Double-Network Interpenetrating Bone Cement via in situ Hybridization Protocol. Adv. Funct. Mater. 2010, 20, 3997–4011. [Google Scholar] [CrossRef]
- Schamel, M.; Groll, J.; Gbureck, U. Simultaneous formation and mineralization of star-P (EO-stat-PO) hydrogels. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Christel, T.; Kuhlmann, M.; Vorndran, E.; Groll, J.; Gbureck, U. Dual setting α-tricalcium phosphate cements. J. Mater. Sci. Mater. Med. 2013, 24, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Rödel, M.; Teßmar, J.; Groll, J.; Gbureck, U. Highly flexible and degradable dual setting systems based on PEG-hydrogels and brushite cement. Acta Biomater. 2018, 79, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, A.S.; Pathak, C.P.; Hubbell, J.A. Bioerodible hydrogels based on photopolymerized poly (ethylene glycol)-co-poly (α-hydroxy acid) diacrylate macromers. Macromolecules 1993, 26, 581–587. [Google Scholar] [CrossRef]
- Marie, A.; Fournier, F.; Tabet, J. Characterization of synthetic polymers by MALDI-TOF/MS: Investigation into new methods of sample target preparation and consequence on mass spectrum finger print. Anal. Chem. 2000, 72, 5106–5114. [Google Scholar] [CrossRef] [PubMed]
- Abbadessa, A.; Mouser, V.H.M.; Blokzijl, M.M.; Gawlitta, D.; Dhert, W.J.A.; Hennink, W.E.; Malda, J.; Vermonden, T. A synthetic thermo-sensitive hydrogel for cartilage bioprinting and its biofunctionalization with polysaccharides. Biomacromolecules 2016, 17, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Clapper, J.D.; Skeie, J.M.; Mullins, R.F.; Guymon, C.A. Development and characterization of photopolymerizable biodegradable materials from PEG–PLA–PEG block macromonomers. Polymer 2007, 48, 6554–6564. [Google Scholar] [CrossRef]
- Hurle, K.; Christel, T.; Gbureck, U.; Moseke, C.; Neubauer, J.; Goetz-Neunhoeffer, F. Reaction kinetics of dual setting α-tricalcium phosphate cements. J. Mater. Sci. Mater. Med. 2016, 27, 1. [Google Scholar] [CrossRef]
- Geffers, M.; Barralet, J.E.; Groll, J.; Gbureck, U. Dual-setting brushite–silica gel cements. Acta Biomater. 2015, 11, 467–476. [Google Scholar] [CrossRef]
- Wenisch, S.; Stahl, J.P.; Horas, U.; Heiss, C.; Kili, O.; Trinkaus, K.; Hild, A.; Schnettler, R. In vivo mechanisms of hydroxyapatite ceramic degradation by osteoclasts: Fine structural microscopy. J. Biomed. Mater. Res. Part A 2003, 67, 713–718. [Google Scholar] [CrossRef]
- Browning, M.B.; Cereceres, S.N.; Luong, P.T.; Cosgriff-Hernandez, E.M. Determination of the in vivo degradation mechanism of PEGDA hydrogels. J. Biomed. Mater. Res. Part A 2014, 102, 4244–4251. [Google Scholar]
- Metters, A.T.; Bowman, C.N.; Anseth, K.S. A statistical kinetic model for the bulk degradation of PLA-b-PEG-b-PLA hydrogel networks. J. Phys. Chem. B 2000, 104, 7043–7049. [Google Scholar] [CrossRef]












| Substances | Percentage by Weight/% | ||
|---|---|---|---|
| Abbreviations | H40 | P10 | H40P10 |
| HEMA | 40 | – | 40 |
| Cross-linker | – | 10 | 10 |
| 2.5 % Na2HPO4-solution | 59.25 | 89.25 | 49.25 |
| APS | 0.5 | ||
| TEMED | 0.25 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rödel, M.; Teßmar, J.; Groll, J.; Gbureck, U. Tough and Elastic α-Tricalcium Phosphate Cement Composites with Degradable PEG-Based Cross-Linker. Materials 2019, 12, 53. https://doi.org/10.3390/ma12010053
Rödel M, Teßmar J, Groll J, Gbureck U. Tough and Elastic α-Tricalcium Phosphate Cement Composites with Degradable PEG-Based Cross-Linker. Materials. 2019; 12(1):53. https://doi.org/10.3390/ma12010053
Chicago/Turabian StyleRödel, Michaela, Jörg Teßmar, Jürgen Groll, and Uwe Gbureck. 2019. "Tough and Elastic α-Tricalcium Phosphate Cement Composites with Degradable PEG-Based Cross-Linker" Materials 12, no. 1: 53. https://doi.org/10.3390/ma12010053






