Preparation of Luminescent Thermotropic Liquid Crystal from Benzodiathiazole Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Design and Synthesis
2.2. The Photophysical Properties of BTC0 and BTC6 in Solution
2.3. PXRD Patterns of the Crystalline Powders of BTC0 and BTC6
2.4. Electrochemical Properties of BTC0 and BTC6
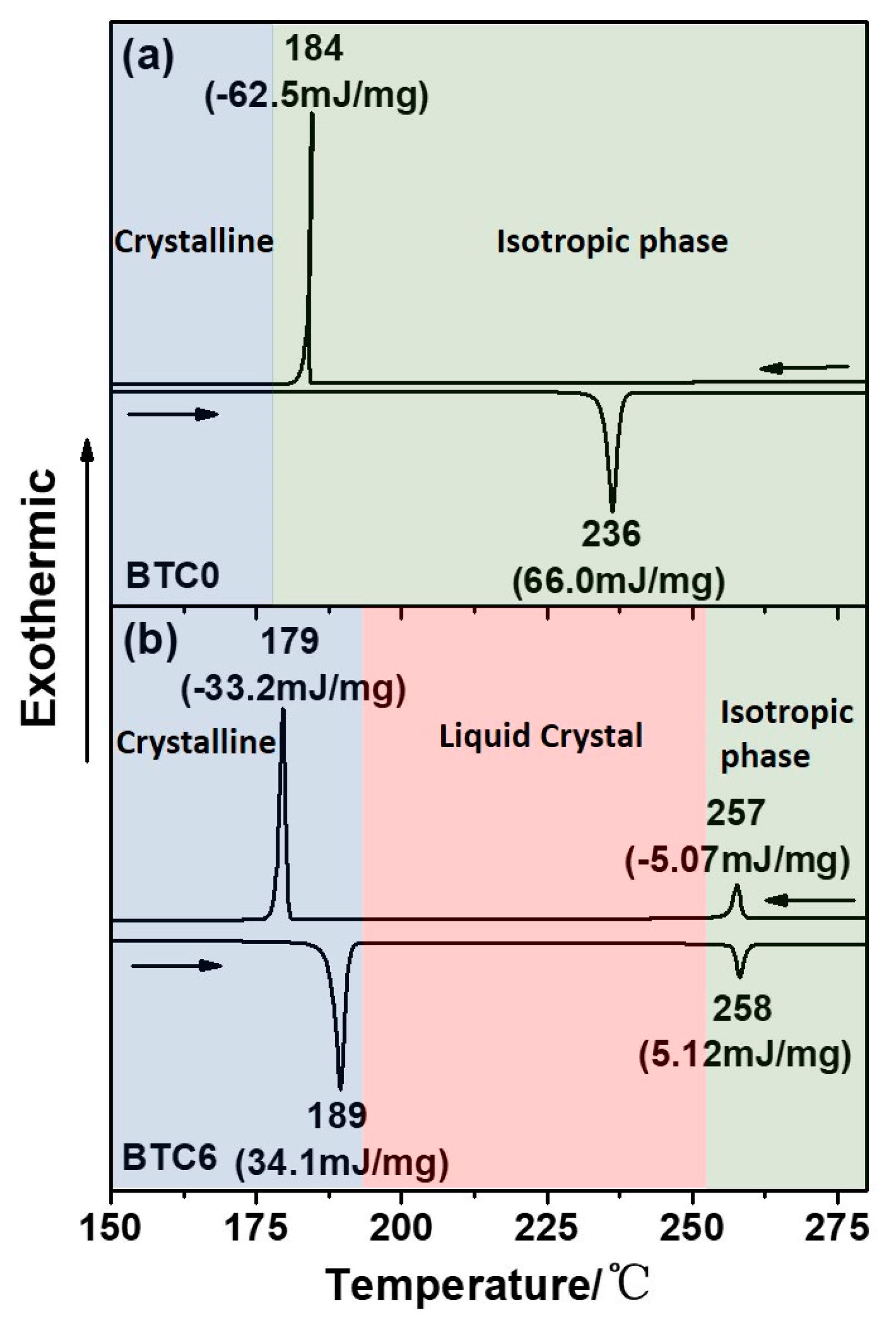
2.5. Thermal Properties and the Liquid Crystalline Phase
3. Experimental Section
3.1. Measurements
3.2. Materials and Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Friend, R.H.; Gymer, R.W.; Holmes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.; Bradley, D.D.C.; Santos, D.A.; Brèdas, J.L.; LÖgdlund, M.; et al. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Wakamiya, A.; Mori, K.; Yamaguchi, S. 3-boryl-2,2′-bithiophene as a versatile core skeleton for full-color highly emissive organic solids. Angew. Chem. Int. Ed. 2007, 46, 4273–4276. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Takeda, Y.; Higashi, M.; Hiyama, T. 1,4-Bis(alkenyl)-2,5-dipiperidinobenzenes: Minimal fluorophores exhibiting highly efficient emission in the solid state. Angew. Chem. Int. Ed. 2009, 48, 3653–3656. [Google Scholar] [CrossRef] [PubMed]
- Westrup, J.L.; Oenning, L.W.; Silva Paula, M.M.; Costa Duarte, R.; Rodembusch, F.S.; Frizon, T.E.A.; Silva, L.; Dal-BÓ, A. New photoactive D-π-A-π-D benzothiadiazole derivatives: Synthesis, thermal and phtophysical properties. Dyes Pigments 2016, 126, 209–217. [Google Scholar] [CrossRef]
- Hasan, Z.A.; Woon, K.L.; Wong, W.S.; Ariffin, A.; Chen, S.A. Solution processed multilayer red, green and blue phosphorescent organic light emitting diodes using carbazole dendrimer as a host. J. Lumin. 2017, 183, 150–158. [Google Scholar] [CrossRef]
- McGehee, M.D.; Heeger, A.J. Semiconducting (conjugated) polymers as materials for solid-state lasers. Adv. Mater. 2000, 12, 1655–1668. [Google Scholar] [CrossRef]
- Samuel, I.D.W.; Turnbull, G.A. Organic semiconductor lasers. Chem. Rev. 2007, 107, 1272–1295. [Google Scholar] [CrossRef]
- Zhang, S.W.; Swager, T.M. Fluorescent detection of chemical warfare agents: Functional group specific ratiometric chemosensors. J. Am. Chem. Soc. 2003, 125, 3420–3421. [Google Scholar] [CrossRef]
- Knapton, D.; Burnworth, M.; Rowan, S.J.; Weder, C. Fluorescent organometallic sensors for the detection of chemical-warfare-agent mimics. Angew. Chem. Int. Ed. 2006, 45, 5825–5829. [Google Scholar] [CrossRef]
- Zhou, X.; Lee, S.; Xu, Z.; Yoon, J. Recent progress on the development of chemosensors for gases. Chem. Rev. 2015, 115, 7944–8000. [Google Scholar] [CrossRef]
- Pan, C.; Sugiyasu, K.; Wakayama, Y.; Sato, A.; Takeuchi, M. Thermoplastic fluorescent conjugated polymers: Benefits of preventing p-p stacking. Angew. Chem. Int. Ed. 2013, 52, 10775–10779. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Sugiyasu, K.; Aimi, J.; Takeuchi, M. Picket-fence polythiophene and its diblock copolymers that afford microphase separations comprising a stacked and an isolated polythiophene ensemble. Angew. Chem. Int. Ed. 2014, 53, 8870–8875. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Sugiyasu, K.; Takeuchi, M. Blending conjugated polymers without phase separation for fluorescent colour tuning of polymeric materials through FRET. Chem. Commun. 2014, 50, 11814–11817. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gong, W.; Tong, Y.; Wei, D.; Wang, Y.; Ding, J.; Hou, H.; Song, Y. Synthesis and properties of benzothiadiazole-pyridine system: The modulation of optical feature. Dyes Pigments 2017, 137, 135–142. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Hiyama, T. Organic fluorophores exhibiting highly efficient photoluminescence in the solid state. Chem. Asian J. 2010, 5, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yamaguchi, R.; Moriyama, K.; Kadowaki, M.; Kobayashi, K.; Ishi-i, T.; Thiemanna, T.; Mataka, S. Highly dichroic benzo-2,1,3-thiadiazole dyes containing five linearly p-conjugated aromatic residues, with fluorescent emission ranging from green to red, in a liquid crystal guest-host system. J. Mater. Chem. 2006, 16, 736–740. [Google Scholar] [CrossRef]
- Sun, X.; Xu, X.; Qiu, W.; Yu, G.; Zhang, H.; Gao, X.; Chen, S.; Song, Y.; Liu, Y. A non-planar pentaphenylbenzene functionalized benzo [2,1,3] thiadiazole derivative as a novel red molecular emitter for non-doped organic light-emitting diodes. J. Mater. Chem. 2008, 18, 2709–2715. [Google Scholar] [CrossRef]
- Lai, W.Y.; Xia, W.; He, Q.Y.; Levermore, P.A.; Huang, W.; Bradley, D.D.C. Enhanced solid-state luminescence and low-threshold lasing from starburst macromolecular materials. Adv. Mater. 2009, 21, 355–360. [Google Scholar] [CrossRef]
- Chen, C.T. Evolution of red organic light-emitting diodes: Materials and devices. Chem. Mater. 2004, 16, 4389–4400. [Google Scholar] [CrossRef]
- Nakazono, S.; Imazaki, Y.; Yoo, H.; Yang, J.; Sasamori, T.; Tokitoh, N.; Cédric, T.; Kageyama, H.; Kim, D.; Shinokubo, H.; et al. Regioselective ru-catalyzed direct 2,5,8,11-alkylation of perylene bisimides. Chem. Eur. J. 2009, 15, 7530–7533. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, M.; Kalinowski, J.; Virgili, D.; Williams, J.A.G. Excimer-based red/near-infrared organic light-emitting diodes with very high quantum efficiency. Appl. Phys. Lett. 2008, 92, 113302. [Google Scholar] [CrossRef]
- Vollbrecht, J.; Wiebeler, C.; Neuba, A.; Bock, H.; Schumacher, S.; Kitzerow, H. Bay-Extended, Distorted perylene esters showing visible luminescence after ultraviolet excitation: Photophysical and electrochemical analysis. J. Phys. Chem. C 2016, 120, 7839–7848. [Google Scholar] [CrossRef]
- Vollbrecht, J. Excimers in organic electronics. New J. Chem. 2018, 42, 11249–11254. [Google Scholar] [CrossRef]
- Hariharan, P.; Gayathri, P.; Kundu, A.; Karthikeyan, S.; Moon, D.; Anthony, S.P. Red fluorescent aggregation-enhanced emissive organic fluorophores: Stimuli-responsive high contrast off–on fluorescence switching. CrystEngComm 2018, 20, 643–651. [Google Scholar] [CrossRef]
- Kim, M.; Whang, D.R.; Gierschner, J.; Park, S.Y. A distyrylbenzene based highly efficient deep red/near-infrared emitting organic solid. J. Mater. Chem. C 2015, 3, 231–234. [Google Scholar] [CrossRef]
- Cheng, X.; Li, D.; Zhang, Z.; Zhang, H.; Wang, Y. Organoboron compounds with morphology-dependent NIR emissions and dual-channel fluorescent ON/OFF switching. Org. Lett. 2014, 16, 880–883. [Google Scholar] [CrossRef]
- Shen, X.Y.; Yuan, W.Z.; Liu, Y.; Zhao, Q.; Lu, P.; Ma, Y.; Williams, I.D.; Qin, A.; Sun, J.Z.; Tang, B.Z. Fumaronitrile-based fluorogen: Red to near-infrared fluorescence, aggregation-induced emission, solvatochromism, and twisted intramolecular charge transfer. J. Phys. Chem. C 2012, 116, 10541–10547. [Google Scholar] [CrossRef]
- Stegemeyer, H.; Stöckel, F.; Bunsenges, B. Anisotropic structures in aqueous solutions of aggregated pseudoisocyanine dyes. Phys. Chem. 1996, 100, 9–14. [Google Scholar] [CrossRef]
- Harrison, W.J.; Mateer, D.L.; Tiddy, G.J.T. Structure of J-Aggregates of pseudoisocyanine dye in aqueous solution. J. Phys. Chem. 1996, 100, 2310–2321. [Google Scholar] [CrossRef]
- Berlepsch, H.V.; Böttcher, C.; Ouart, A.; Burger, C.; Dähne, S.; Kirstein, S. Liquid-crystalline J-Aggregates formed by aqueous ionic cyanine dyes. J. Phys. Chem. B 2000, 104, 5255–5262. [Google Scholar]
- Emerson, E.S.; Conlin, M.A.; Rosenoff, A.E.; Norland, K.S.; Rodriguez, H.; Bird, G.R. The geometrical structure and absorption spectrum of a cyanine dye aggregate. J. Phys. Chem. 1967, 71, 2396–2403. [Google Scholar] [CrossRef]
- Czikkely, V.; Försterling, H.D.; Kuhn, H. Extended dipole model for aggregates of dye molecule. Chem. Phys. Lett. 1970, 6, 11–14. [Google Scholar] [CrossRef]
- Lapkowski, M.; Data, P.; Golba, S.; Soloducho, J.; Nowakowska-Oleksy, A. Unusual band-gap migration of N-alkylcarbazole-thiophene derivative. Opt. Mater. 2011, 22, 1445. [Google Scholar] [CrossRef]
- Cristiano, R.; Vieira, A.A.; Ely, F.; Gallardo, H. Synthesis and characterization of luminescent hockey stick-shaped liquid crystalline compounds. Liq. Cryst. 2006, 33, 381–390. [Google Scholar] [CrossRef]
- Frein, S.; Camerel, F.; Ziessel, R.; Barbera, J.; Deschenaux, R. Highly fluorescent liquid-crystalline dendrimers based on borondipyrromethene dyes. Chem. Mater. 2009, 21, 3950–3959. [Google Scholar] [CrossRef]
- Lu, H.B.; Zhang, S.N.; Ding, A.X.; Yuan, M.; Zhang, G.Y.; Xu, W.; Zhang, G.B.; Wang, X.H.; Qiu, L.Z.; Yang, J.X. A luminescent liquid crystal with multistimuli tunable emission colors based on different molecular packing structures. New. J. Chem. 2014, 38, 3429–3433. [Google Scholar] [CrossRef]






| Sample | Solution a | |||||||
| λabs | λem | ε | Stokes Shift | ΦF | <τf> e,f | kr b | knr c | |
| BTC0 | 342 nm 489 nm | 627 nm | 5.0 × 104 cm−1·M−1 3.7 × 104 cm−1·M−1 | 138 nm | 0.81 | 7.83 ns | 1.03 × 108 s−1 | 2.43 × 107 s−1 |
| BTC6 | 348 nm 500 nm | 637 nm | 4.3 × 104 cm−1·M−1 3.2 × 104 cm−1·M−1 | 137 nm | 0.78 | 7.30 ns | 1.07 × 108 s−1 | 3.01 × 107 s−1 |
| Sample | Powder | |||||||
| λabs | λem | Stokes Shift | ΦF | τf (fi) d | <τf> e,f | kr b | knr c | |
| BTC0 | 336 nm 473 nm | 669 nm | 196 nm | 0.10 | τ1 = 0.79 ns (15%) τ2 = 2.46 ns (77%) τ3 = 92.78 ns (8%) | 1.97 ns | 5.07 × 107 s−1 | 4.57 × 108 s−1 |
| BTC6 | 346 nm 470 nm | 678 nm | 208 nm | 0.26 | τ1 = 6.33 ns (78%) τ2 = 13.36 ns (22%) | 7.17 ns | 3.63 × 107 s−1 | 1.03 × 108 s−1 |
| Sample | EOxa | ERedb | EHOMOc | ELUMOd | EGe |
|---|---|---|---|---|---|
| BTC0 | 1.08 V | −1.14 V | −5.40 eV | −3.18 eV | 2.22 eV |
| BTC6 | 1.07 V | −1.16 V | −5.39 eV | −3.16 eV | 2.23 eV |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Yu, H.; Xie, D.; Zhu, Y.; Zhong, X.; Pan, C.; Shao, G. Preparation of Luminescent Thermotropic Liquid Crystal from Benzodiathiazole Derivatives. Materials 2019, 12, 1919. https://doi.org/10.3390/ma12121919
Feng Y, Yu H, Xie D, Zhu Y, Zhong X, Pan C, Shao G. Preparation of Luminescent Thermotropic Liquid Crystal from Benzodiathiazole Derivatives. Materials. 2019; 12(12):1919. https://doi.org/10.3390/ma12121919
Chicago/Turabian StyleFeng, Yuchen, Huijuan Yu, Dexun Xie, Yi Zhu, Xinhao Zhong, Chengjun Pan, and Guang Shao. 2019. "Preparation of Luminescent Thermotropic Liquid Crystal from Benzodiathiazole Derivatives" Materials 12, no. 12: 1919. https://doi.org/10.3390/ma12121919




