Analysis of Physical and Mechanical Properties of the Mortar in the Historic Retaining Wall of the Gediminas Castle Hill (Vilnius, Lithuania)
Abstract
:1. Introduction
2. Retaining Wall of the Gediminas Castle Hill
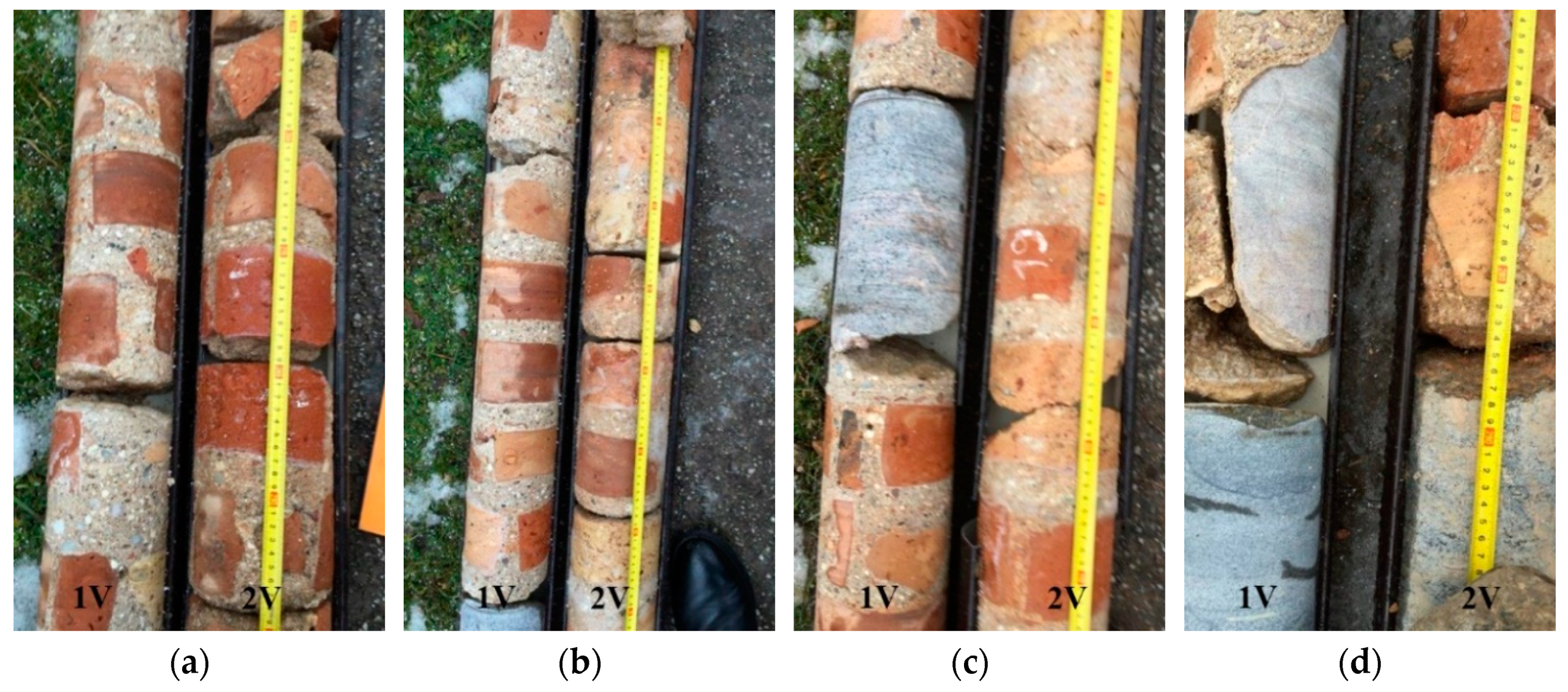
3. Specimens and Test Methods
4. Analysis of the Test Results
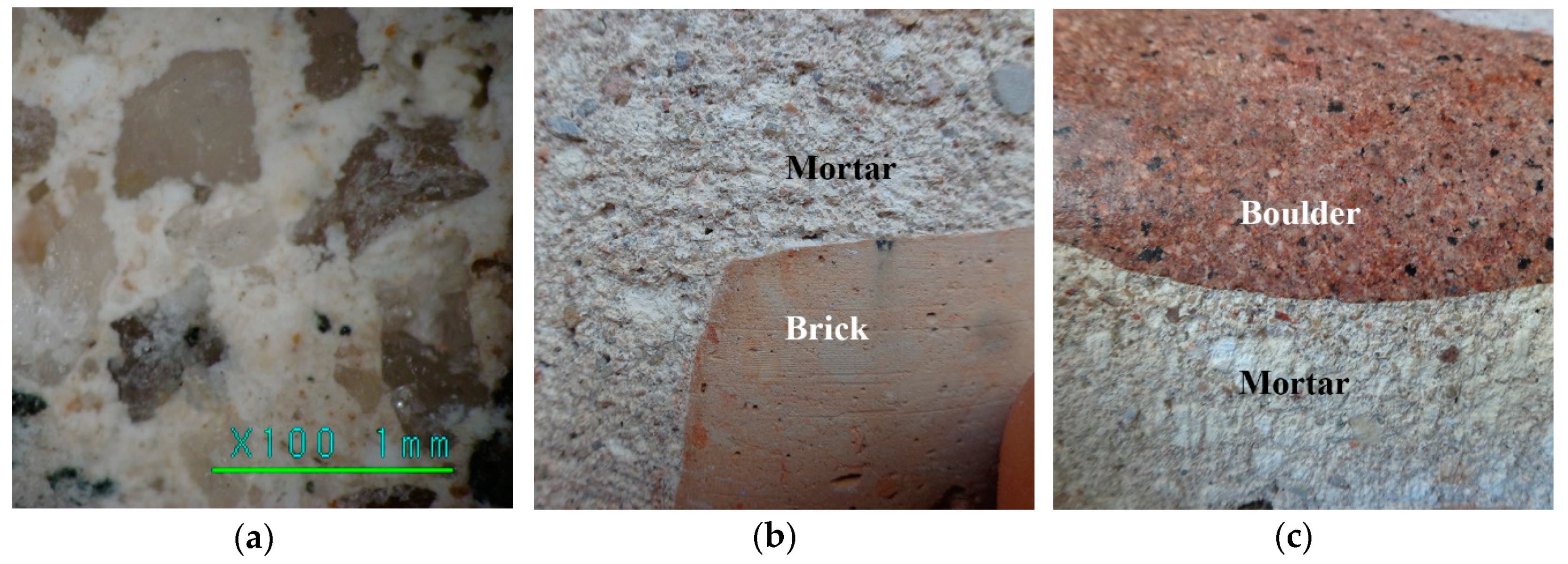
4.1. Physical Mechanical Properties of Brickwork Components
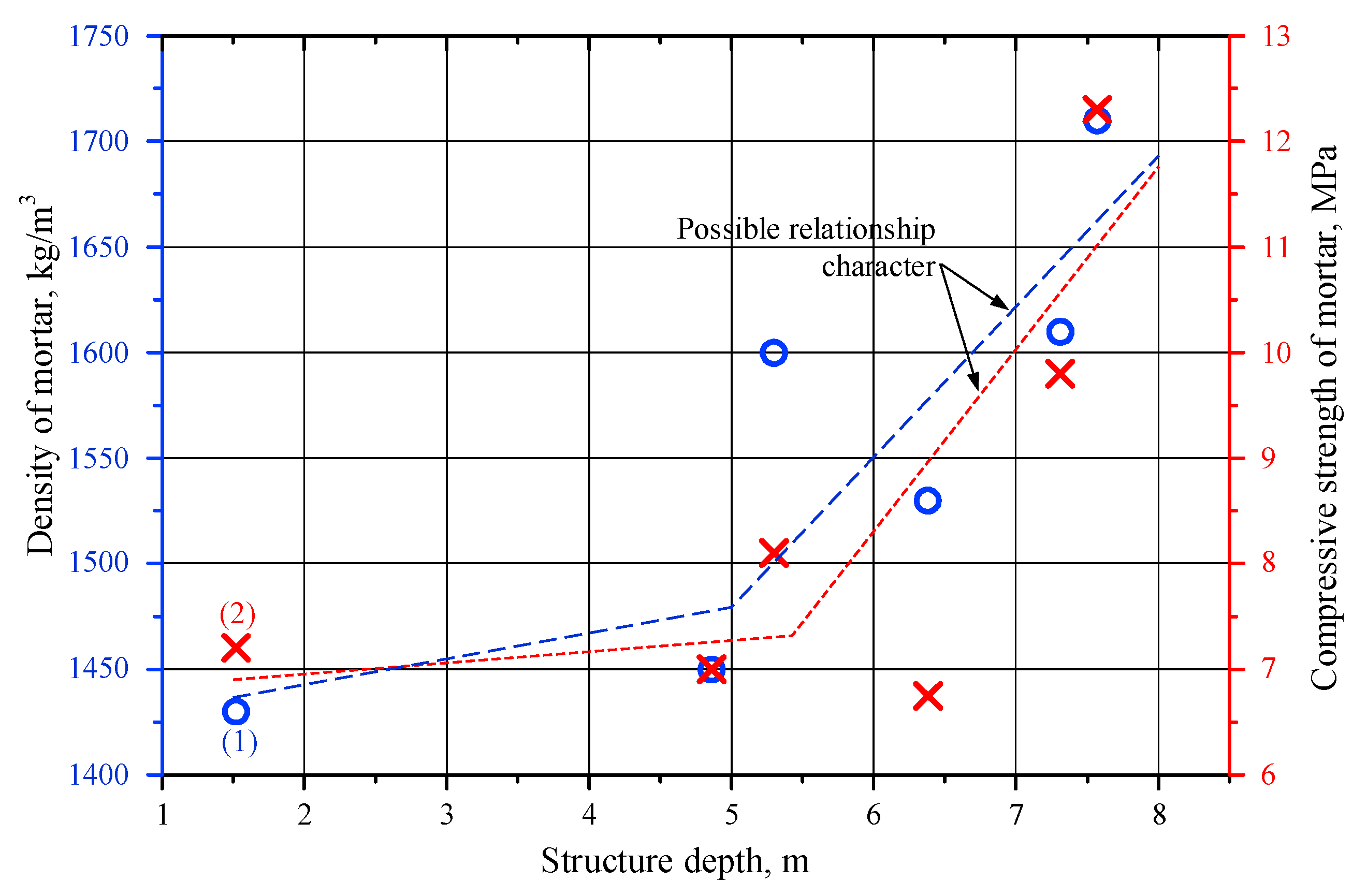
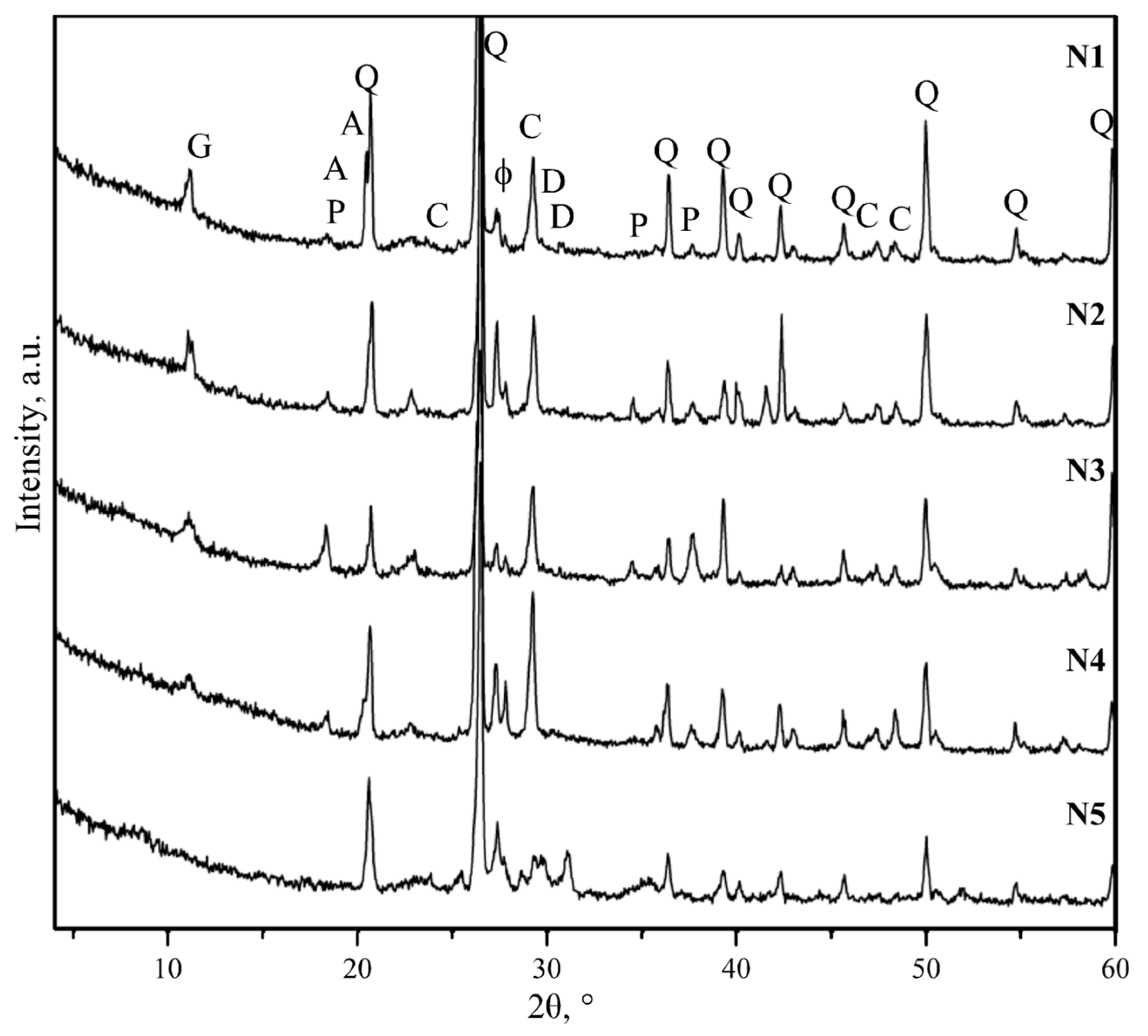
4.2. Mortar Component Tests
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stefanidou, M.; Papayianni, I. The role of aggregates on the structure and properties of lime mortars. Cem. Concr. Compos. 2005, 27, 914–919. [Google Scholar] [CrossRef]
- Faria, P.; Henriques, F.; Rato, V. Comparative evaluation of lime mortars for architectural conservation. J. Cult. Herit. 2008, 9, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Moropoulou, A.; Polikreti, K.; Bakolas, A.; Michailidis, P. Correlation of physicochemical and mechanical properties of historical mortars and classification by multivariate statistics. Cem. Concr. Res. 2003, 33, 891–898. [Google Scholar] [CrossRef]
- Moropoulou, A.; Bakolas, A.; Anagnostopoulou, S. Composite materials in ancient structures. Cem. Concr. Compos. 2005, 27, 295–300. [Google Scholar] [CrossRef]
- Blaeuer, C.; Kueng, A. Examples of microscopic analysis of historic mortars by means of polarising light microscopy of dispersions and thin sections. Mater. Charact. 2007, 58, 1199–1207. [Google Scholar] [CrossRef]
- Levandauskas, V. History of Masonry in Lithuania (in Lithuanian); Vytauto Didžiojo Universiteto Leidykla: Kaunas, Lithuania, 2012. [Google Scholar]
- Válek, J.; Veiga, R. Characterisation of mechanical properties of historic mortars: Testing of irregular samples. Trans. Built Environ. 2005, 83, 365–374. [Google Scholar]
- Binici, H.; Kapur, S. The physical, chemical, and microscopic properties of masonry mortars from Alhambra Palace (Spain) in reference to their earthquake resistance. Front. Archit. Res. 2016, 5, 101–110. [Google Scholar] [CrossRef]
- Solak, A. Experimental Investigation of Lime Mortar Used in Historical Buildings in Becin, Turkey. Mater. Sci. 2016, 22, 105–112. [Google Scholar] [CrossRef]
- Černý, R.; Kunca, A.; Tydlitát, V.; Drchalová, J.; Rovnaníková, P. Effect of pozzolanic admixtures on mechanical, thermal and hygric properties of lime plasters. Constr. Build. Mater. 2006, 20, 849–857. [Google Scholar] [CrossRef]
- Böke, H.; Akkurt, S.; İpekoğlu, B.; Uğurlu, E. Characteristics of brick used as aggregate in historic brick-lime mortars and plasters. Cem. Concr. Res. 2006, 36, 1115–1122. [Google Scholar] [CrossRef]
- Žvironaitė, J.; Gnip, I.; Krušinskienė, A. Determination of calcic lime properties by different methods (in Lithuanian). Cheminė Technol. 2003, 28, 40–45. [Google Scholar]
- Lanas, J.; Bernal, J.L.P.; Bello, M.A.A.; Galindo, J.I.A.; Pérez Bernal, J.L.; Bello, M.A.A.; Alvarez Galindo, J.I. Mechanical properties of natural hydraulic lime-based mortars. Cem. Concr. Res. 2004, 34, 2191–2201. [Google Scholar] [CrossRef] [Green Version]
- Middendorf, B.; Hughes, J.J.; Callebaut, K.; Baronio, G.; Papayianni, I. Investigative methods for the characterisation of historic mortars. Part 1: Mineralogical characterisation. Mater. Struct. 2005, 38, 761–769. [Google Scholar] [CrossRef]
- Middendorf, B.; Hughes, J.J.; Callebaut, K.; Baronio, G.; Papayianni, I. Investigative methods for the characterisation of historic mortars. Part 2: Chemical characterisation. Mater. Struct. 2005, 38, 771–780. [Google Scholar] [CrossRef]
- Lawrence, R.M.; Mays, T.J.; Rigby, S.P.; Walker, P.; D’Ayala, D. Effects of carbonation on the pore structure of non-hydraulic lime mortars. Cem. Concr. Res. 2007, 37, 1059–1069. [Google Scholar] [CrossRef]
- Kirilovica, I.; Gulbe, L.; Vitina, I.; Igaune-Blumberga, S. Chemical characterization of lime-based binders in historic buildings of Latvia. IOP Conf. Ser. Mater. Sci. Eng. 2015, 96. [Google Scholar] [CrossRef]
- Bianchini, G.; Marrocchino, E.; Vaccaro, C. Chemical and mineralogical characterisation of historic mortars in Ferrara (northeast Italy). Cem. Concr. Res. 2004, 34, 1471–1475. [Google Scholar] [CrossRef]
- Petrėnas, T. Development and Application of New Methods for the Conservation of Ceramics (in Lithuanian). PhD. Thesis, Vilnius University, Vilnius, Lithuania, 2017. [Google Scholar]
- Kitkauskas, N. Vilnius Castles (in Lithuanian); Mokslo ir Enciklopedijų Leidybos Centras: Vilnius, Lithuania, 2012; ISBN 978-5-420-01716-6. [Google Scholar]
- LST EN 12504-1:2009 Testing Concrete in Structures—Part 1: Cored Specimens—Taking, Examining and Testing in Compression; Lithuanian Standards Board: Vilnius, Lithuania, 2009.
- LST EN 12390-7:2009/P:2011 Testing Hardened Concrete—Part 7: Density of Hardened Concrete; Lithuanian Standards Board: Vilnius, Lithuania, 2009.
- LST EN 772-1:2011+A1:2015 Methods of Test for Masonry Units—Part 1: Determination of Compressive Strength; Lithuanian Standards Board: Vilnius, Lithuania, 2011.
- LST EN 772-21:2011 Methods of Test for Masonry Units—Part 21: Determination of Water Absorption of Clay and Calcium Silicate Masonry Units by Cold Water Absorption; Lithuanian Standards Board: Vilnius, Lithuania, 2011.
- LST EN 1015-11:2002/P:2004 Methods of Test for Mortar for Masonry. Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar; Lithuanian Standards Board: Vilnius, Lithuania, 2002.
- LST EN 1015-10:2002/P:2004 Methods of Test for Mortar for Masonry. Part 10: Determination of Dry Bulk Density of Hardened Mortar; Lithuanian Standards Board: Vilnius, Lithuania, 2002.
- Baston, G.M.N.; Clacher, A.P.; Heath, T.G.; Hunter, F.M.I.; Smith, V.; Swanton, S.W. Calcium silicate hydrate (C-S-H) gel dissolution and pH buffering in a cementitious near field. Mineral. Mag. 2012, 76, 3045–3053. [Google Scholar] [CrossRef]
- Moropoulou, A.; Bakolas, A.; Bisbikou, K. Characterization of ancient, byzantine and later historic mortars by thermal and X-ray diffraction techniques. Thermochim. Acta 1995, 269–270, 779–795. [Google Scholar] [CrossRef]
- Ukrainczyk, N.; Matusinovic, T.; Kurajica, S.; Zimmermann, B.; Sipusic, J. Dehydration of a layered double hydroxide—C2AH8. Thermochim. Acta 2007, 464, 7–15. [Google Scholar] [CrossRef]
- Cardoso, F.A.; Innocentini, M.D.M.; Akiyoshi, M.M.; Pandolfelli, V.C. Effect of curing time on the properties of CAC bonded refractory castables. J. Eur. Ceram. Soc. 2004, 24, 2073–2078. [Google Scholar] [CrossRef]
- Bakolas, A.; Aggelakopoulou, E.; Moropoulou, A. Evaluation of pozzolanic activity and physico-mechanical characteristics in ceramic powder-lime pastes. J. Therm. Anal. Calorim. 2008, 92, 345–351. [Google Scholar] [CrossRef]
- Steinberg, R.H.; Belic, H.J. Silica Refractories. Anal. Chem. 1949, 21, 730–731. [Google Scholar] [CrossRef]
- Goswami, G.; Sanu, P.; Panigrahy, P.K. Estimation of thermal expansion of silica refractory based on its mineralogy. InterCeram Int. Ceram. Rev. 2015, 64, 174–176. [Google Scholar] [CrossRef]
- Binal, A. Investigation of Hydraulic Binding Characteristics of Lime Based Mortars Used in Historical Masonry Structures. IOP Conf. Ser. Mater. Sci. Eng. 2017, 245. [Google Scholar] [CrossRef]
- Cizer, Ö.; Van Balen, K.; Elsen, J.; Van Gemert, D. Real-time investigation of reaction rate and mineral phase modifications of lime carbonation. Constr. Build. Mater. 2012, 35, 741–751. [Google Scholar] [CrossRef]
- Kubiliūtė, R.; Kaminskas, R. The Pozzolanic Activity of Calcined Clay—Silica Gel Composites. Mater. Sci. 2013, 19, 453–460. [Google Scholar] [CrossRef]
- Valanciene, V.; Siauciunas, R.; Baltusnikaite, J. The influence of mineralogical composition on the colour of clay body. J. Eur. Ceram. Soc. 2010, 30, 1609–1617. [Google Scholar] [CrossRef]



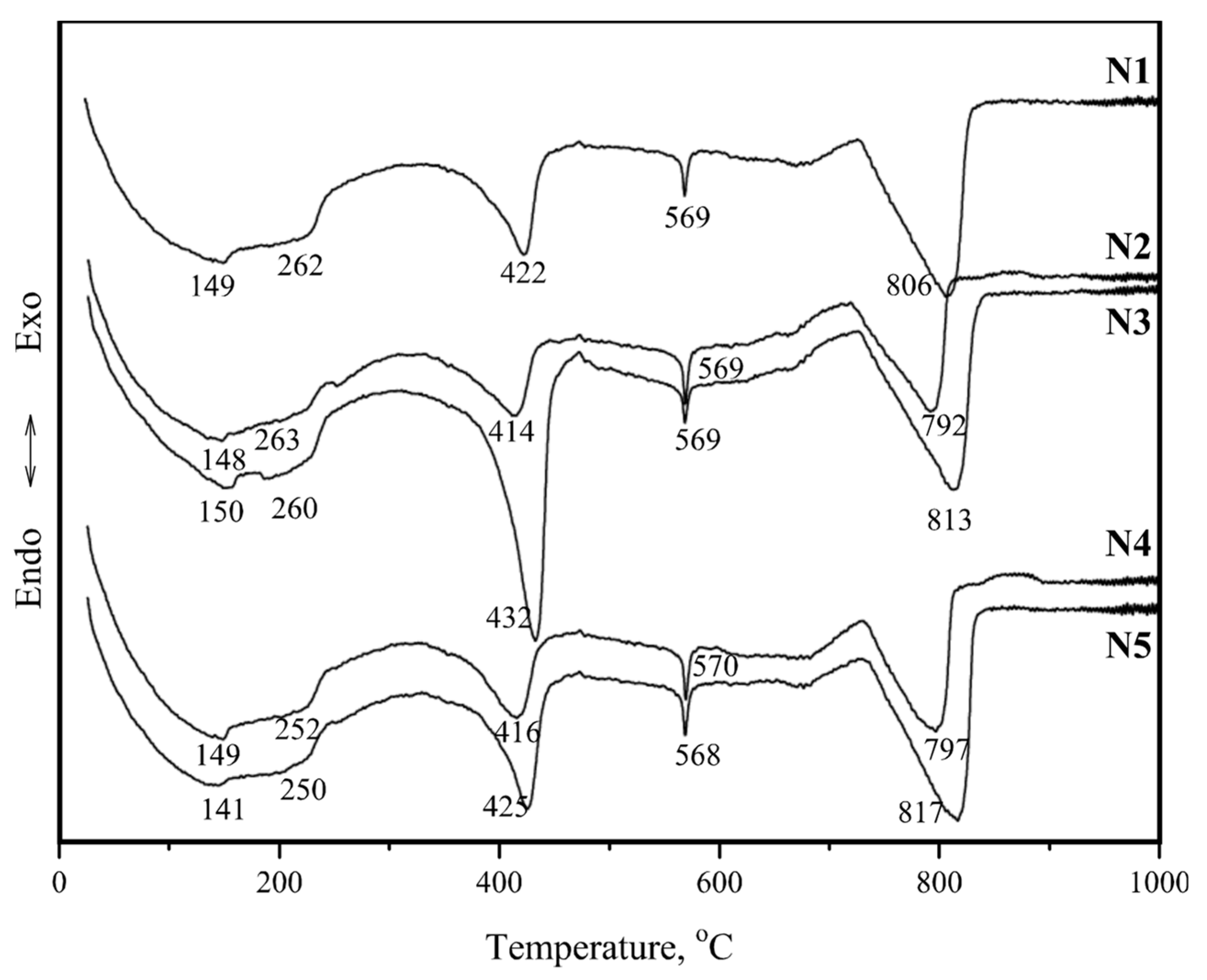


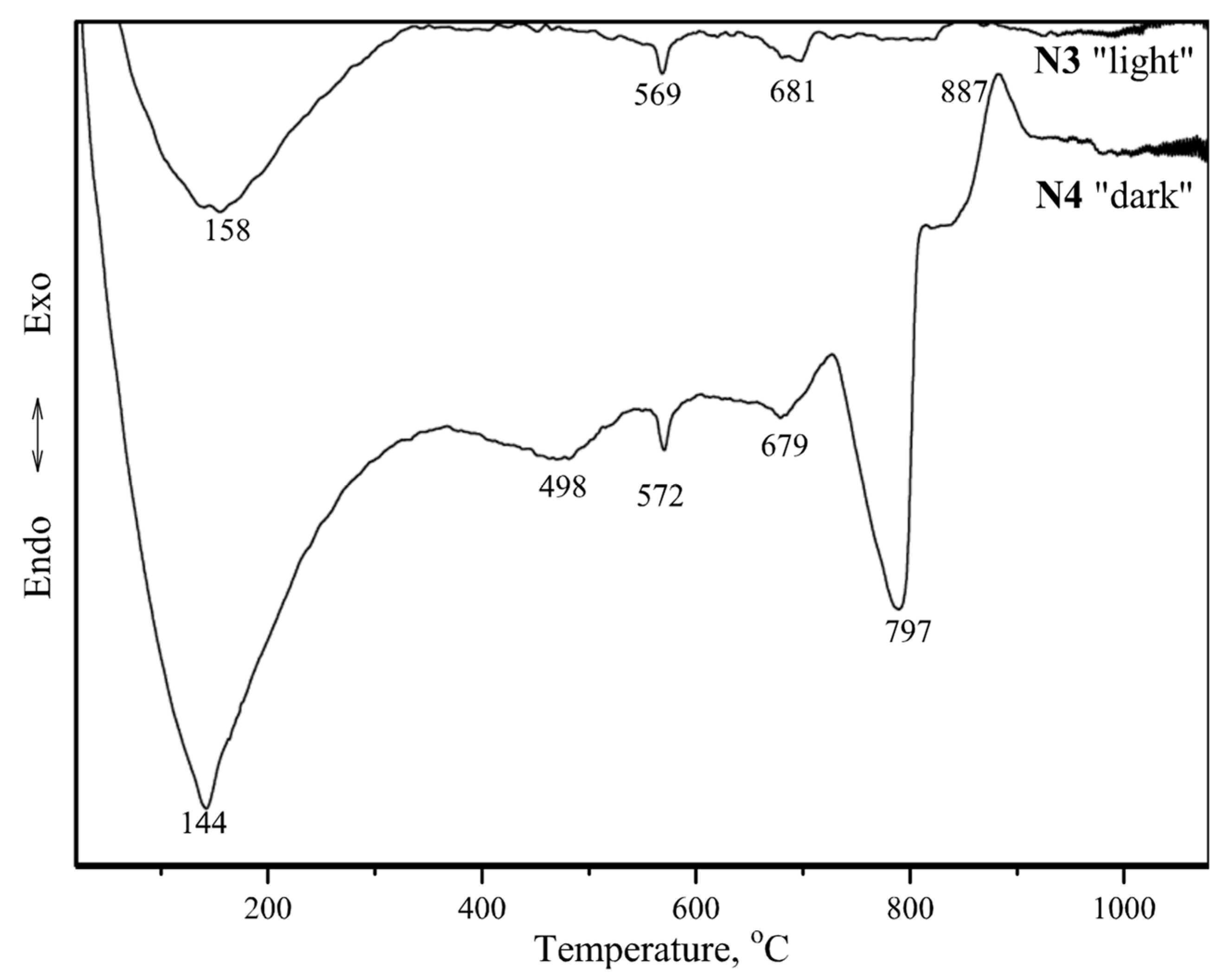

| Core Sample Code | Core Sampling Depth, cm | Compressive Strength, MPa | Volumetric Density, kg/m3 | ||
|---|---|---|---|---|---|
| Specimen | Average | Specimen | Average | ||
| N2 | 280–320 | 7.4 | 10.2 20 * | 1650 | 1720 2.7 * |
| N3 | 320–380 | 9.2 | 1720 | ||
| 11.5 | 1780 | ||||
| N4 | 390–440 | 7.8 | 1720 | ||
| N6 | 670–700 | 11.0 | 1780 | ||
| N10 | 700–730 | 11.8 | 1700 | ||
| N12 | 200–220 | 12.7 | 1710 | ||
| Note: specimens are cylinders Ø =115 mm, h =115 mm * variation, % | |||||
| Core Sample Code | Core Sampling Depth, cm | Compressive Strength, MPa | Water Absorption, wt.% | Volumetric Density, kg/m3 | |||
|---|---|---|---|---|---|---|---|
| Specimen fi | Normalised ** fbi | Specimen | Average | Specimen | Average | ||
| N2 | 280–320 | 12.9 | 10.7 | 17 | 16 8.4 * | 1870 | 1840 4.2 * |
| N7 | 200–220 | 18.0 | 14.4 | 15 | 1850 | ||
| 30.2 | 24.1 | 14 | 1750 | ||||
| N8 | 420–450 | 36.4 | 29.5 | 17 | 1760 | ||
| 31.0 | 25.5 | 17 | 1830 | ||||
| N10 | 700–730 | 22.1 | 18.5 | 15 | 1950 | ||
| Note: specimens are 50 × 50 × 50 mm cubes * variation, % ** the normalised compressive strength fbi was calculated according to LST EN 772-1 standard [23] by applying the capacity reduction factor d (fbi = fi·d). Average normalised compressive strength fb = 20.5 MPa (variation 33.5%) | |||||||
| Core Sample Code | Core Sampling Depth, cm | Compressive Strength, MPa | Water Absorption, wt.% | Volumetric Density, kg/m3 | |||
|---|---|---|---|---|---|---|---|
| Specimen | Average | Specimen | Average | Specimen | Average | ||
| N1 | 152–180 | 7.2 | 8.5 25 * | 6.0 | 15.7 0.081 * | 1430 | 1560 6.8 * |
| N9 | 486–530 | 8.1 | 14.4 | 1600 | |||
| 7.0 | 16.6 | 1450 | |||||
| N11 | 731–757 | 9.8 | 14.9 | 1610 | |||
| 12.3 | 14.0 | 1710 | |||||
| N13 | 638–658 | 6.7 | 16.1 | 1530 | |||
| Note: specimens are 40 × 40 × 40 mm cut cubes * variation, % | |||||||
| Specimen Marking | |||||
|---|---|---|---|---|---|
| N1 | N2 | N3 | N4 | N5 | |
| Oxide, wt.% | |||||
| CaO | 21.58 | 24.32 | 27.42 | 27.06 | 30.67 |
| MgO | 6.67 | 6.00 | 6.76 | 6.55 | 10.76 |
| Al2O3 | 4.00 | 5.26 | 4.34 | 4.87 | 4.16 |
| SiO2 | 53.15 | 50.12 | 47.55 | 47.26 | 41.60 |
| Fe2O3 | 1.03 | 1.20 | 1.33 | 1.68 | 1.38 |
| K2O | 0.88 | 1.00 | 1.00 | 1.05 | 0.99 |
| Na2O | 0.66 | 0.50 | 0.43 | 0.45 | 0.39 |
| O | 11.55 | 11.06 | 10.36 | 10.57 | 9.37 |
| Other * | 0.48 | 0.55 | 0.81 | 0.53 | 0.67 |
| Specimen Marking | Weight Loss in a Certain Temperature Range (%) | Total Weight Loss (%) | |||
|---|---|---|---|---|---|
| <120 °C | 120–200 °C | 200–600 °C | >600 °C | 1000 °C | |
| N1 | 1.18 | 1.01 | 4.66 | 8.70 | 15.56 |
| N2 | 0.95 | 0.80 | 5.40 | 5.56 | 11.90 |
| N3 | 0.96 | 1.08 | 7.59 | 8.93 | 18.56 |
| N4 | 1.15 | 1.03 | 4.62 | 6.84 | 13.64 |
| N5 | 0.95 | 0.84 | 4.57 | 8.36 | 14.72 |
| Specimen Marking | ||||
|---|---|---|---|---|
| White Inclusions | Brick Crumbles | |||
| N1 | N2 | N3 Light | N4 Dark | |
| Chemical Element, wt.%: | ||||
| O | 58.22 | 51.80 | 50.56 | 51.57 |
| Si | 9.60 | 10.36 | 25.34 | 23.86 |
| Al | 2.98 | 2.17 | 10.54 | 8.80 |
| Ca | 24.14 | 29.47 | 8.95 | 9.86 |
| Mg | 0.97 | 1.76 | 1.08 | 1.16 |
| C | 4.11 | 4.45 | - | - |
| K | - | - | 1.20 | 1.33 |
| Fe | - | - | 1.96 | 2.91 |
| Na | - | - | 0.37 | 0.51 |
| Total, wt.% | 100.0 | 100.0 | 100.0 | 100.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonaitis, B.; Antonovič, V.; Šneideris, A.; Boris, R.; Zavalis, R. Analysis of Physical and Mechanical Properties of the Mortar in the Historic Retaining Wall of the Gediminas Castle Hill (Vilnius, Lithuania). Materials 2019, 12, 8. https://doi.org/10.3390/ma12010008
Jonaitis B, Antonovič V, Šneideris A, Boris R, Zavalis R. Analysis of Physical and Mechanical Properties of the Mortar in the Historic Retaining Wall of the Gediminas Castle Hill (Vilnius, Lithuania). Materials. 2019; 12(1):8. https://doi.org/10.3390/ma12010008
Chicago/Turabian StyleJonaitis, Bronius, Valentin Antonovič, Arnoldas Šneideris, Renata Boris, and Robertas Zavalis. 2019. "Analysis of Physical and Mechanical Properties of the Mortar in the Historic Retaining Wall of the Gediminas Castle Hill (Vilnius, Lithuania)" Materials 12, no. 1: 8. https://doi.org/10.3390/ma12010008






