N-Acetyl-D-Glucosamine-Loaded Chitosan Filaments Biodegradable and Biocompatible for Use as Absorbable Surgical Suture Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
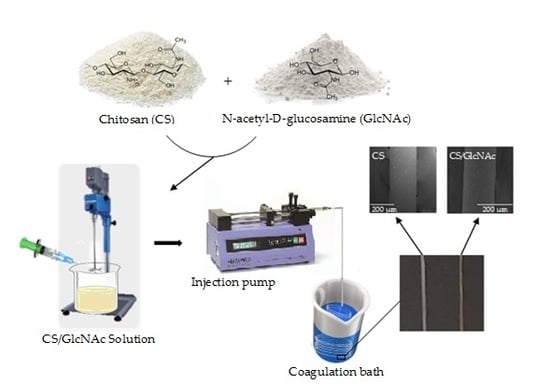
2.2. Fabrication of GlcNAc-Loaded CS Filaments
2.3. Characterization of the Filaments
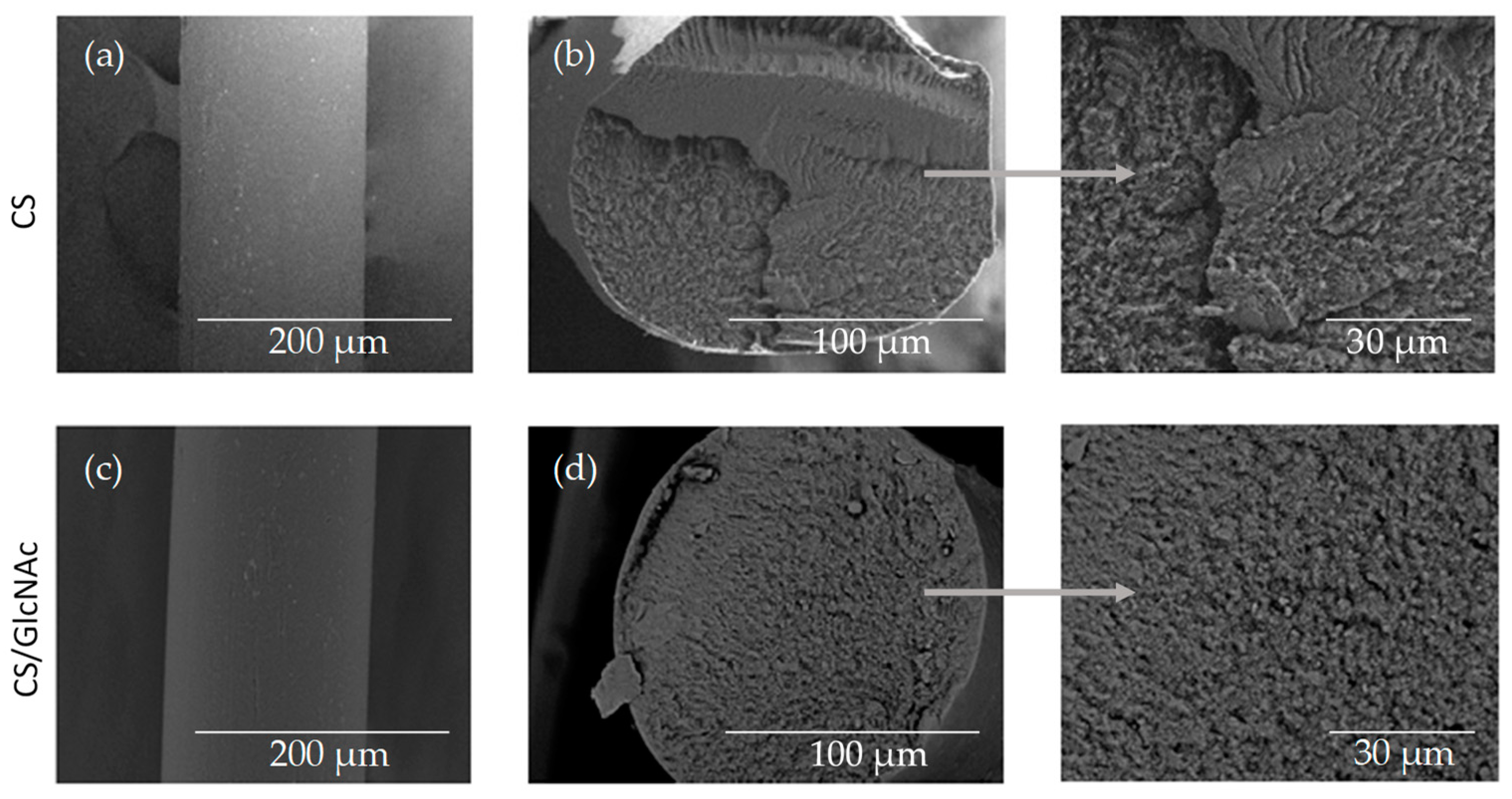
2.3.1. Scanning Electron Microscopy (SEM)
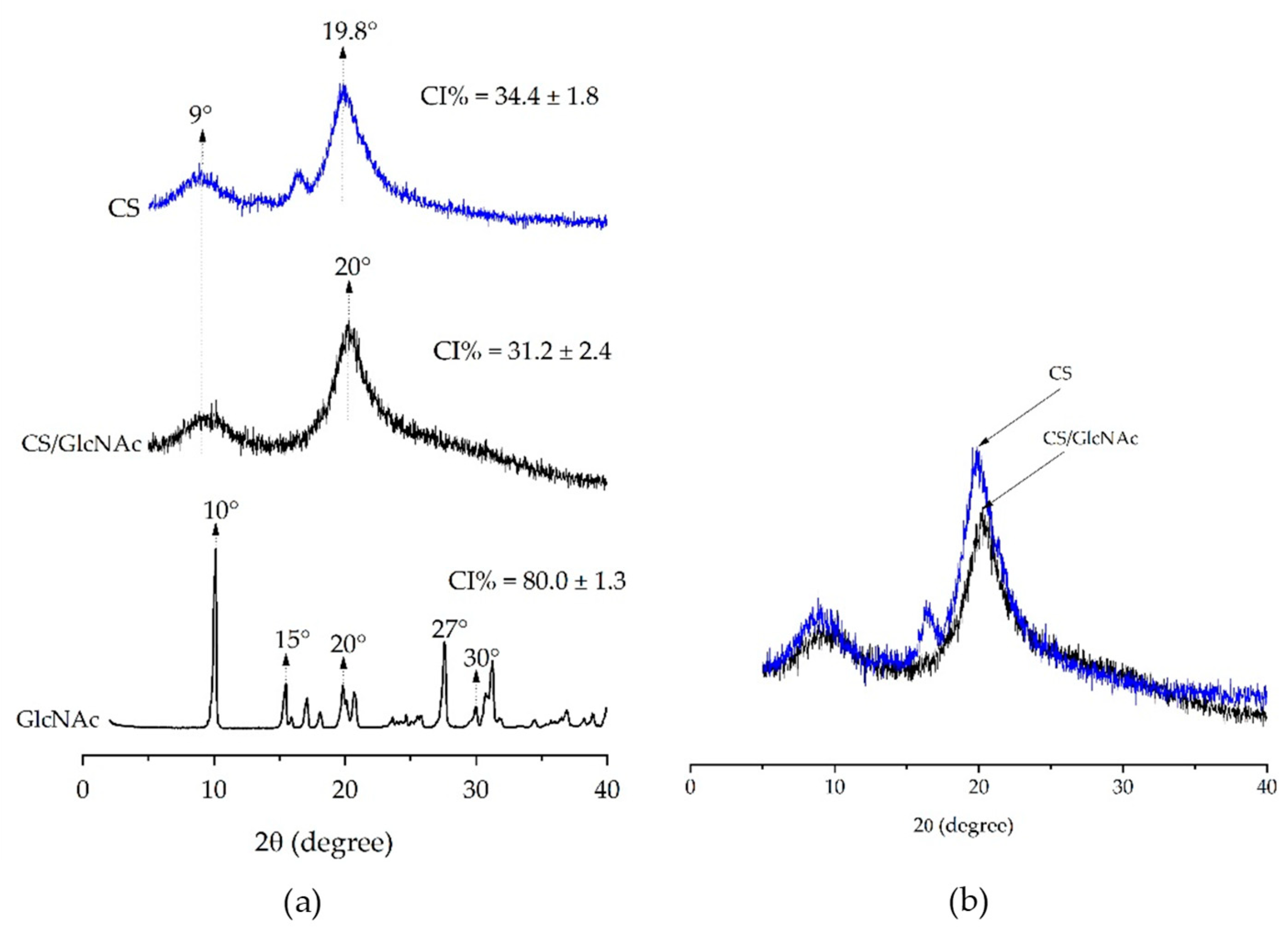
2.3.2. XRD Analysis
2.3.3. Mechanical Properties
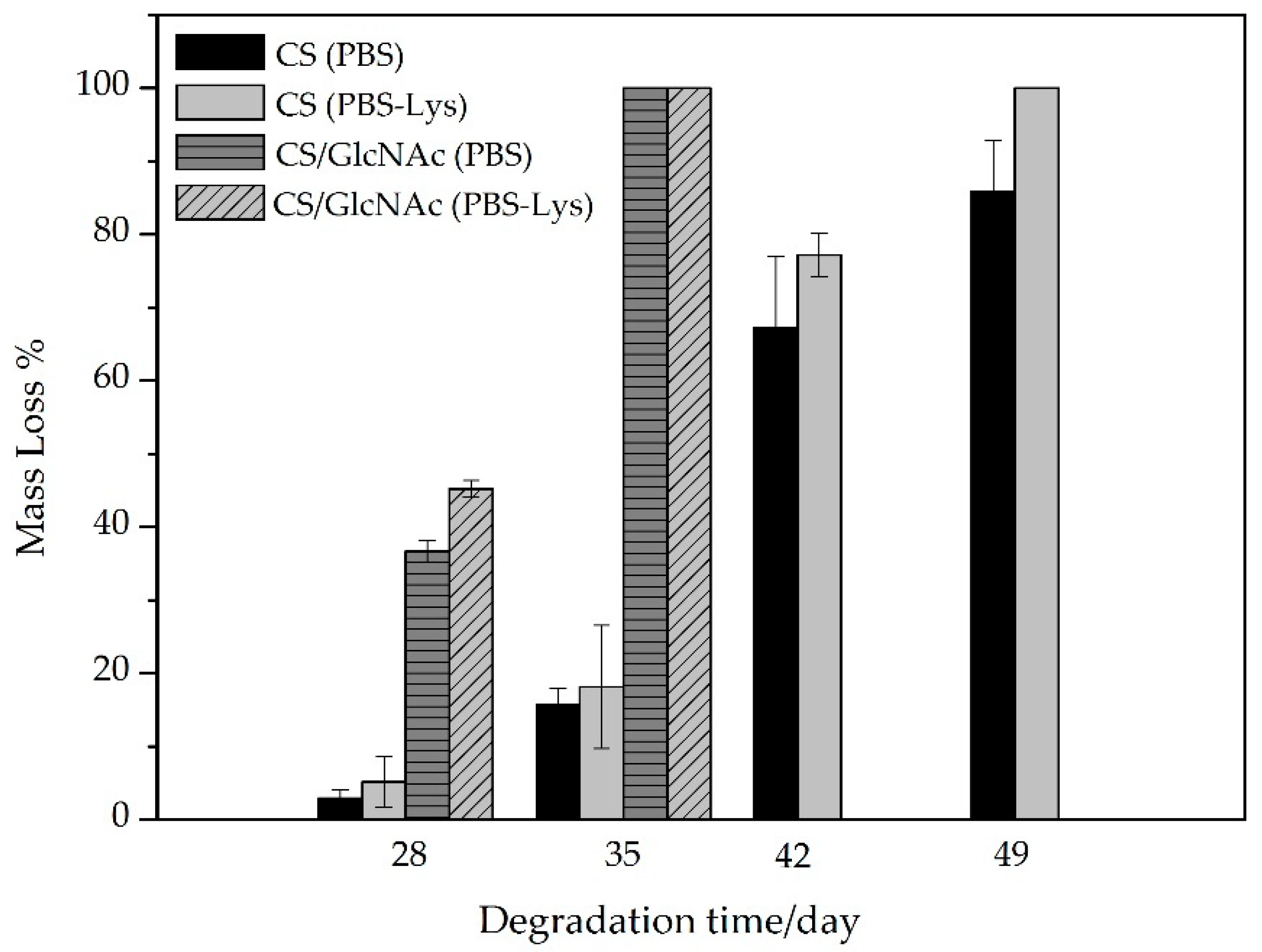
2.3.4. In Vitro Biodegradation
2.3.5. In Vitro Drug Release
2.3.6. In Vitro Cytotoxicity Studies
2.4. Statistical Analysis
3. Results and Discussion
3.1. Filament Morphology
3.2. XRD Analysis
3.3. Mechanical Test
3.4. In Vitro Biodegration
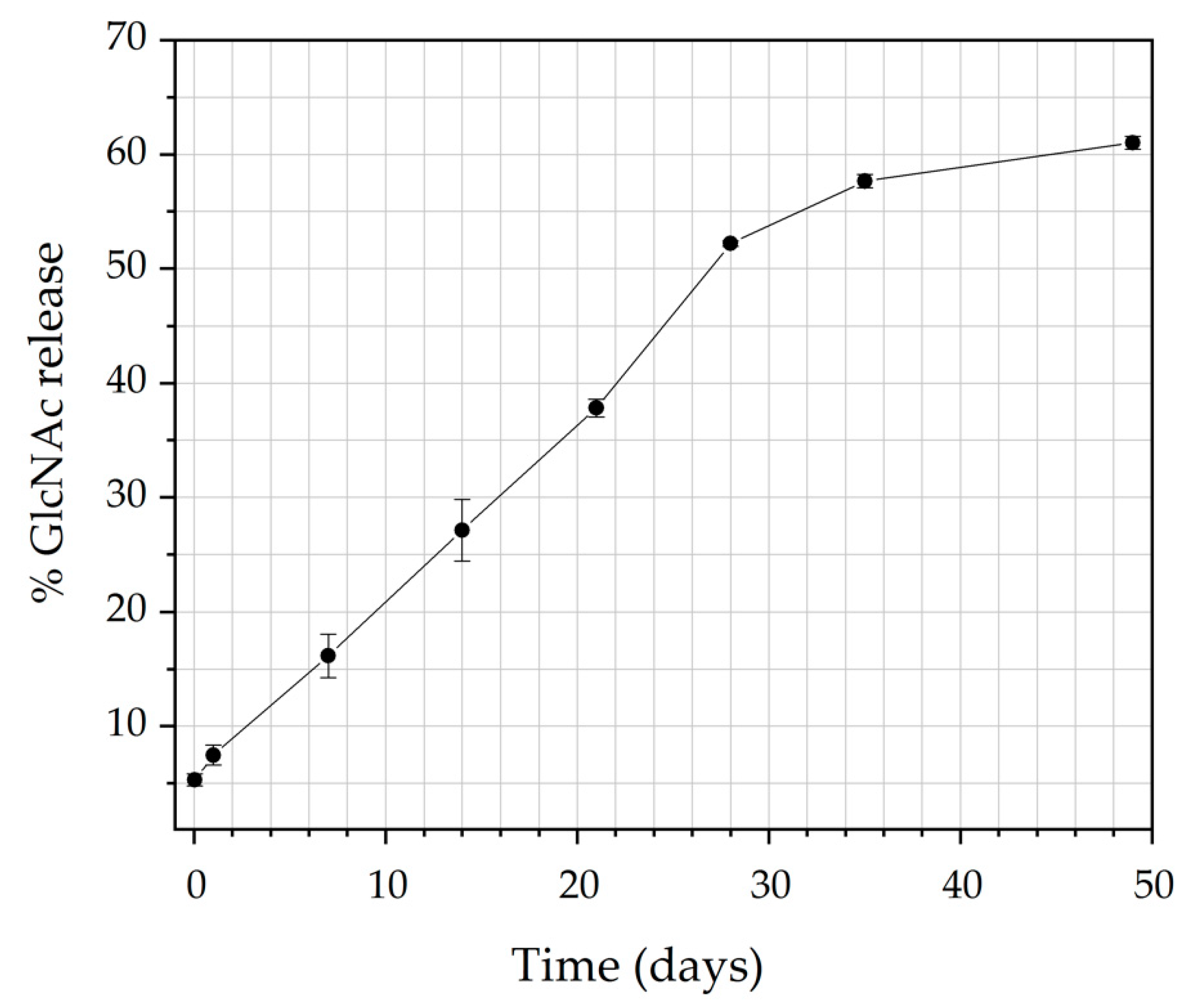
3.5. In Vitro Drug Release
3.6. Kinetics of Release
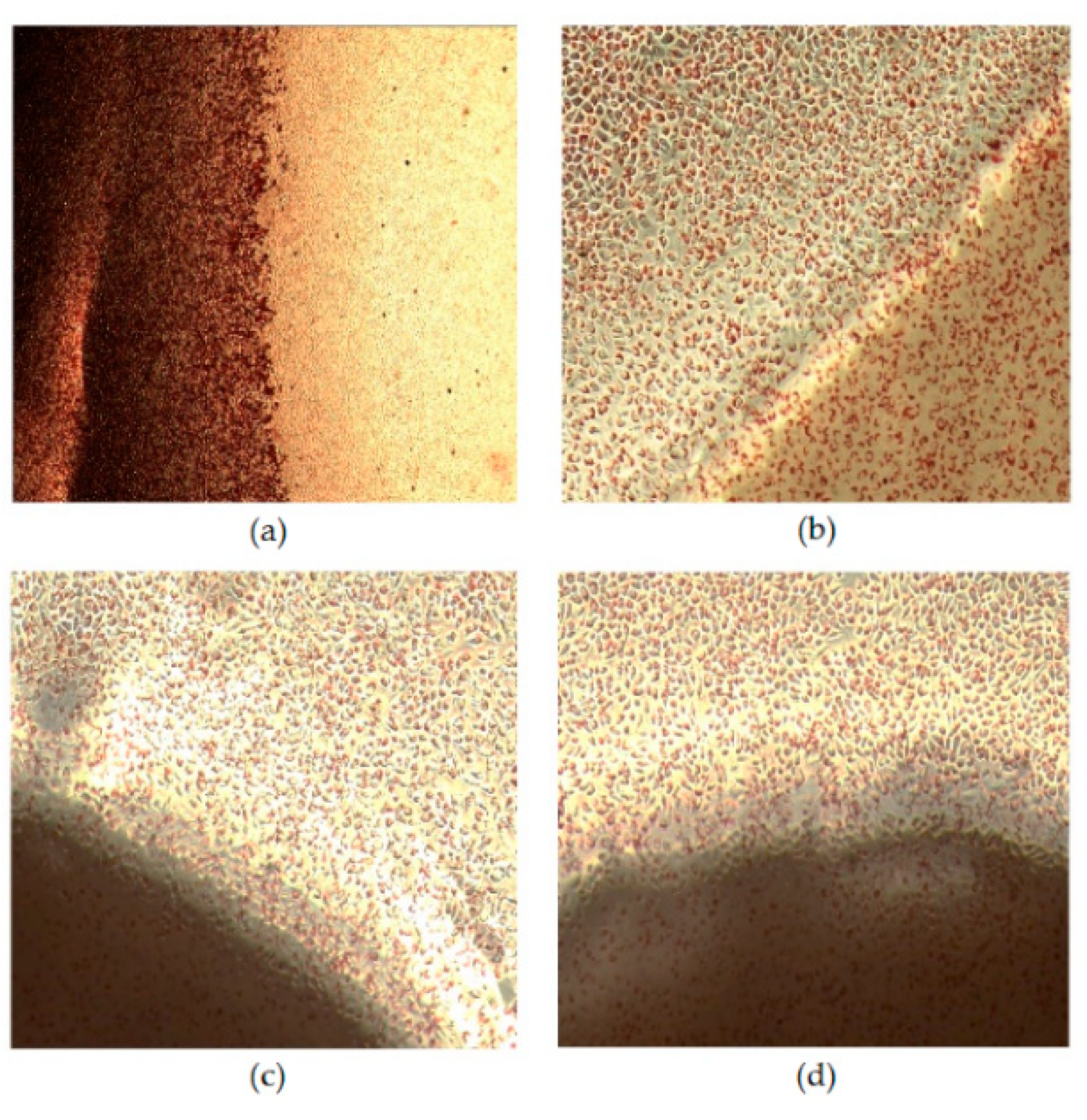
3.7. Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chu, C.C. 10—Types and Properties of Surgical Sutures; Woodhead Publishing Limited: Cambridge, UK, 2013; ISBN 9781845694395. [Google Scholar]
- Kladakis, S. Choosing Sutures in Small Animal Surgery. J. Dairy Vet. Anim. Res. 2015, 1, 68–71. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, Y.K.; Lim, B.S.; Rhee, S.H.; Yang, H.C. Comparison of tensile and knot security properties of surgical sutures. J. Mater. Sci. Mater. Med. 2007, 18, 2363–2369. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.K.S.; Sharma, C.P. Review Paper: Absorbable Polymeric Surgical Sutures: Chemistry, Production, Properties, Biodegradability, and Performance. J. Biomater. Appl. 2010, 25, 291–366. [Google Scholar] [CrossRef] [PubMed]
- Viju, S.; Thilagavathi, G. Fabrication and characterization of silk braided sutures. Fibers Polym. 2012, 13, 782–789. [Google Scholar] [CrossRef]
- Gogoi, D.; Choudhury, A.J.; Chutia, J.; Pal, A.R.; Khan, M.; Choudhury, M.; Pathak, P.; Das, G.; Patil, D.S. Development of advanced antimicrobial and sterilized plasma polypropylene grafted MUGA (antheraea assama) silk as suture biomaterial. Biopolymers 2014, 101, 355–365. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Clark, R.M. Advances in suture material for obstetric and gynecologic surgery. Rev. Obstet. Gynecol. 2009, 2, 146–158. [Google Scholar]
- Debus, E.S.; Geiger, D.; Sailer, M.; Ederer, J.; Thiede, A. Physical, Biological and Handling Characteristics of Surgical Suture Material: A Comparison of Four Different Multifilament Absorbable Sutures. Eur. Surg. Res. 1997, 29, 52–61. [Google Scholar] [CrossRef]
- Lapointe, S.; Zhim, F.; Sidéris, L.; Drolet, P.; Célestin-Noël, S.; Dubé, P. Effect of chemotherapy and heat on biomechanical properties of absorbable sutures. J. Surg. Res. 2016, 200, 59–65. [Google Scholar] [CrossRef]
- Carter, A.; Skilbeck, C.J. Sutures, ligatures and knots. Surgery 2014, 32, 117–120. [Google Scholar] [CrossRef]
- Rethinam, S.; Thotapalli Parvathaleswara, S.; Nandhagobal, G.; Alagumuthu, T.; Robert, B. Preparation of absorbable surgical suture: Novel approach in biomedical application. J. Drug Deliv. Sci. Technol. 2018, 47, 454–460. [Google Scholar] [CrossRef]
- Nakajim, M.; Atsum, K.; Kifune, K.; Miura, K.; Kanamaru, H. Chitin is an Effective Material for Sutures. Jpn. J. Surg. 1986, 16, 418–424. [Google Scholar] [CrossRef]
- Montenegro, R.; Gordeiro, J.R.G. Chitosan Based Suture Focusing on the Real Advantages of an Outstanding. Adv. Chitin Sci. 2012, 14, 211–216. [Google Scholar]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar]
- Prudden, J.F.; Balassa, L.; Friedrich, L.; Hanson, P.; Migel, P. The discovery of a potent pure chemical wound-healing accelerator. Am. J. Surg. 1970, 119, 560–564. [Google Scholar] [CrossRef]
- Vert, M.; Hellwich, K.H.; Hess, M. Terminology for biorelated polymers and applications. Pure Appl. Chem 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, S.; Park, M.; Kima, M.H.; Park, C.G.; Lee, S.H.; Choi, S.Y.; Kim, B.H.; Park, H.J.; Park, J.-H.; et al. Surgical suture assembled with polymeric drug-delivery sheet for sustained, local pain relief. Acta Biomater. 2013, 9, 8318–8327. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Kamdem, D.P. Development and characterization of biodegradable chitosan films containing two essential oils. Int. J. Biol. Macromol. 2015, 74, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Yu, H.; Zain-ul-Abdin; Chen, Y.; Chen, Q.; Zhou, W.; Zhang, H.; Chen, X. Recent progress on synthesis, property and application of modified chitosan: An overview. Int. J. Biol. Macromol. 2016, 88, 333–344. [Google Scholar] [CrossRef]
- Gu, B.K.; Park, S.J.; Kim, M.S.; Lee, Y.J.; Kim, J.-I.; Kim, C.-H. Gelatin blending and sonication of chitosan nanofiber mats produce synergistic effects on hemostatic functions. Int. J. Biol. Macromol. 2016, 82, 89–96. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Masoud Hosseini, S. Effect of chitosan molecular weight as micro and nanoparticles on antibacterial activity against some soft rot pathogenic bacteria. LWT Food Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of chitosan—A challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- De la Fuente, M.; Raviña, M.; Paolicelli, P.; Sanchez, A.; Seijo, B.; Alonso, M.J. Chitosan-based nanostructures: A delivery platform for ocular therapeutics. Adv. Drug Deliv. Rev. 2010, 62, 100–117. [Google Scholar] [CrossRef]
- Saber, A.; Strand, S.P.; Ulfendahl, M. Use of the biodegradable polymer chitosan as a vehicle for applying drugs to the inner ear. Eur. J. Pharm. Sci. 2010, 39, 110–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Pellá, M.C.G.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, D.; Hu, C.; Sun, F.; Gustave, W.; Tian, H.; Yang, S. Flexible fibers wet-spun from formic acid modified chitosan. Carbohydr. Polym. 2016, 136, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Gokarneshan, N.; Dhatchayani, U. Performance Evaluation of Newer Types of Silk Surgical Sutures. J. Gerontol. Geriatr. Med. 2018, 4. [Google Scholar]
- Casalini, T.; Masi, M.; Perale, G. Drug eluting sutures: A model for in vivo estimations. Int. J. Pharm. 2012, 429, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; George, A.; Gopi, S.; Kalarikkal, N.; Thomas, S. Polymer sutures for simultaneous wound healing and drug delivery—A review. Int. J. Pharm. 2017, 524, 454–466. [Google Scholar] [CrossRef]
- Padmakumar, S.; Joseph, J.; Neppalli, M.H.; Mathew, S.E.; Nair, S.V.; Shankarappa, S.A.; Menon, D. Electrospun Polymeric Core-sheath Yarns as Drug Eluting Surgical Sutures. ACS Appl. Mater. Interfaces 2016, 8, 6925–6934. [Google Scholar] [CrossRef] [PubMed]
- Weldon, C.B.; Tsui, J.H.; Shankarappa, S.A.; Nguyen, V.T.; Ma, M.; Anderson, D.G.; Kohane, D.S. Electrospun drug-eluting sutures for local anesthesia. J. Control. Release 2012, 161, 903–909. [Google Scholar] [CrossRef]
- Mccarty, M.F. Glucosamine for Wound Healing. Med. Hypotheses 1996, 47, 273–275. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Human enzymatic activities related to the ther- apeutic administration of chitin derivatives. Cell. Mol. Life Sci. 1997, 53, 131–140. [Google Scholar] [CrossRef]
- Schoukens, G. Bioactive Dressings to Promote Wound Healing, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9781845692711. [Google Scholar]
- Ashkani-Esfahani, S. Glucosamine Enhances Tissue Regeneration In The Process Of Wound Healing In Rats As Animal Model; A Stereological Study. J. Cytol. Histol. 2012, 03, 3–7. [Google Scholar] [CrossRef]
- Hyun, H.; Park, M.; Jo, G.; Kim, S.; Chun, H.; Yang, D. Photo-Cured Glycol Chitosan Hydrogel for Ovarian Cancer Drug Delivery. Mar. Drugs 2019, 17, 41. [Google Scholar] [CrossRef]
- Dalirfardouei, R.; Karimi, G.; Jamialahmadi, K. Molecular mechanisms and biomedical applications of glucosamine as a potential multifunctional therapeutic agent. Life Sci. 2016, 152, 21–29. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer (Guildf) 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Electrospun Fibers from Polysaccharides. Innov. Biofibers Renew. Resour. 2015, 1–454. [Google Scholar]
- Yudin, V.E.; Dobrovolskaya, I.P.; Neelov, I.M.; Dresvyanina, E.N.; Popryadukhin, P.V.; Ivan’Kova, E.M.; Elokhovskii, V.Y.; Kasatkin, I.A.; Okrugin, B.M.; Morganti, P. Wet spinning of fibers made of chitosan and chitin nanofibrils. Carbohydr. Polym. 2014, 108, 176–182. [Google Scholar] [CrossRef]
- Foroughi, J.; Mirabedini, A.; Warren, H. Hydrogels Fibers, 1st ed.; IntechOpen: Melbourne, Australia, 2018; pp. 75–105. [Google Scholar]
- Cruz, R.d.C.A.L.; Diniz, L.G.M.; Lisboa, H.M.; Fook, M.V.L. Effect of different carboxylic acids as solvent on chitosan fibers production by wet spinning. Rev. Matér. 2016, 525–531. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]
- ISO-10993-5 Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity; The International Organization for Standardization: Geneva, Switzerland, 2009.
- Rissanen, M.; Puolakka, A.; Nousiainen, P.; Kellomaki, M.; Ella, V. Solubility and Phase Separation of Poly(L,D-Lactide) Copolymers. J. Appl. Polym. Sci. 2008, 110, 2399–2404. [Google Scholar] [CrossRef]
- Lavin, D.M.; Zhang, L.; Furtado, S.; Hopkins, R.A.; Mathiowitz, E. Effects of protein molecular weight on the intrinsic material properties and release kinetics of wet spun polymeric microfiber delivery systems. Acta Biomater. 2013, 9, 4569–4578. [Google Scholar] [CrossRef]
- Dart, A.J.; Dart, C.M. Suture Material: Conventional and Stimuli Responsive. Compr. Biomater. II 2017, 7, 746–771. [Google Scholar]
- Madihally, S.V.; Matthew, H.W.T. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Madihally, S.V.; Flake, A.W.; Matthew, H.W.T. Maintenance of CD34 expression during proliferation of CD34+ cord blood cells on glycosaminoglycan surfaces. Stem Cells 1999, 17, 295–305. [Google Scholar] [CrossRef]
- Lavin, D.M.; Stefani, R.M.; Zhang, L.; Furtado, S.; Hopkins, R.A.; Mathiowitz, E. Multifunctional polymeric microfibers with prolonged drug delivery and structural support capabilities. Acta Biomater. 2012, 8, 1891–1900. [Google Scholar] [CrossRef]
- Baklagina, Y.G.; Klechkovskaya, V.V.; Kononova, S.V.; Petrova, V.A.; Poshina, D.N.; Orekhov, A.S.; Skorik, Y.A. Polymorphic Modifications of Chitosan. Crystallogr. Rep. 2018, 63, 303–313. [Google Scholar] [CrossRef]
- Heward, A.G.; Laing, R.M.; Carr, D.J.; Niven, B.E. Tensile Performance of Nonsterile Suture Monofilaments Affected by Test Conditions. Text. Res. J. 2004, 74, 83–90. [Google Scholar] [CrossRef]
- Muffly, T.M.; Boyce, J.; Kieweg, S.L.; Bonham, A.J. Tensile Strength of a Surgeon’s or a Square Knot. J. Surg. Educ. 2010, 67, 222–226. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Goldman, R.H. Barbed suture: a review of the technology and clinical uses in obstetrics and gynecology. Rev. Obstet. Gynecol. 2013, 6, 107–115. [Google Scholar]
- Dart, A.J.; Dart, C.M. Suture Material: Conventional and Stimuli Responsive. Comprehrnsive Biomater. 2011, 6, 573–587. [Google Scholar]
- Vehoff, T.; Glišović, A.; Schollmeyer, H.; Zippelius, A.; Salditt, T. Mechanical properties of spider dragline silk: Humidity, hysteresis, and relaxation. Biophys. J. 2007, 93, 4425–4432. [Google Scholar] [CrossRef]
- Judawisastra, H.; Hadyiswanto, I.O.C.; Winiati, W. The Effects of Demineralization Process on Diameter, Tensile Properties and Biodegradation of Chitosan Fiber. Procedia Chem. 2012, 4, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Notin, L.; Viton, C.; David, L.; Alcouffe, P.; Rochas, C.; Domard, A. Morphology and mechanical properties of chitosan fibers obtained by gel-spinning: Influence of the dry-jet-stretching step and ageing. Acta Biomater. 2006, 2, 387–402. [Google Scholar] [CrossRef]
- Barros, M.; Gorgal, R.; Machado, A.P.; Correia, A.; Montenegro, N. Princípios básicos em cirurgia: Fios de sutura. Acta Med. Port. 2011, 24, 1051–1056. [Google Scholar]
- Alves, A.P.; Sá, M.J.C.; Fook, M.V.L.; Felipe, G.C.; Henrique, F.V.; Albuquerque, E.E.; Medeiros, L.K.G.; Alexandre, P.R.S. Avaliação biomecânica e dimensional do fio de sutura à base de quitosana. Arq. Bras. Med. Vet. Zootec. 2017, 69, 896–900. [Google Scholar] [CrossRef]
- Albanna, M.Z.; Bou-Akl, T.H.; Blowytsky, O.; Walters, H.L.; Matthew, H.W.T. Chitosan fibers with improved biological and mechanical properties for tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2013, 20, 217–226. [Google Scholar] [CrossRef]
- Su, B.; Sun, S.; Zhao, C. Polyethersulfone Hollow Fiber Membranes for Hemodialysis. In Progress in Hemodialysis—From Emergent Biotechnology to Clinical Practice; Capri, A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 65–92. [Google Scholar]
- AIBA, S.-I. Studies on chitosan: 4. Lysozymic hydrolysis of partially N-acetylated chitosans. Int. J. Biol. Macromol. 1992, 14, 225–228. [Google Scholar] [CrossRef]
- Nordtveit, R.J.; Vhrum, K.M.; Smidsrod, O. Degradation of fully water-soluble, partially N-acetylated chitosans with lysozyme. Carbohydr. Polym. 1994, 23, 253–260. [Google Scholar] [CrossRef]
- Lee, K.Y.; Ha, W.S.; Park, W.H. Blood compatibility and biodegradability of partially N-acylated chitosan derivatives. Biomaterials 1995, 16, 1211–1216. [Google Scholar] [CrossRef]
- Nordtveit, R.J.; Vårum, K.M.; Smidsrød, O. Degradation of partially N-acetylated chitosans with hen egg white and human lysozyme. Carbohydr. Polym. 1996, 29, 163–167. [Google Scholar] [CrossRef]
- Onishi, H.; Machida, Y. Biodegradation and distribution of water-soluble chitosan in mice. Biomaterials 1999, 20, 175–182. [Google Scholar] [CrossRef]
- Sun, L.; Wanasekara, N.; Chalivendra, V.; Calvert, P. Nano-Mechanical Studies on Polyglactin Sutures Subjected to In Vitro Hydrolytic and Enzymatic Degradation. J. Nanosci. Nanotechnol. 2015, 15, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Laranjeira, M.C.M.; Fávere, V.T. de Quitosana:Biopolímero Funcional com Potencial Industrial Biomédico. Quim. Nova 2009, 32, 672–678. [Google Scholar] [CrossRef]
- Lizardi-Mendoza, J.; Argüelles Monal, W.M.; Goycoolea Valencia, F.M. Chapter 1—Chemical Characteristics and Functional Properties of Chitosan. In Chitosan in the Preservation Agricultural Commodities; Academic Press: Cambridge, MA, USA, 2016; pp. 3–31. ISBN 9780128027356. [Google Scholar]
- Li, Q.; Dunn, E.T.; Grandmaison, E.W.; Goosen, M.F.A. Applications and Properties of Chitosan. J. Bioact. Compat. Polym. 1992, 7, 370–397. [Google Scholar] [CrossRef]
- Wang, X.H.; Cui, F.Z.; Feng, Q.L.; Li, J.C.; Zhang, Y.H. Preparation and Characterization of Collagen/Chitosan Matrices As Potential Biomaterials. J. Bioact. Compat. Polym. 2003, 18, 453–467. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Kumar , M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Qian, S.; Zhang, Q.; Wang, Y.; Lee, B.; Betageri, G.V.; Chow, M.S.S.; Huang, M.; Zuo, Z. Bioavailability enhancement of glucosamine hydrochloride by chitosan. Int. J. Pharm. 2013, 455, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Dredán, J.; Zelkó, R.; Antal, I.; Bihari, E.; Rácz, I. Effect of chemical properties on drug release from hydrophobic matrices. Int. J. Pharm. 1998, 190, 257–260. [Google Scholar] [CrossRef]
- Vueba, M.L.; Batista de Carvalho, L.A.E.; Veiga, F.; Sousa, J.J.; Pina, M.E. Influence of cellulose ether polymers on ketoprofen release from hydrophilic matrix tablets. Eur. J. Pharm. Biopharm. 2004, 58, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Sood, A.; Panchagnula, R. Drug release evaluation of diltiazem CR preparations. Int. J. Pharm. 1998, 175, 95–107. [Google Scholar] [CrossRef]
- NAJIB, N.; SULEIMAN, M. The kinetics of drug release from ethylcellulose solid dispersions. Drug Dev. Ind. Pharm. 1985, 11, 2169–2181. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef]
- Siegel, R.A.; Rathbone, M.J. Fundamentals and Applications of Controlled Release. In Drug Delivery; Siepmann, J., Siegel, R.A., Rathbone, M.J., Eds.; Springer: New York, NY, USA, 2012; pp. 19–43. [Google Scholar]
- Vulcani, V.A.S.; Bizarria, M.T.M.; d’Ávila, M.A.; Mei, L.H.I.; Bernal, C.; Perussi, J.R. Cytotoxicity tests for nanostructured chitosan/PEO membranes using the agar diffusion method. Mater. Res. 2012, 15, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Rejinold, N.S.; Muthunarayanan, M.; Muthuchelian, K.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Saponin-loaded chitosan nanoparticles and their cytotoxicity to cancer cell lines in vitro. Carbohydr. Polym. 2011, 84, 407–416. [Google Scholar] [CrossRef]
- Mansouri, S.; Lavigne, P.; Corsi, K.; Benderdour, M.; Beaumont, E.; Fernandes, J.C. Chitosan-DNA nanoparticles as non-viral vectors in gene therapy: strategies to improve transfection efficacy. Eur. J. Pharm. Biopharm. 2004, 57, 1–8. [Google Scholar] [CrossRef]
- Temtem, M.; Silva, L.M.C.; Andrade, P.Z.; dos Santos, F.; da Silva, C.L.; Cabral, J.M.S.; Abecasis, M.M.; Aguiar-Ricardo, A. Supercritical CO2 generating chitosan devices with controlled morphology. Potential application for drug delivery and mesenchymal stem cell culture. J. Supercrit. Fluids 2009, 48, 269–277. [Google Scholar] [CrossRef]
- Archana, D.; Dutta, J.; Dutta, P.K. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ao, Q.; Wang, A.; Gong, Y.; Zhao, N.; Zhang, X. In Vitro Cytotoxicity and Protein Drug Release Properties of Chitosan/Heparin Microspheres. Tsinghua Sci. Technol. 2007, 12, 361–365. [Google Scholar] [CrossRef]
- Xu, Y.; Han, J.; Chai, Y.; Yuan, S.; Lin, H.; Zhang, X. Development of porous chitosan/tripolyphosphate scaffolds with tunable uncross-linking primary amine content for bone tissue engineering. Mater. Sci. Eng. C 2018, 85, 182–190. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Zhang, Y.; Zhang, D.; Tang, Z.; Chen, X.; Wang, C. Electrospun chitosan/sericin composite nanofibers with antibacterial property as potential wound dressings. Int. J. Biol. Macromol. 2014, 68, 92–97. [Google Scholar] [CrossRef] [PubMed]



| Grade | Reactivity | Reactivity Zone Description |
|---|---|---|
| 0 | None | No detectable zone around or under the specimen |
| 1 | Slight | Some malformed or degenerated cells under the specimen |
| 2 | Mild | Zone limited to the area under the specimen |
| 3 | Moderate | Zone extending the specimen size up to 1.0 cm |
| 4 | Severe | Zone extending farther than 1.0 cm beyond the specimen |
| Filament Type | Sample | Breaking Strength (N) | Elongation at Break (%) | Young Modulus (MPa) | Stress at Break (MPa) |
|---|---|---|---|---|---|
| Non-knotted | CS Dry | 4.3 ± 0.5 | 8.9 ± 1.1 | 25.2 ± 0.1 | 261.0 ± 25.1 |
| CS Wet | 2.9 ± 0.5 | 15.9 ± 5.3 | 6.2 ± 0.3 | 170.9 ± 29.8 | |
| CS/GlcNAc Dry | 3.4 ± 0.7 | 8.6 ± 1.0 | 21.7 ± 2.0 | 181.9 ± 71.5 | |
| CS/GlcNAc Wet | 2.8 ± 0.8 | 17.2 ± 3.9 | 7.2 ± 0.2 | 159.4 ± 39.0 | |
| Knotted | CS Dry | 2.4 ± 0.5 | 10.5 ± 4.3 | 17.8 ± 0.2 | 127.1 ± 32.8 |
| CS Wet | 2.7 ± 0.7 | 11.4 ± 2.8 | 1.2 ± 0.3 | 104.4 ± 22.3 | |
| CS/GlcNAc Dry | 1.9 ± 0.2 | 9.2 ± 2.6 | 9.1 ± 0.2 | 95.4 ± 26.1 | |
| CS/GlcNAc Wet | 1.8 ± 0.7 | 16.6 ± 5.4 | 4.8 ± 1.4 | 88.3 ± 51.1 |
| Models | r2 | AIC | MSC |
|---|---|---|---|
| Zero-order | 0.994 | 30.52 | 4.08 |
| Higuchi | 0.908 | 46.08 | 2.14 |
| Peppas–Sahlin | 0.987 | 35.72 | 3.43 |
| Hopfenberg | 0.981 | 33.85 | 3.66 |
| Reading the Plates Average (cm) | Cytotoxicity Degree | |
|---|---|---|
| Negative Control | 0.00 | 0 |
| Positive Control | 0.92 | 3 |
| Chitosan | 0.00 | 0 |
| Chitosan/GlcNAc | 0.00 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, M.C.; da Silva, H.N.; Alves Leal Cruz, R.d.C.; Sagoe Amoah, S.K.; de Lima Silva, S.M.; Lia Fook, M.V. N-Acetyl-D-Glucosamine-Loaded Chitosan Filaments Biodegradable and Biocompatible for Use as Absorbable Surgical Suture Materials. Materials 2019, 12, 1807. https://doi.org/10.3390/ma12111807
da Silva MC, da Silva HN, Alves Leal Cruz RdC, Sagoe Amoah SK, de Lima Silva SM, Lia Fook MV. N-Acetyl-D-Glucosamine-Loaded Chitosan Filaments Biodegradable and Biocompatible for Use as Absorbable Surgical Suture Materials. Materials. 2019; 12(11):1807. https://doi.org/10.3390/ma12111807
Chicago/Turabian Styleda Silva, Milena Costa, Henrique Nunes da Silva, Rita de Cássia Alves Leal Cruz, Solomon Kweku Sagoe Amoah, Suédina Maria de Lima Silva, and Marcus Vinícius Lia Fook. 2019. "N-Acetyl-D-Glucosamine-Loaded Chitosan Filaments Biodegradable and Biocompatible for Use as Absorbable Surgical Suture Materials" Materials 12, no. 11: 1807. https://doi.org/10.3390/ma12111807