Percolative Composites with Carbon Nanohorns: Low-Frequency and Ultra-High Frequency Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CNH/PS Composites
2.3. Methods
3. Results and Discussion
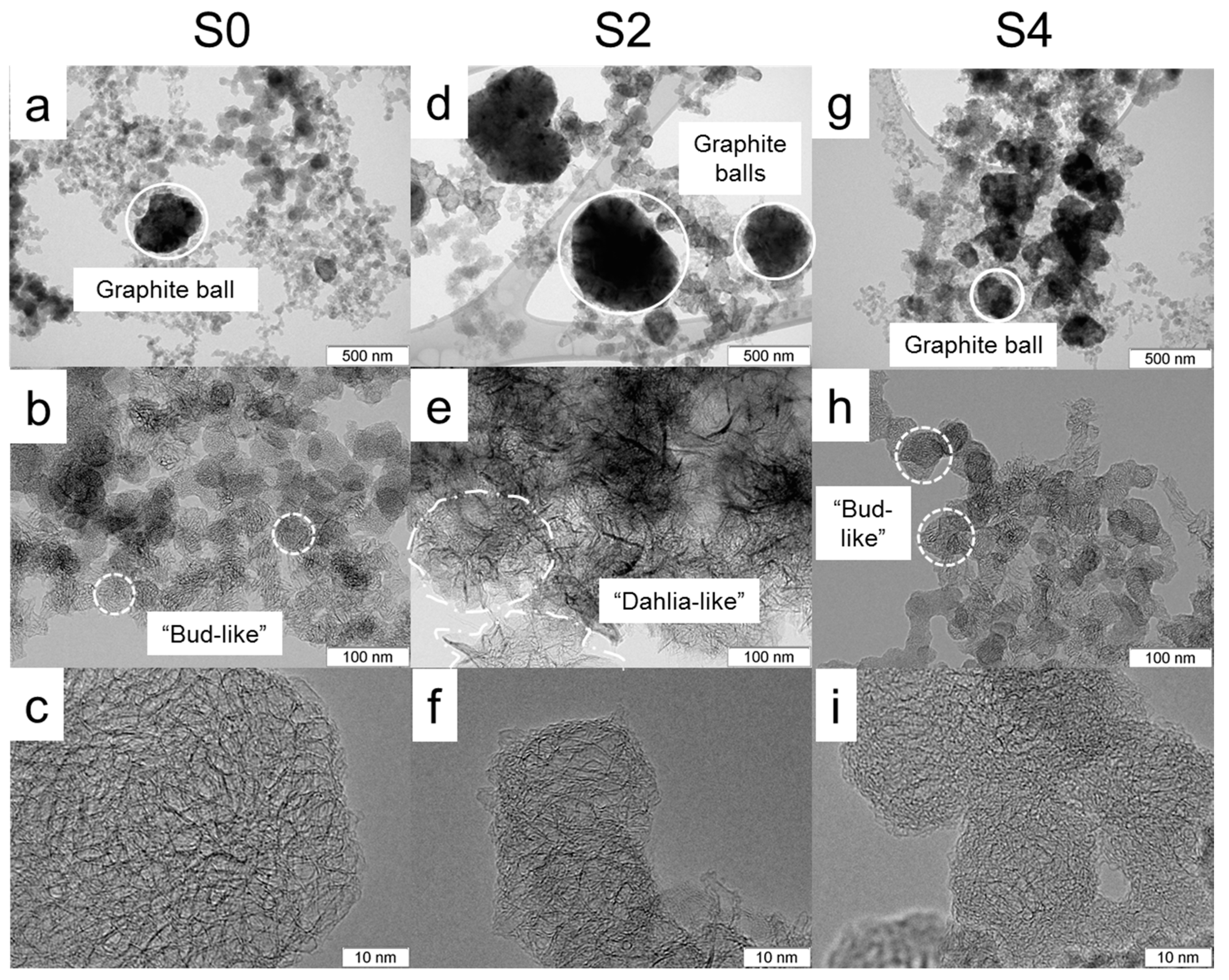
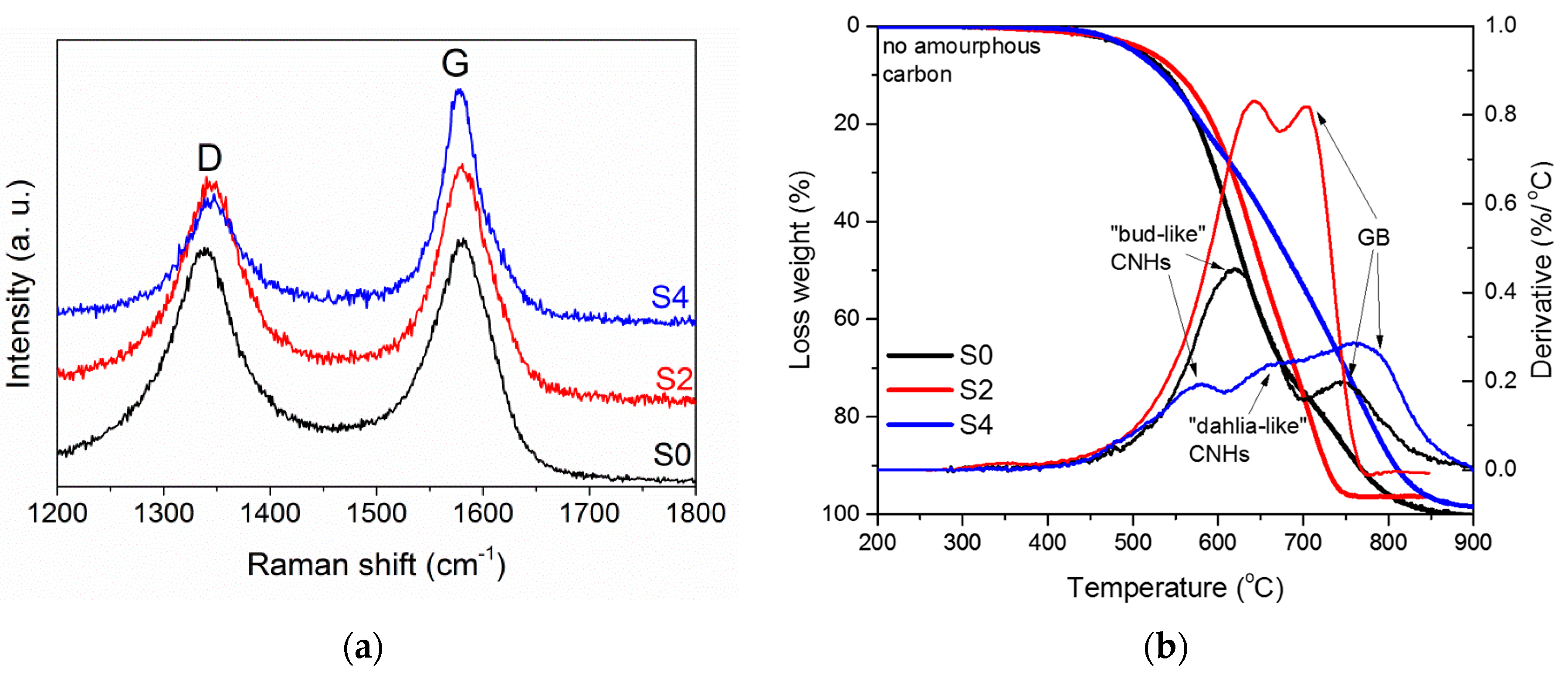
3.1. Structural Characterisation
3.2. Electromagnetic Properties of CNHs
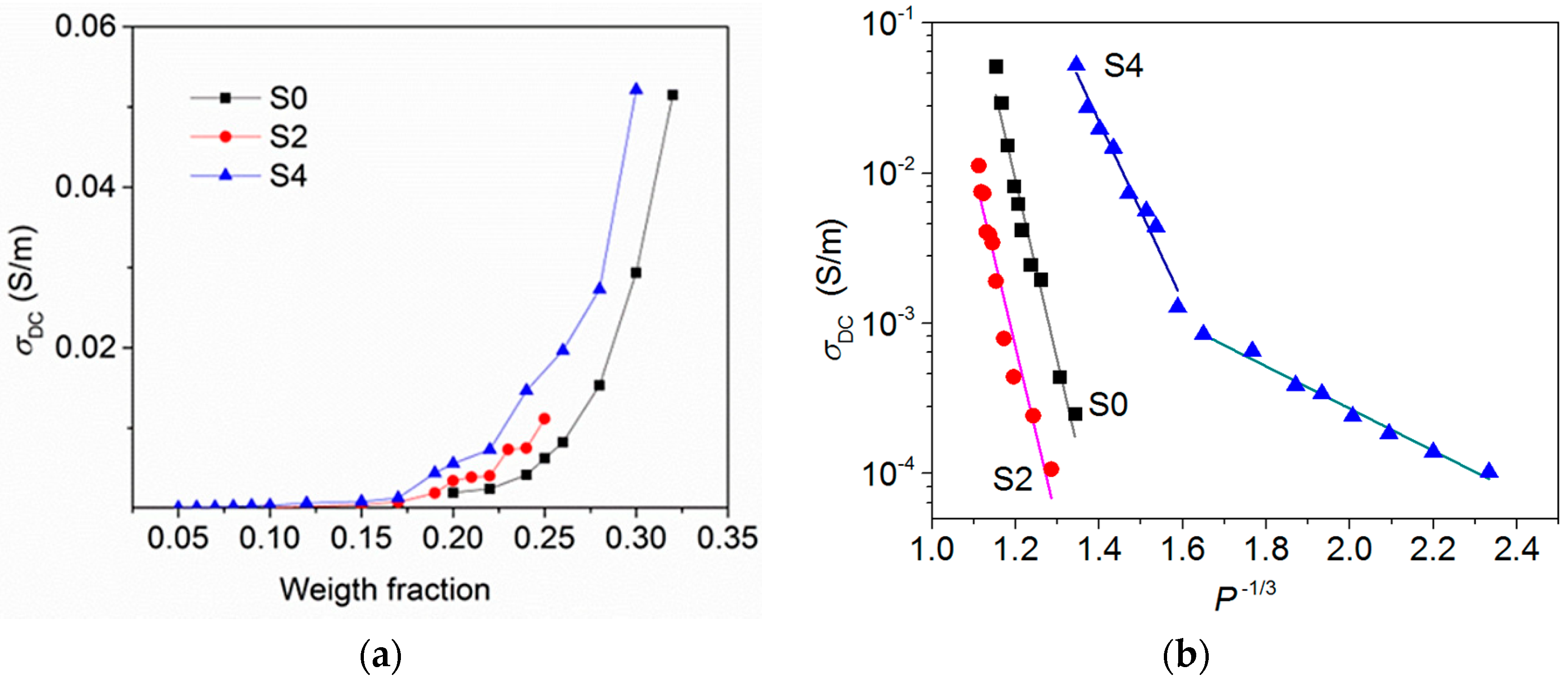
3.3. DC Conductivity of CNH/PS Composites
3.4. AC Permittivity and Conductivity of CNH/PS Composites
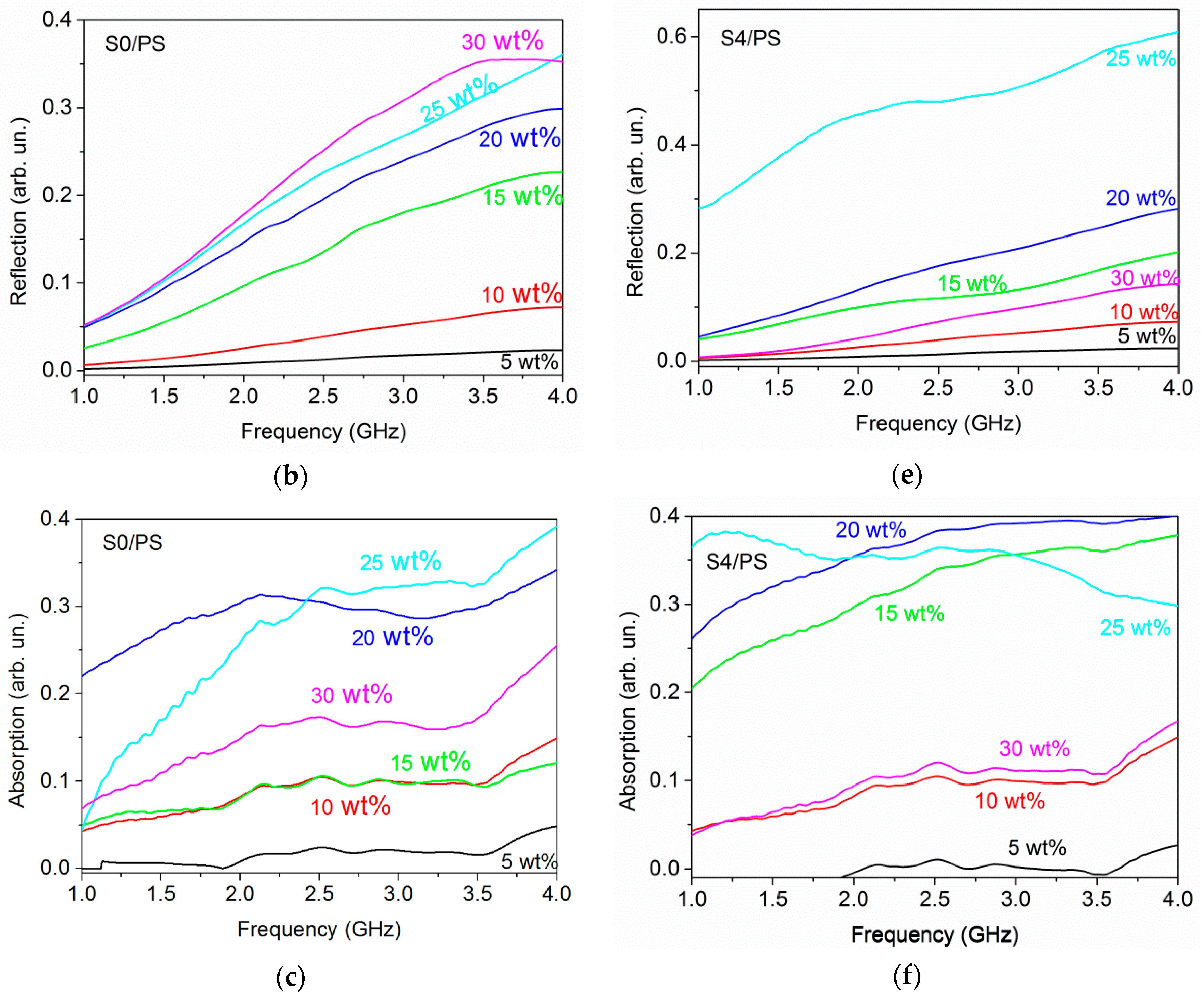
3.5. Electromagnetic Shielding Effectiveness of CNH/PS Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dang, Z.-M.; Yuan, J.-K.; Zha, J.-W.; Zhou, T.; Li, S.-T.; Hu, G.-H. Fundamentals, processes and applications of high-permittivity polymer–matrix composites. Prog. Mater. Sci. 2012, 57, 660–723. [Google Scholar] [CrossRef]
- Yaun, J. Percolation of carbon materials for high-k polymer nanocomposites. Chin. Chem. Lett. 2017, 28, 2036–2044. [Google Scholar] [CrossRef]
- Nan, C.-W.; Shen, Y.; Ma, J. Physical properties of composites near percolation. Annu. Rev. Matter. Res. 2010, 40, 131–152. [Google Scholar] [CrossRef]
- Yuan, J.-K.; Yao, S.-H.; Dang, Z.-M.; Sylvestre, A.; Genestoux, M.; Bai, J. Giant Dielectric permittivity nanocomposites: Realizing true potential of pristine carbon nanotubes in polyvinylidene fluoride matrix through an enhanced interfacial interaction. J. Phys. Chem. C 2011, 115, 5515–5521. [Google Scholar] [CrossRef]
- Wu, G.; Huang, X.; Xie, L.; Wu, X.; Yu, J.; Jiang, P. Morphology-controllable graphene—TiO2 nanorod hybrid nanostructures for polymer composites with high dielectric performance. J. Chem. Phys. 2011, 21, 17729. [Google Scholar] [CrossRef]
- Micheli, D.; Vricella, A.; Pastore, R.; Marchetti, M. Synthesis and electromagnetic characterization of frequency selective radar absorbing materials using carbon nanopowders. Carbon 2014, 77, 756–774. [Google Scholar] [CrossRef]
- Selvi, M.; Devaraju, S.; Sethuraman, K.; Alagar, M. Carbon black–polybenzoxazine nanocomposites for high k dielectric applications. Polym. Compos. 2014, 35, 2121–2128. [Google Scholar] [CrossRef]
- Gavrilov, N.N.; Okotrub, A.V.; Bulusheva, L.G.; Sedelnikova, O.V.; Yushina, I.V.; Kuznetsov, V.L. Dielectric properties of polystyrene/onion-like carbon composites in frequency range of 0.5-500 kHz. Compos. Sci. Technol. 2010, 70, 719–724. [Google Scholar] [CrossRef]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, properties, functionalization, and applications of carbon nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef]
- Yudasaka, M.; Iijima, S.; Crespi, V.H. Single-wall carbon nanohorns and nanocones. In Carbon Nanotubes: Advanced Topics in the Synthesis, Structure, Properties and Applications, 2nd ed.; Jorio, A., Dresselhaus, G., Dresselhaus, M.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 111, pp. 605–629. [Google Scholar]
- Yamaguchi, T.; Bandow, S.; Iijima, S. Synthesis of carbon nanohorn particles by simple pulsed arc discharge ignited between pre-heated carbon rods. Chem. Phys. Lett 2004, 389, 181–185. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Zhao, K.; Shi, Z.; Gu, Z.; Xu, S. Synthesis of single-wall carbon nanohorns by arc-discharge in air and their formation mechanism. Carbon 2010, 48, 1580–1585. [Google Scholar] [CrossRef]
- Gattia, D.M.; Vittori Antisari, M.; Marazzi, R.; Pilloni, L.; Contini, V.; Montone, A. Arc-discharge synthesis of carbon nano horns and multiwalled carbon nanotubes. Mater. Sci. Forum 2006, 518, 23–28. [Google Scholar] [CrossRef]
- Gattia, D.M.; Vittori Antisari, M.; Marazzi, R. AC arc discharge synthesis of single-walled nanohorns and highly convoluted graphene sheets. Nanotechnology 2007, 18, 255604. [Google Scholar] [CrossRef]
- Khoerunnisa, F.; Fujimori, T.; Itoh, T.; Kanoh, H.; Ohba, T.; Yudasak, M.; Iijima, S.; Kaneko, K. Electronically modified single wall carbon nanohorns with iodine adsorption. Chem. Phys. Lett. 2011, 501, 485–490. [Google Scholar] [CrossRef]
- Tao, Y.; Noguchi, D.; Yang, C.-M.; Kanoh, H.; Tanaka, H.; Yadasaka, M.; Iijima, S.; Janeko, K. Conductive and mesoporous single-wall carbon nanohorn/organic aerogel composites. Langmuir 2007, 23, 9155–9157. [Google Scholar] [CrossRef] [PubMed]
- Unni, S.M.; Bhange, S.N.; Illathvalappil, R.; Mutneja, N.; Patil, K.R.; Kurungot, S. Nitrogen-induced surface area and conductivity modulation of carbon nanohorn and its function as an efficient metal-free oxygen reduction electrocatalyst for anion-exchange membrane fuel cells. Small 2015, 11, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Fraczek-Szczypta, A.; Blazewicz, S. Manufacturing and physico-mechanical characterization of carbon nanohorns/polyacrylonitrile nanocomposites. J. Mater. Sci. 2011, 46, 5680–5689. [Google Scholar] [CrossRef]
- Famengo, A.; Ferrario, A.; Boldrini, S.; Battiston, S.; Fiameni, S.; Pagura, C.; Fabrizio, M. Polyaniline-carbon nanohorn composites as thermoelectric materials. Polym. Int. 2017, 66, 1724–1730. [Google Scholar] [CrossRef]
- Bera, R.; Suin, S.; Maiti, S.; Shrivastava, N.K.; Khatua, B.B. Carbon nanohorn and graphene nanoplate based polystyrene nanocomposites for superior electromagnetic interference shielding applications. J. Appl. Polym. Sci. 2015, 132, 42803. [Google Scholar] [CrossRef]
- Bera, R.; Karan, S.K.; Das, A.K.; Paria, S.; Khatua, B.B. Single wall carbon nanohorn (SWCNH)/graphene nanoplate/poly (methyl methacrylate) nanocomposites: A promising material for electromagnetic interference shielding applications. RSC Adv. 2015, 5, 70482–70493. [Google Scholar] [CrossRef]
- Bera, R.; Das, A.K.; Maitra, A.; Paria, S.; Karan, S.K.; Khatua, B.B. Salt leached viable porous Fe3O4 decorated polyaniline-SWCNH/PVDF composite spectacles as an admirable electromagnetic shielding efficiency in extended Ku-band region. Compos. Part B Eng. 2017, 129, 210–220. [Google Scholar] [CrossRef]
- Bera, R.; Maitra, A.; Paria, S.; Karan, S.K.; Das, A.K.; Bera, A.; Si, S.K.; Halder, L.; De, A.; Khatua, B.B. An approach to widen the electromagnetic shielding efficiency in PDMS/ferrous ferric oxide decorated RGO–SWCNH composite through pressure induced tunability. Chem. Eng. J. 2018, 335, 501–509. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Shevtsov, Y.V.; Nasonova, L.I.; Sinyakov, D.E.; Chuvilin, A.V.; Gutakovskii, A.K.; Mazalov, L.N. Arc-discharge synthesis of single-shell carbon particles. Inorg. Mater. 1996, 32, 858–861. [Google Scholar]
- Plyushch, A.O.; Sokol, A.A.; Lapko, K.N.; Kuzhir, P.P.; Fedoseeva, Y.V.; Romanenko, A.I.; Anikeeva, O.B.; Bulusheva, L.G.; Okotrub, A.V. Electromagnetic properties of phosphate composite materials with boron-containing carbon nanotubes. Phys. Solid State 2014, 56, 2537–2542. [Google Scholar] [CrossRef]
- Shuba, M.V.; Yuko, D.I.; Kuzhir, P.P.; Maksimenko, S.A.; Chigir, G.G.; Pyatlitski, A.N.; Sedelnikova, O.V.; Okotrub, A.V.; Lambin, P. Localized plasmon resonance in boron-doped multiwalled carbon nanotubes. Phys. Rev. B 2018, 97, 205427. [Google Scholar] [CrossRef] [Green Version]
- Sedelnikova, O.V.; Fedoseeva, Yu.V.; Romanenko, A.I.; Gusel’nikov, A.V.; Vilkov, O.Y.; Maksomenko, E.A.; Bychanok, D.S.; Kuzhir, P.P.; Bulusheva, L.G.; Okotrub, A.V. Effect of boron and nitrogen additives on structure and transport properties of arc-produced carbon. Carbon 2019, 142, 660–668. [Google Scholar] [CrossRef]
- Gavrilov, N.N.; Okotrub, A.V.; Bulusheva, L.G.; Kuznetsov, V.L.; Moseenkov, S.I. Low-frequency (10–50 kHz) impedance of polystyrene-onion-like-carbon composites. Tech. Phys. Lett. 2009, 35, 85–88. [Google Scholar] [CrossRef]
- Sedelnikova, O.V.; Gavrilov, N.N.; Bulusheva, L.G.; Okotrub, A.V. Maxwell-Garnett description of oermittivity of onion-like carbon-polystyrene composites. J. Nanoelectron. Optoelectron. 2009, 4, 267–270. [Google Scholar] [CrossRef]
- Sedelnikova, O.V.; Kanygin, M.A.; Korovib, E.Y.; Bulusheva, L.G.; Suslyaev, V.I.; Okotrub, A.V. Effect of fabrication method on the structure and electromagnetic response of carbon nanotube/polystyrene composites in low-frequency and Ka bands. Compos. Sci. Technol. 2014, 102, 59–64. [Google Scholar] [CrossRef]
- Kanygin, M.A.; Sedelnikova, O.V.; Bulusheva, L.G.; Okotrub, A.V. Polymer-assisted forge-rolling disaggregation of detonation nanodiamonds and onion-like carbon. Int. J. Nanotechnol. 2015, 12, 1820191. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Fan, J.; Yudasaka, M.; Kasuya, D.; Azami, T.; Yuge, R.; Imai, H.; Kubo, Y.; Iijima, S. Micrometer-sized graphitic balls produced together with single-wall carbon nanohorns. J. Phys. Chem. B 2005, 109, 10756–10759. [Google Scholar] [CrossRef] [PubMed]
- Azami, T.; Kasuya, D.; Yuge, R.; Yudasaka, M.; Iijima, S.; Yoshitake, T.; Kubo, Y. Large-scale production of single-wall carbon nanohorns with high purity. J. Phys. Chem. C 2008, 112, 1330–1334. [Google Scholar] [CrossRef]
- Jonscher, A.K. The ‘universal’ dielectric response. Nature 1977, 267, 673–679. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Berrau, S.; Demont, P.; Peigney, A.; Laurent, C.; Lacabanne, C. DC and AC conductivity of carbon nanotubes−polyepoxy composites. Macromolecules 2003, 36, 5187–5194. [Google Scholar] [CrossRef]
- Bryning, M.B.; Islam, M.F.; Kikkawa, J.M.; Yogh, A.G. Very Low Conductivity Threshold in Bulk Isotropic Single-Walled Carbon Nanotube–Epoxy Composites. Adv. Mater. 2005, 17, 1186–1191. [Google Scholar] [CrossRef]
- Rainindi, M.; Guadagno, L.; Vertuccio, L.; Naddeo, C.; Barra, G.; Spinelli, G.; Lamberti, P.; Tucci, V.; Lafdi, K. Electrical conductivity of carbon nanofiber reinforced resins: Potentiality of Tunneling Atomic Force Microscopy (TUNA) technique. Compos. Part B Eng. 2018, 143, 148–160. [Google Scholar]
- Macutkevic, J.; Kranauskaite, I.; Banys, J.; Moseenkov, S.; Kuznetsov, V.; Shenderova, O. Metal-insulator transition and size dependent electrical percolation in onion-like carbon/polydimethylsiloxane composites. J. Appl. Phys. 2014, 115, 213702. [Google Scholar] [CrossRef]
- Connor, M.T.; Roy, S.; Ezquerra, T.A.; Balta Calleja, F. Broadband ac conductivity of conductor-polymer composites. J. Phys. Rev. B 1998, 57, 2286. [Google Scholar] [CrossRef]
- Zhang, W.; Blackburn, R.S.; Dehghani-Sanij, A. Effect of carbon black concentration on electrical conductivity of epoxy resin–carbon black–silica nanocomposites. J. Mater. Sci. 2007, 42, 7861–7865. [Google Scholar] [CrossRef]
- Grimaldi, C.; Kecsenovity, E.; Majidian, M.; Kuznetsov, V.L.; Magrez, A.; Forró, L. Electrical transport in onion-like carbon-PMMA nanocomposites. Appl. Phys. Lett. 2019, 114, 103102. [Google Scholar] [CrossRef]
- Efros, A.L.; Shklovskii, B.I. Critical behaviour of conductivity and dielectric constant near the metal-non-metal transition threshold. Phys. Status Solidi B 1976, 76, 475–785. [Google Scholar] [CrossRef]
- McLachlan, D.S. Analytical functions for the DC and AC conductivity of conductor-insulator composites. J. Electroceramics 2000, 5, 93–110. [Google Scholar] [CrossRef]
- Sumita, M.; Sakata, K.; Hayakawa, Y.; Asai, S.; Miyasaka, K.; Tanemura, M. Double percolation effect on the electrical conductivity of conductive particles filled polymer blends. Colloid Polym. Sci. 1992, 270, 134139. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, Y.K.; Hong, C.E.; Kim, N.H.; Li, P.; Lee, H.K. Effect of carbon fillers on properties of polymer composite bipolar plates of fuel cells. J. Pow. Sources 2009, 193, 523–529. [Google Scholar] [CrossRef]




| S0/PS | S2/PS | S4/PS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| p, wt % | σDC, S/m | s | P, vol% | p, wt % | σDC, S/m | s | P, vol% | p, wt % | σDC, S/m | s | P, vol% |
| 10 | 6∙× 10−5 | 0.11 | 31 | 10 | 2∙× 10−5 | 0.31 | 47 | 12 | 4 × 10−5 | 0.16 | 18 |
| 15 | 6 ×∙10−4 | 0.24 | 41 | 15 | 1 × 10−4 | 0.44 | 59 | 15 | 1 × 10−4 | 0.24 | 22 |
| 17 | 2∙× 10−3 | 0.35 | 45 | 16 | 5 ×∙10−3 | 0.56 | 60 | 19 | 7 × 10−4 | 0.25 | 28 |
| 20 | 3∙× 10−3 | 0.39 | 50 | 17 | 1 × 10−2 | 0.58 | 62 | 20 | 3∙× 10−3 | 0.32 | 29 |
| 25 | 8∙× 10−3 | 0.51 | 57 | 24 | 1 × 10−2 | 0.34 | 34 | ||||
| 27 | 1 × 10−2 | 0.61 | 60 | ||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedelnikova, O.V.; Baskakova, K.I.; Gusel’nikov, A.V.; Plyusnin, P.E.; Bulusheva, L.G.; Okotrub, A.V. Percolative Composites with Carbon Nanohorns: Low-Frequency and Ultra-High Frequency Response. Materials 2019, 12, 1848. https://doi.org/10.3390/ma12111848
Sedelnikova OV, Baskakova KI, Gusel’nikov AV, Plyusnin PE, Bulusheva LG, Okotrub AV. Percolative Composites with Carbon Nanohorns: Low-Frequency and Ultra-High Frequency Response. Materials. 2019; 12(11):1848. https://doi.org/10.3390/ma12111848
Chicago/Turabian StyleSedelnikova, Olga V., Kseniya I. Baskakova, Artem V. Gusel’nikov, Pavel E. Plyusnin, Lyubov G. Bulusheva, and Alexander V. Okotrub. 2019. "Percolative Composites with Carbon Nanohorns: Low-Frequency and Ultra-High Frequency Response" Materials 12, no. 11: 1848. https://doi.org/10.3390/ma12111848





