Microwave-assisted Synthesis of Hexagonal Gold Nanoparticles Reduced by Organosilane (3-Mercaptopropyl)trimethoxysilane
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis
2.3. Characterization
3. Results and discussion
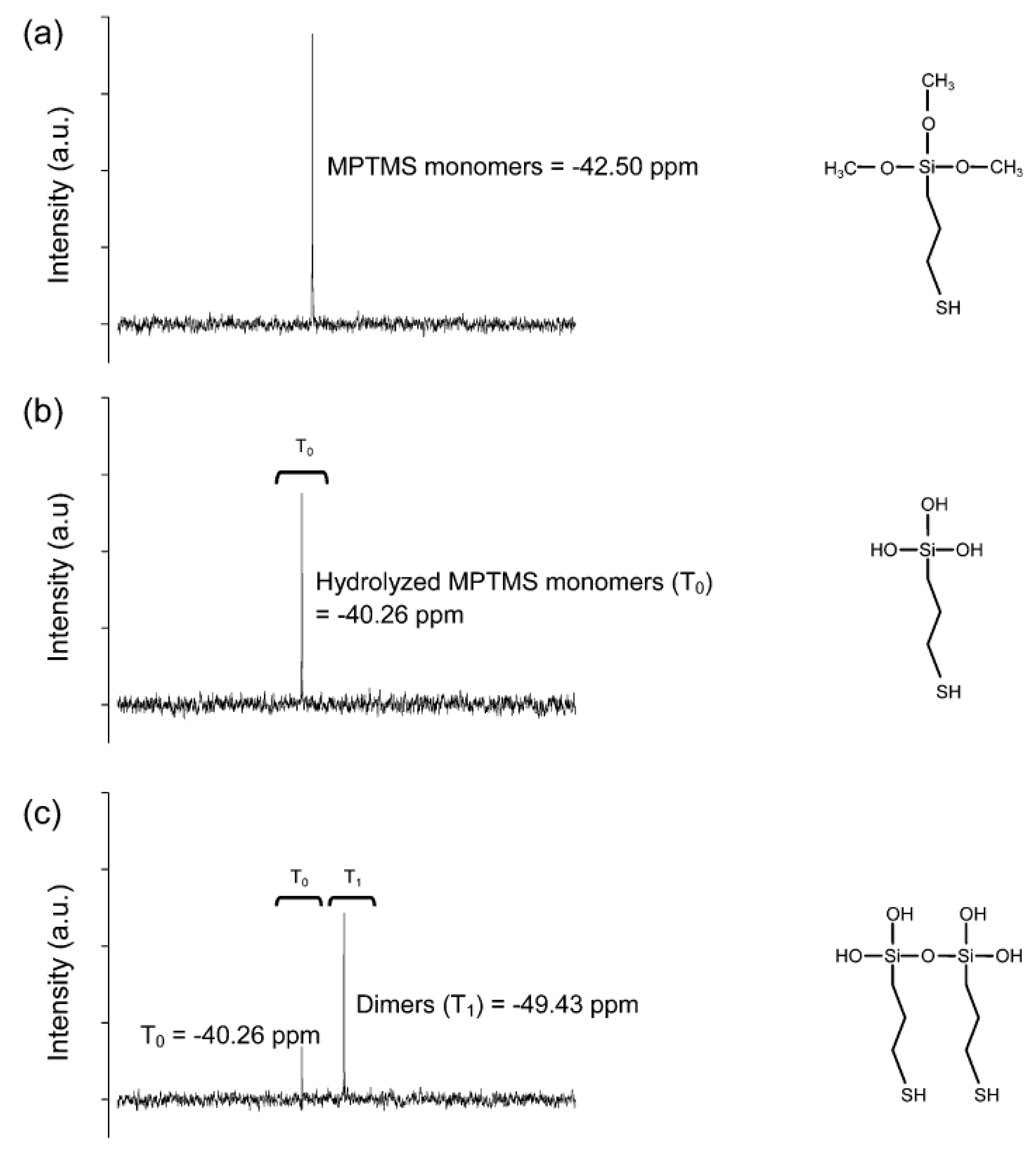
3.1. Formation of Au NPs
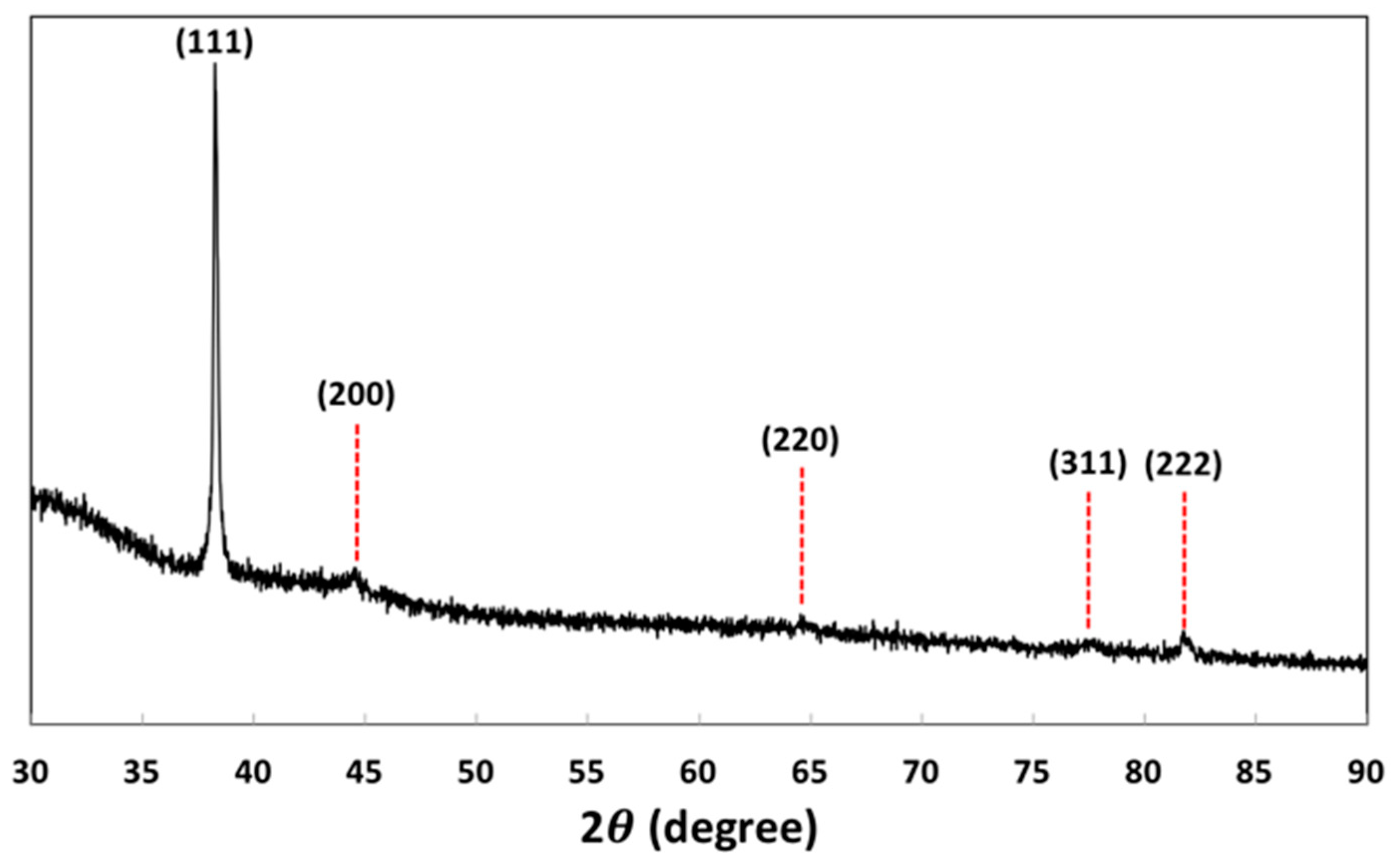
3.2. Characteristics of As-synthesized Au NPs
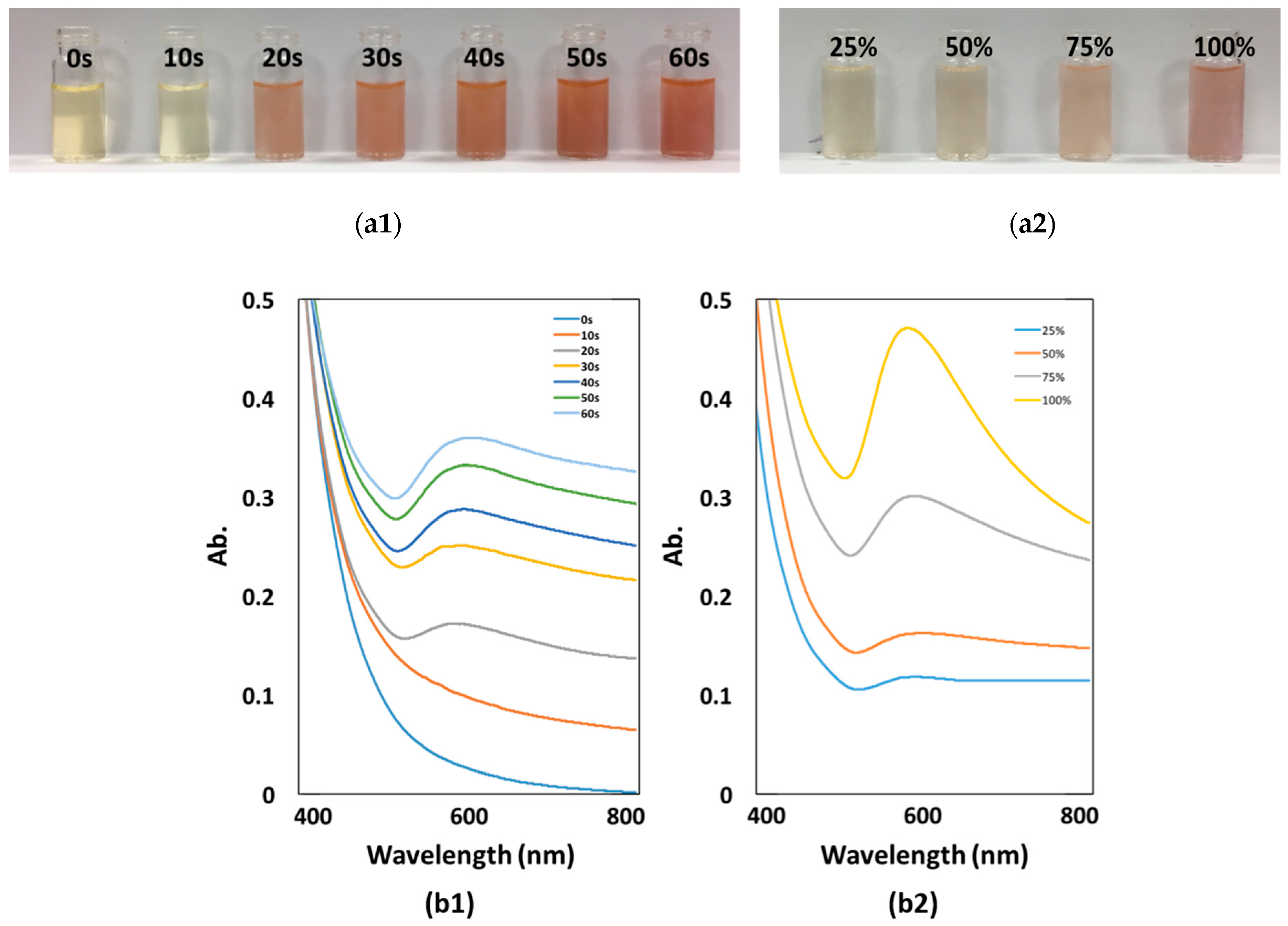
3.3. Effect of Microwave Irradiation
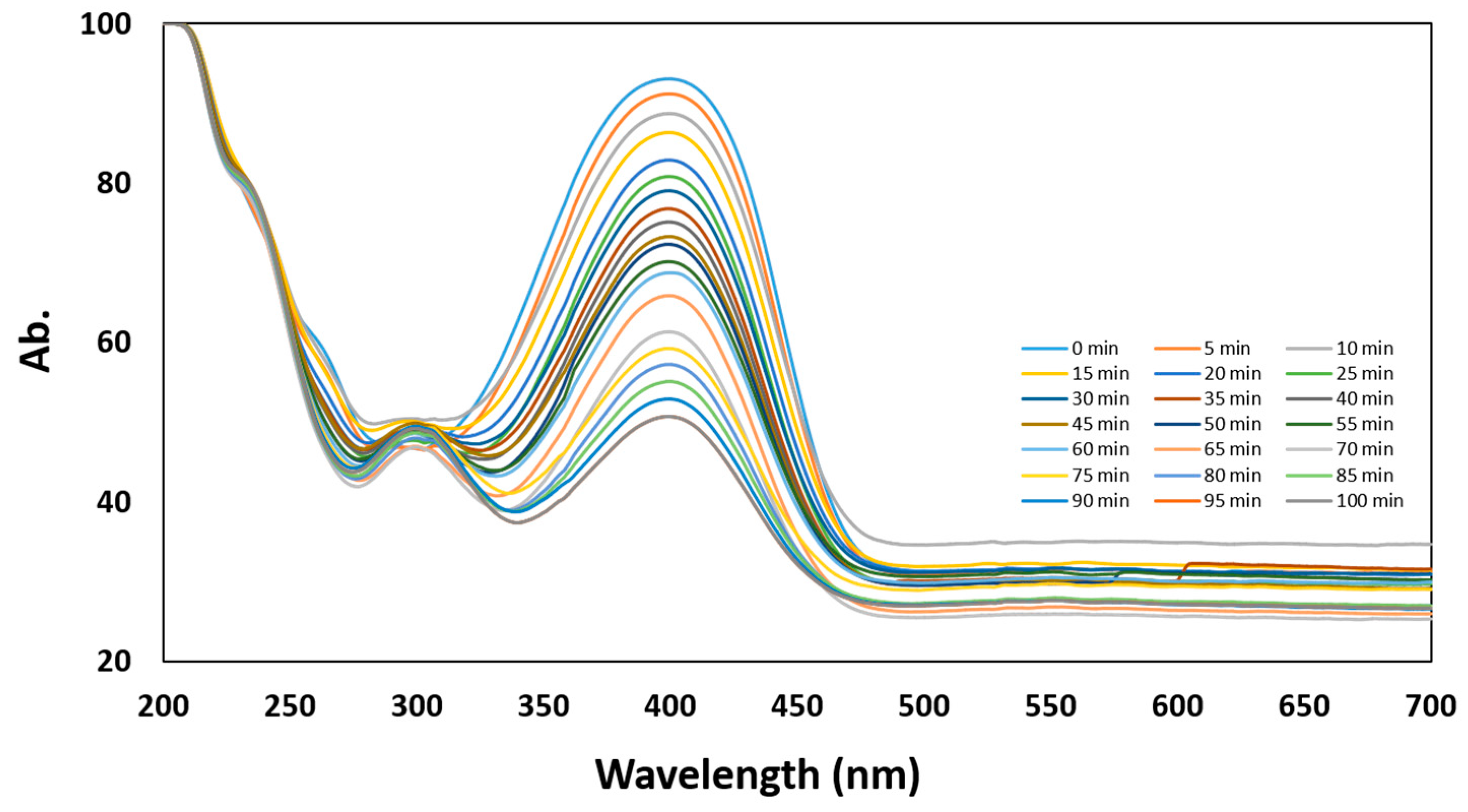
3.4. Catalytic Reduction of 4-Nitrophenol
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohamed, M.B.; AbouZeid, K.M.; Abdelsayed, V.; Aljarash, A.A.; El-Shall, M.S. Growth mechanism of anisotropic gold nanocrystals via microwave synthesis: formation of dioleamide by gold nanocatalysis. ACS nano 2010, 4, 2766–2772. [Google Scholar] [CrossRef]
- Iwakoshi, A.; Nanke, T.; Kobayashi, T. Coating materials containing gold nanoparticles. Gold. Bull. 2005, 38, 107–112. [Google Scholar] [CrossRef]
- Kuo, C.-N.; Chen, H.-F.; Lin, J.-N.; Wan, B.-Z. Nano-gold supported on TiO2 coated glass-fiber for removing toxic CO gas from air. Catal. Today 2007, 122, 270–276. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, X.; Shi, F.; Deng, Y. Nano-gold catalysis in fine chemical synthesis. Chem. Rev. 2011, 112, 2467–2505. [Google Scholar]
- Chakraborty, S.; Ansar, S.M.; Stroud, J.G.; Kitchens, C.L. Comparison of Colloidal versus Supported Gold Nanoparticle Catalysis. J. Phys. Chem. C 2018, 122, 7749–7758. [Google Scholar] [CrossRef]
- Min, B.K.; Friend, C.M. Heterogeneous gold-based catalysis for green chemistry: low-temperature CO oxidation and propene oxidation. Chem. Rev. 2007, 107, 2709–2724. [Google Scholar] [CrossRef] [PubMed]
- Storhoff, J.J.; Lucas, A.D.; Garimella, V.; Bao, Y.P.; Müller, U.R. Homogeneous detection of unamplified genomic DNA sequences based on colorimetric scatter of gold nanoparticle probes. Nat. Biotechnol. 2004, 22, 883. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, D.; Nörbel, L.; Shen, J.; Zhao, Z.; Dou, Y.; Peng, T.; Shi, J.; Mathur, S.; Fan, C. Multicolor gold–silver nano-mushrooms as ready-to-use SERS probes for ultrasensitive and multiplex DNA/miRNA detection. Anal. Chem. 2017, 89, 2531–2538. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Samia, A.C.; Meyers, J.D.; Panagopoulos, I.; Fei, B.; Burda, C. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J. Am. Chem. Soc. 2008, 130, 10643–10647. [Google Scholar] [CrossRef]
- Kennedy, L.C.; Bickford, L.R.; Lewinski, N.A.; Coughlin, A.J.; Hu, Y.; Day, E.S.; West, J.L.; Drezek, R.A. A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small 2011, 7, 169–183. [Google Scholar] [CrossRef]
- Rengan, A.K.; Bukhari, A.B.; Pradhan, A.; Malhotra, R.; Banerjee, R.; Srivastava, R.; De, A. In vivo analysis of biodegradable liposome gold nanoparticles as efficient agents for photothermal therapy of cancer. Nano Lett. 2015, 15, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2014, 43, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205. [Google Scholar] [CrossRef]
- Jankovic, V.; Yang, Y.; You, J.; Dou, L.; Liu, Y.; Cheung, P.; Chang, J.P.; Yang, Y. Active layer-incorporated, spectrally tuned Au/SiO2 core/shell nanorod-based light trapping for organic photovoltaics. ACS nano 2013, 7, 3815–3822. [Google Scholar] [CrossRef] [PubMed]
- Oo, T.Z.; Mathews, N.; Xing, G.; Wu, B.; Xing, B.; Wong, L.H.; Sum, T.C.; Mhaisalkar, S.G. Ultrafine gold nanowire networks as plasmonic antennae in organic photovoltaics. J. Phys. Chem. C 2012, 116, 6453–6458. [Google Scholar] [CrossRef]
- Xu, X.; Stevens, M.; Cortie, M. In situ precipitation of gold nanoparticles onto glass for potential architectural applications. Chem. Mater. 2004, 16, 2259–2266. [Google Scholar] [CrossRef]
- Hrapovic, S.; Liu, Y.; Enright, G.; Bensebaa, F.; Luong, J.H. New strategy for preparing thin gold films on modified glass surfaces by electroless deposition. Langmuir 2003, 19, 3958–3965. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Controlling the optical properties of lemongrass extract synthesized gold nanotriangles and potential application in infrared-absorbing optical coatings. Chem. Mater. 2005, 17, 566–572. [Google Scholar] [CrossRef]
- Wei, D.; Qian, W.; Shi, Y.; Ding, S.; Xia, Y. Mass synthesis of single-crystal gold nanosheets based on chitosan. Carbohydr. Res. 2007, 342, 2494–2499. [Google Scholar] [CrossRef]
- Kim, F.; Connor, S.; Song, H.; Kuykendall, T.; Yang, P. Platonic gold nanocrystals. Angew. Chem. Int. Ed. 2004, 43, 3673–3677. [Google Scholar] [CrossRef]
- Tsuji, M.; Miyamae, N.; Hashimoto, M.; Nishio, M.; Hikino, S.; Ishigami, N.; Tanaka, I. Shape and size controlled synthesis of gold nanocrystals using oxidative etching by AuCl4− and Cl− anions in microwave-polyol process. Colloids Surf., A 2007, 302, 587–598. [Google Scholar] [CrossRef]
- Lisiecki, I.; Pileni, M.P. Synthesis of copper metallic clusters using reverse micelles as microreactors. J. Am. Chem. Soc. 1993, 115, 3887–3896. [Google Scholar] [CrossRef]
- Cai, M.; Chen, J.; Zhou, J. Reduction and morphology of silver nanoparticles via liquid–liquid method. Appl. Surf. Sci. 2004, 226, 422–426. [Google Scholar] [CrossRef]
- Kuo, P.-L.; Chen, W.-F. Formation of silver nanoparticles under structured amino groups in pseudo-dendritic poly (allylamine) derivatives. J. Phys. Chem. B 2003, 107, 11267–11272. [Google Scholar] [CrossRef]
- Yanagihara, N.; Tanaka, Y.; Okamoto, H. Formation of silver nanoparticles in poly (methyl methacrylate) by UV irradiation. Chem. Lett. 2001, 30, 796–797. [Google Scholar] [CrossRef]
- Chu, H.-C.; Kuo, C.-H.; Huang, M.H. Thermal Aqueous Solution Approach for the Synthesis of Triangular and Hexagonal Gold Nanoplates with Three Different Size Ranges. Inorg. Chem. 2006, 45, 808–813. [Google Scholar] [CrossRef]
- Huang, W.-L.; Chen, C.-H.; Huang, M.H. Investigation of the Growth Process of Gold Nanoplates Formed by Thermal Aqueous Solution Approach and the Synthesis of Ultra-Small Gold Nanoplates. J. Phys. Chem. C 2007, 111, 2533–2538. [Google Scholar] [CrossRef]
- Shang, L.; Yang, L.; Stockmar, F.; Popescu, R.; Trouillet, V.; Bruns, M.; Gerthsen, D.; Nienhaus, G.U. Microwave-assisted rapid synthesis of luminescent gold nanoclusters for sensing Hg 2+ in living cells using fluorescence imaging. Nanoscale 2012, 4, 4155–4160. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z. Rapid synthesis of hexagon-shaped gold nanoplates by microwave assistant method. Mater. Lett. 2007, 61, 4149–4151. [Google Scholar] [CrossRef]
- Kou, J.; Bennett-Stamper, C.; Varma, R.S. Green synthesis of noble nanometals (Au, Pt, Pd) using glycerol under microwave irradiation conditions. ACS. Sustainable Chem. Eng 2013, 1, 810–816. [Google Scholar] [CrossRef]
- Yu, S.; Hachtel, J.A.; Chisholm, M.F.; Pantelides, S.T.; Laromaine, A.; Roig, A. Magnetic gold nanotriangles by microwave-assisted polyol synthesis. Nanoscale 2015, 7, 14039–14046. [Google Scholar] [CrossRef]
- Horikoshi, S.; Abe, H.; Sumi, T.; Torigoe, K.; Sakai, H.; Serpone, N.; Abe, M. Microwave frequency effect in the formation of Au nanocolloids in polar and non-polar solvents. Nanoscale 2011, 3, 1697–1702. [Google Scholar] [CrossRef]
- Lin, G.; Lu, W.; Cui, W.; Jiang, L. A simple synthesis method for gold nano-and microplate fabrication using a tree-type multiple-amine head surfactant. Cryst. Growth Des. 2010, 10, 1118–1123. [Google Scholar] [CrossRef]
- Ren, L.; Meng, L.; Lu, Q.; Fei, Z.; Dyson, P.J. Fabrication of gold nano-and microstructures in ionic liquids—a remarkable anion effect. J. Colloid Interface Sci. 2008, 323, 260–266. [Google Scholar] [CrossRef]
- Mallikarjuna, N.N.; Varma, R.S. Microwave-assisted shape-controlled bulk synthesis of noble nanocrystals and their catalytic properties. Cryst. Growth Des. 2007, 7, 686–690. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Zhang, J.; Han, B.; Du, J.; Gao, Y.; Jiang, T. Synthesis of single-crystal gold nanosheets of large size in ionic liquids. J. Phys. Chem. B 2005, 109, 14445–14448. [Google Scholar] [CrossRef]
- Kawasaki, H.; Kosaka, Y.; Myoujin, Y.; Narushima, T.; Yonezawa, T.; Arakawa, R. Microwave-assisted polyol synthesis of copper nanocrystals without using additional protective agents. Chem. Commun. 2011, 47, 7740–7742. [Google Scholar] [CrossRef] [Green Version]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef]
- Gerbec, J.A.; Magana, D.; Washington, A.; Strouse, G.F. Microwave-enhanced reaction rates for nanoparticle synthesis. J. Am. Chem. Soc. 2005, 127, 15791–15800. [Google Scholar] [CrossRef]
- Millstone, J.E.; Métraux, G.S.; Mirkin, C.A. Controlling the edge length of gold nanoprisms via a seed-mediated approach. Adv. Funct. Mater. 2006, 16, 1209–1214. [Google Scholar] [CrossRef]
- Sau, T.K.; Murphy, C.J. Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. J. Am. Chem. Soc. 2004, 126, 8648–8649. [Google Scholar] [CrossRef]
- Motoyama, Y.; Aoki, M.; Takaoka, N.; Aoto, R.; Nagashima, H. Highly efficient synthesis of aldenamines from carboxamides by iridium-catalyzed silane-reduction/dehydration under mild conditions. Chem. Commun. 2009, 1574–1576. [Google Scholar] [CrossRef]
- Chauhan, B.P.; Thekkathu, R.; Prasanth K, L.; Mandal, M.; Lewis, K. Long-chain silanes as reducing agents part 1: A facile, efficient and selective route to amine and phosphine-stabilized active Pd-nanoparticles. Appl. Organomet. Chem. 2010, 24, 222–228. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, M.; Li, X.; Chan, A.S.; Li, C.-J. Highly Efficient Reduction of Aldehydes with Silanes in Water Catalyzed by Silver. Synlett 2013, 24, 2049–2056. [Google Scholar]
- Mohammadnejad, S.; Provis, J.L.; van Deventer, J.S. Reduction of gold (III) chloride to gold (0) on silicate surfaces. J. Colloid Interface Sci. 2013, 389, 252–259. [Google Scholar] [CrossRef]
- Joseph, S.; Mathew, B. Microwave assisted facile green synthesis of silver and gold nanocatalysts using the leaf extract of Aerva lanata. Spectrochim. Acta Part A 2015, 136, 1371–1379. [Google Scholar] [CrossRef]
- Francis, S.; Joseph, S.; Koshy, E.P.; Mathew, B. Synthesis and characterization of multifunctional gold and silver nanoparticles using leaf extract of Naregamia alata and their applications in the catalysis and control of mastitis. New J. Chem. 2017, 41, 14288–14298. [Google Scholar] [CrossRef]
- Gangula, A.; Podila, R.; Karanam, L.; Janardhana, C.; Rao, A.M. Catalytic reduction of 4-nitrophenol using biogenic gold and silver nanoparticles derived from Breynia rhamnoides. Langmuir 2011, 27, 15268–15274. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, K.W.; Zheng, L. Microwave-assisted Synthesis of Hexagonal Gold Nanoparticles Reduced by Organosilane (3-Mercaptopropyl)trimethoxysilane. Materials 2019, 12, 1680. https://doi.org/10.3390/ma12101680
Shah KW, Zheng L. Microwave-assisted Synthesis of Hexagonal Gold Nanoparticles Reduced by Organosilane (3-Mercaptopropyl)trimethoxysilane. Materials. 2019; 12(10):1680. https://doi.org/10.3390/ma12101680
Chicago/Turabian StyleShah, Kwok Wei, and Long Zheng. 2019. "Microwave-assisted Synthesis of Hexagonal Gold Nanoparticles Reduced by Organosilane (3-Mercaptopropyl)trimethoxysilane" Materials 12, no. 10: 1680. https://doi.org/10.3390/ma12101680





