Effect of Sn Addition on Microstructure and Corrosion Behavior of As-Extruded Mg–5Zn–4Al Alloy
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Sample Preparation
2.2. Microstructural Characterization
2.3. Electrochemical and Immersion Tests
2.4. Corrosion Morphology and Products
3. Results and Discussion
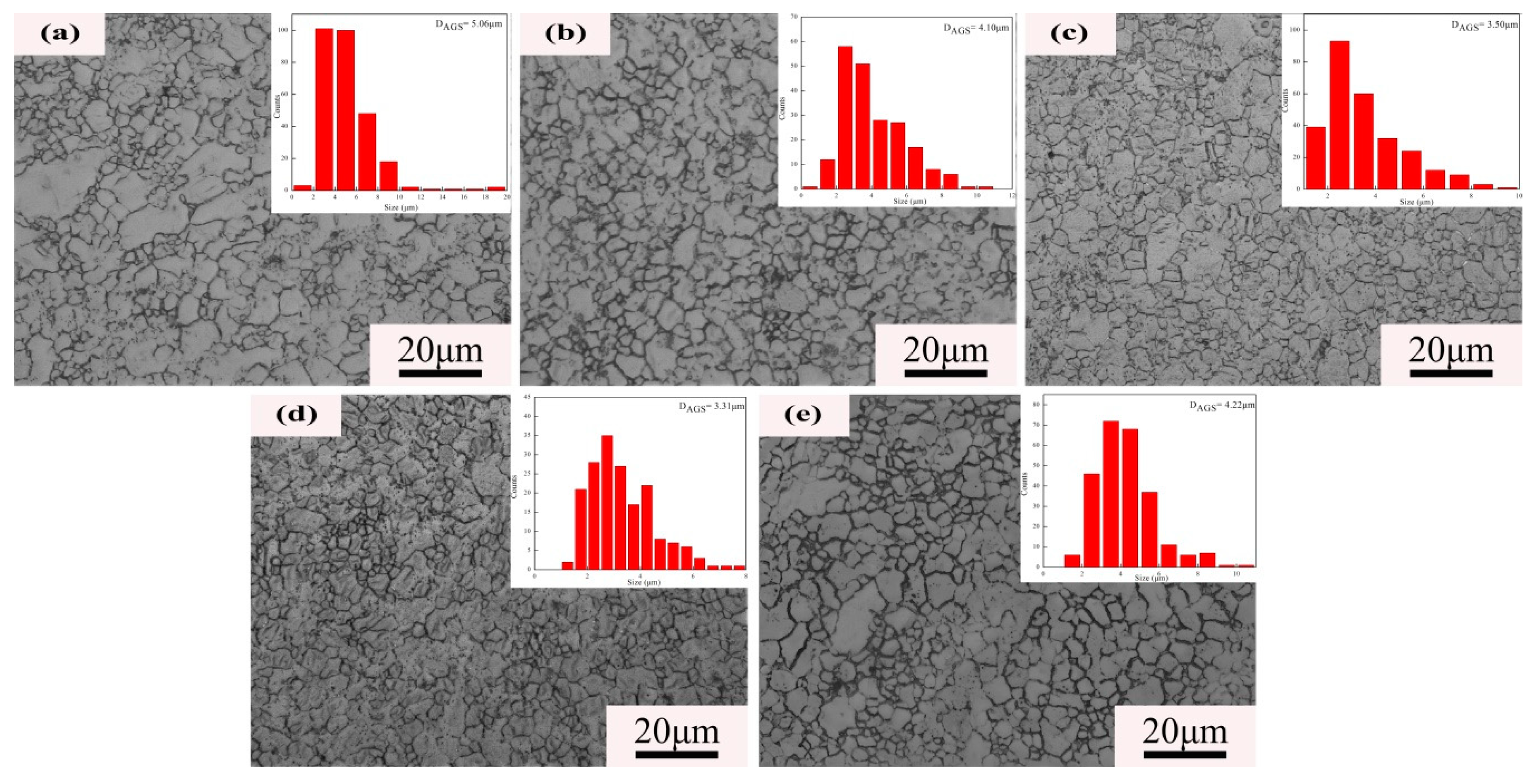
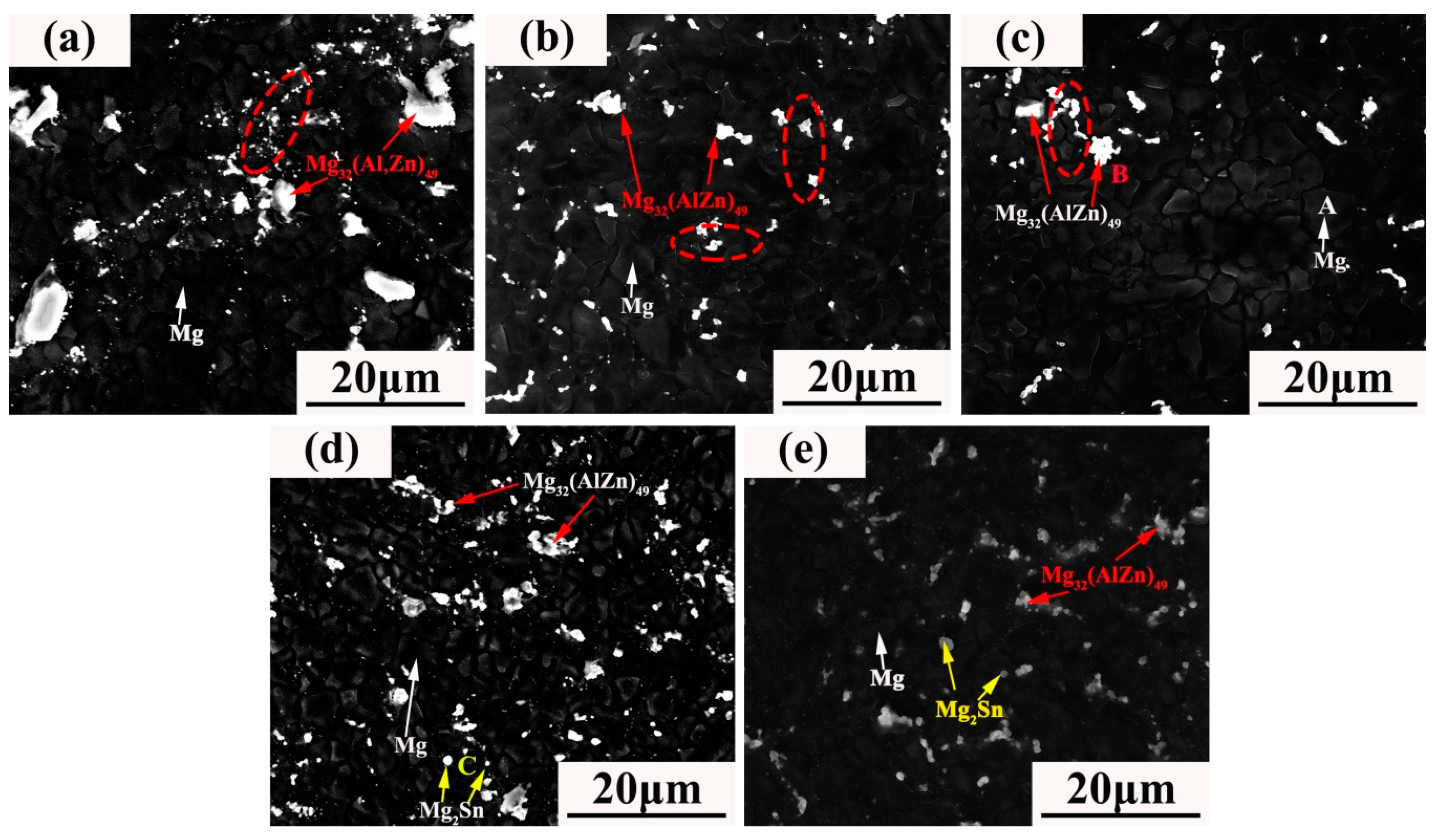
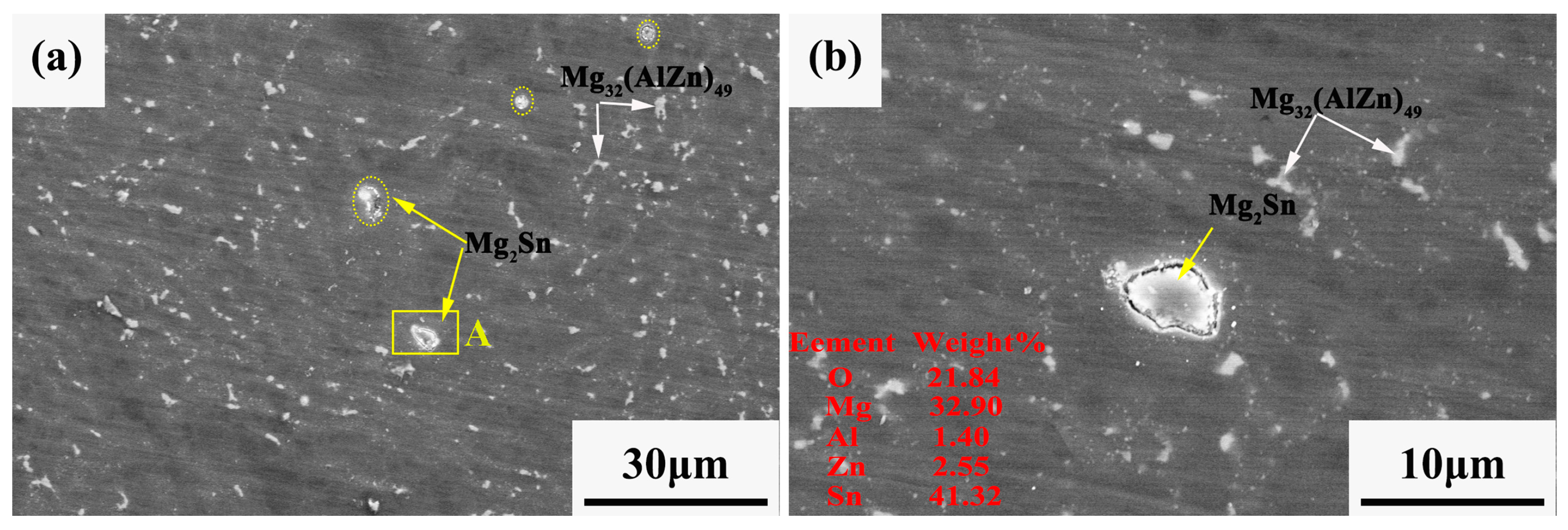
3.1. Microstructure
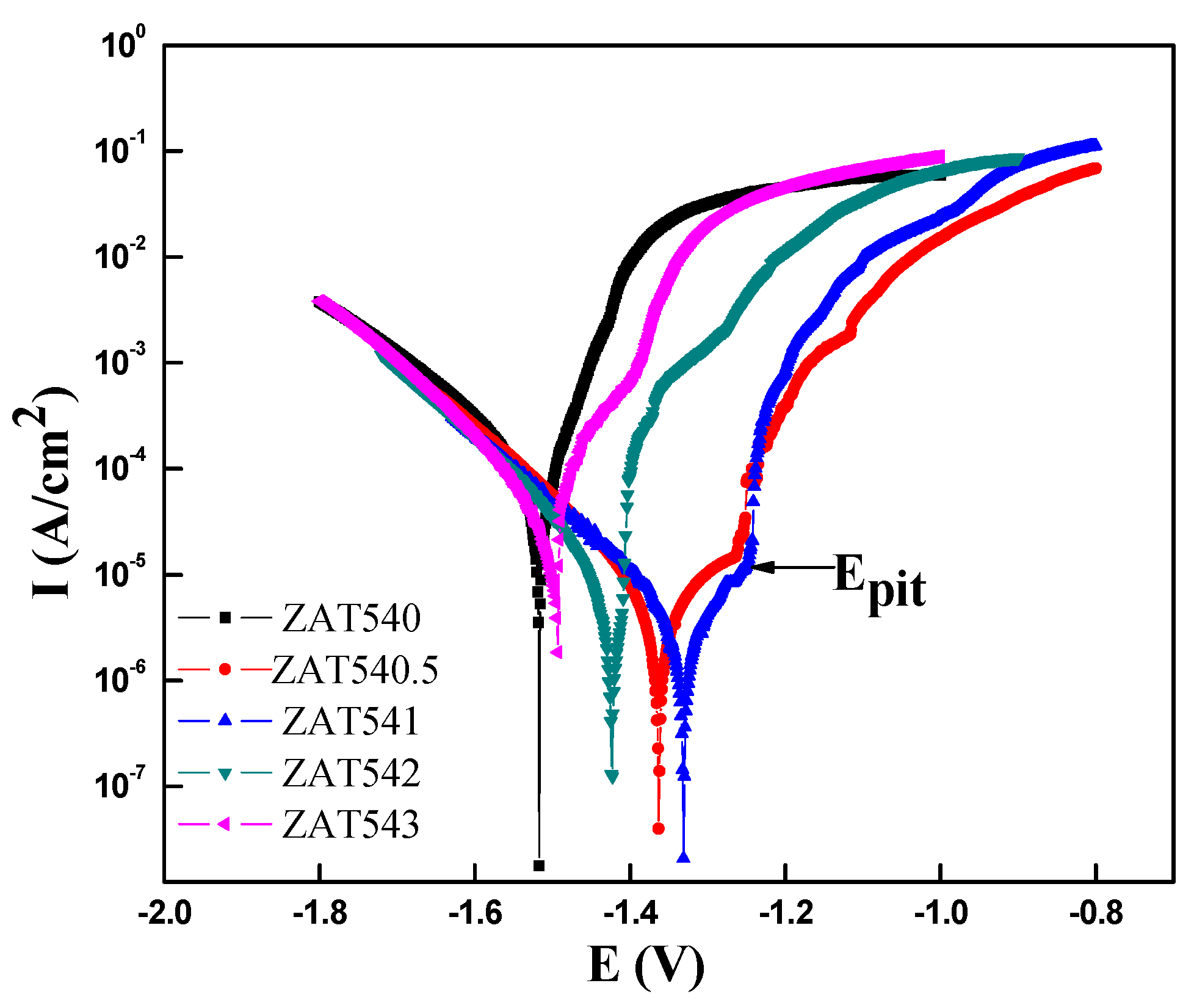
3.2. Potentiodynamic Polarization Test
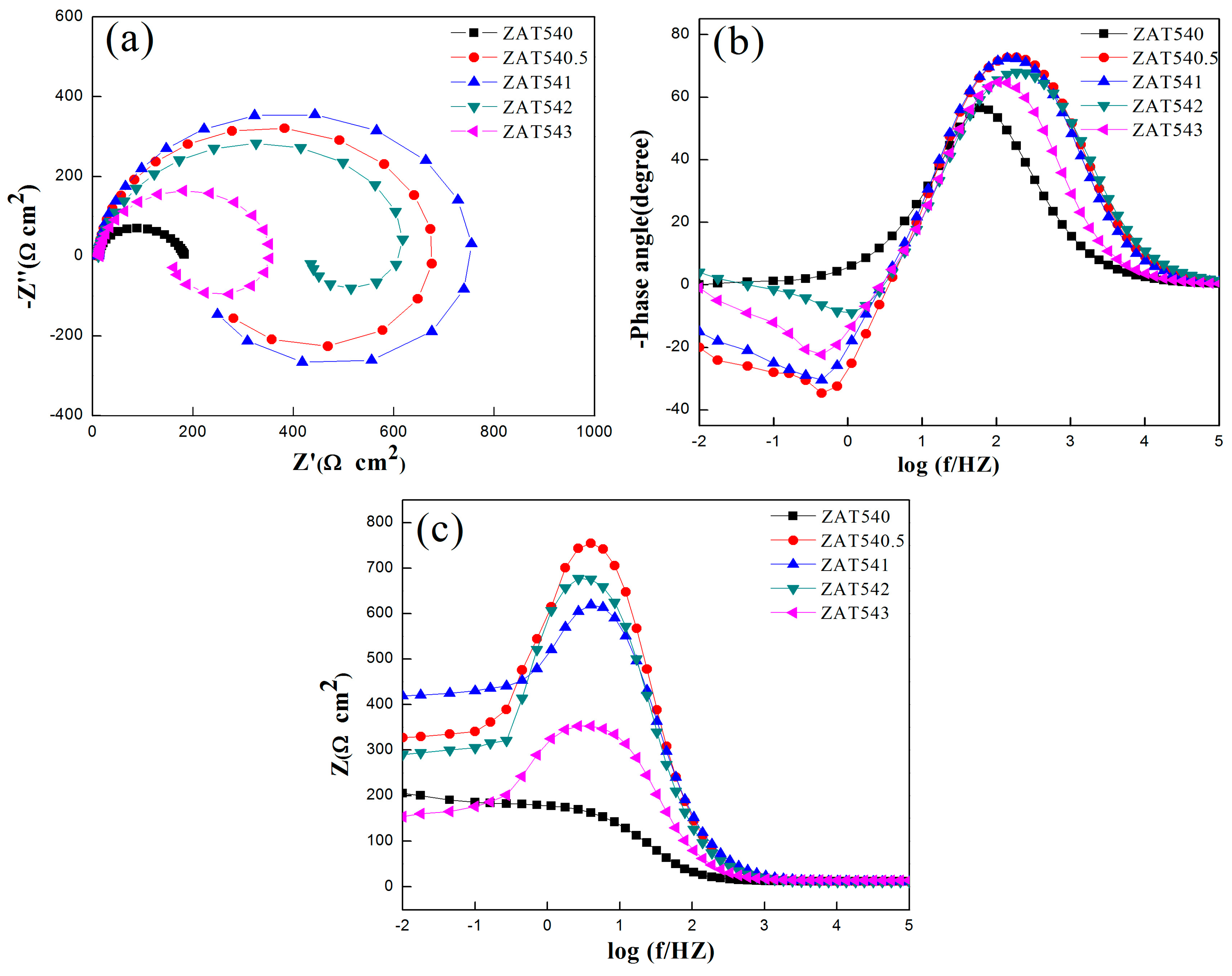
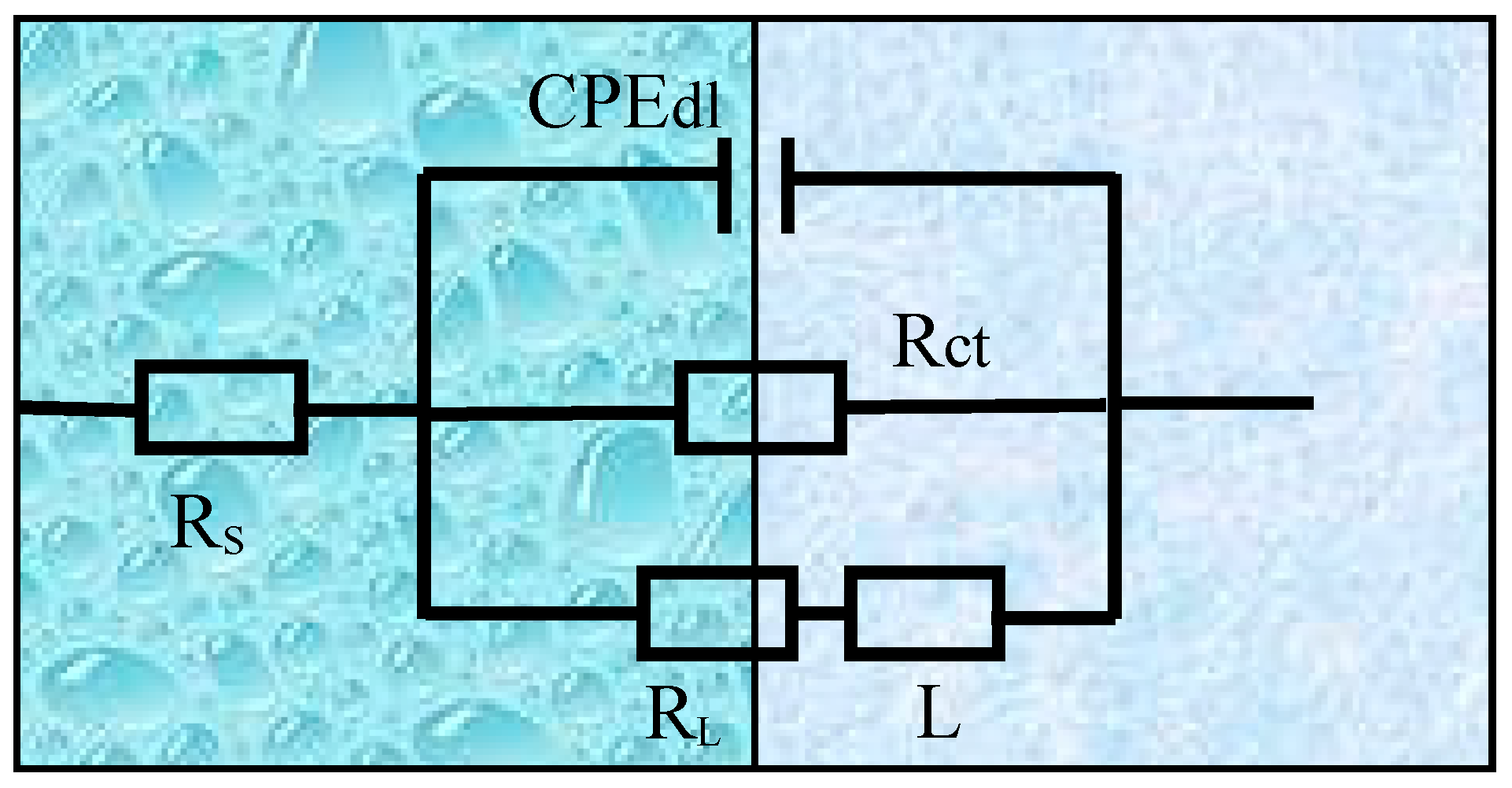
3.3. Electrochemical Impedance Spectroscopy
3.4. Corrosion Behavior
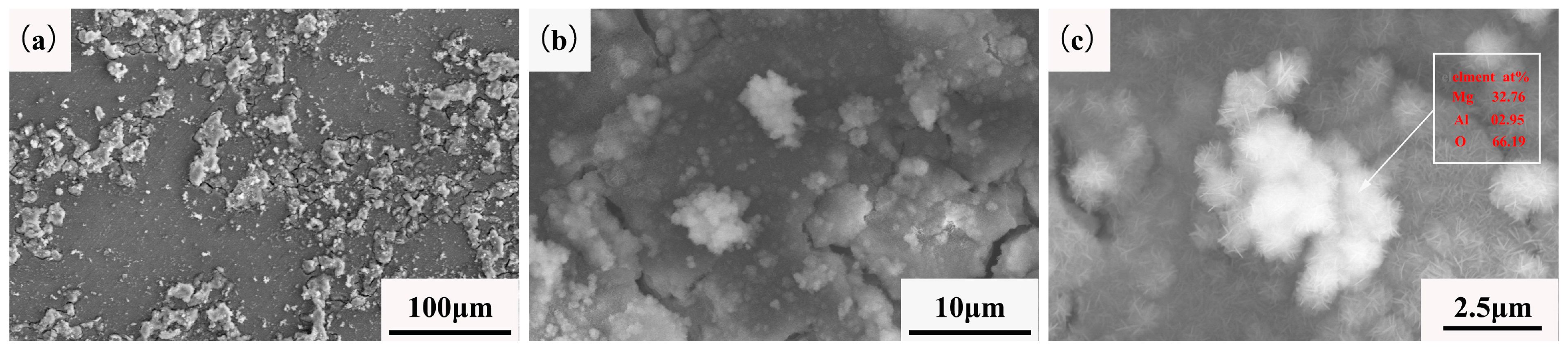
3.5. Corrosion Products Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bian, M.Z.; Sasaki, T.T.; Nakata, T.; Yoshida, Y.; Kawabe, N.; Kamado, S.; Hono, K. Bake-hardenable Mg-Al-Zn-Mn-Ca sheet alloy processed by twin-roll casting. Acta Mater. 2018, 158, 278–288. [Google Scholar] [CrossRef]
- Hu, Y.B.; Zhang, C.; Meng, W.Q.; Pan, F.S.; Zhou, J.P. Microstructure, mechanical and corrosion properties of Mg-4Al-2Sn-xY-0.4Mn alloys. J. Alloys. Compd. 2017, 727, 491–500. [Google Scholar] [CrossRef]
- Sugrib, K.S.; Hamid, J. Characterization of Nanolayer Intermetallics Formed in Cold Sprayed Al Powder on Mg Substrate. Materials 2019, 12, 1317. [Google Scholar] [Green Version]
- Zhu, S.Q.; Yan, H.G.; Liao, X.Z.; Moody, S.J.; Sha, G.; Wu, Y.Z.; Ringer, S.P. Mechanisms for enhanced plasticity in magnesium alloys. Acta Mater. 2015, 82, 344–355. [Google Scholar] [CrossRef]
- Hua, T.; Xiao, W.L.; Wang, F.; Li, Y.; Liu, S.Y.; Zheng, R.X.; Ma, C.L. Improving tensile properties of Mg-Sn-Zn magnesium alloy sheets using pretension and ageing treatment. J. Alloys. Compd. 2018, 735, 1494–1504. [Google Scholar] [CrossRef]
- Yang, J.; Peng, J.; Nyberg, E.A.; Pan, F.S. Effect of Ca addition on the corrosion behavior of Mg-Al-Mn alloy. Appl. Surf. Sci. 2016, 369, 92–100. [Google Scholar] [CrossRef]
- Liu, G.; Xie, W.; Wei, G.B.; Yang, Y.; Liu, J.W.; Xu, T.C.; Xie, W.D.; Peng, X.D. Dynamic Recrystallization Behavior and Corrosion Resistance of a Dual-Phase Mg-Li Alloy. Materials 2018, 11, 408. [Google Scholar] [CrossRef]
- Mingo, B.; Arrabala, R.; Mohedano, M.; Mendis, C.L.; Olmo, R.D.; Matykina, E.; Hort, N.; Merino, M.C.; Pardo, A. Corrosion of Mg-9Al alloy with minor alloying elements (Mn, Nd, Ca, Y and Sn). Mater. Des. 2017, 130, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Nezamdoust, S.; Seifzadeh, D.; Rajabalizadeh, Z. PTMS/OH-MWCNT sol-gel nanocomposite for corrosion protection of magnesium alloy. Surf. Coat. Technol. 2018, 335, 228–240. [Google Scholar] [CrossRef]
- Liu, W.; Yan, Z.J.; Ma, X.L.; Geng, T.; Wu, H.H.; Li, Z.Y. Mg-MOF-74/MgF2 Composite Coating for Improving the Properties of Magnesium Alloy Implants: Hydrophilicity and Corrosion Resistance. Materials 2018, 11, 396. [Google Scholar] [CrossRef]
- Tao, Y.S.; Xiong, T.Y.; Sun, C.; Jin, H.Z.; Du, H.; Li, T.F. Effect of a-Al2O3 on the properties of cold sprayed Al/a-Al2O3 composite coatings on AZ91D magnesium alloy. Appl. Surf. Sci. 2009, 256, 261–266. [Google Scholar] [CrossRef]
- Zhong, Y.; Hu, J.; Zhang, Y.; Tang, Y. The one-step electroposition of superhydrophobic surface on AZ31 magnesium alloy and its time-dependence corrosion resistance in NaCl solution. Appl. Surf. Sci. 2018, 427, 1193–1201. [Google Scholar] [CrossRef]
- Szklarz, Z.; Bisztyga, M.; Krawiec, H.; Lityńska-Dobrzyńska, L.; Rogal, Ł. Global and local investigations of the electrochemical behavior the T6 heat treated Mg-Zn-RE magnesium alloy thixo-cast. Appl. Surf. Sci. 2017, 405, 529–539. [Google Scholar] [CrossRef]
- Jiang, J.; Yin, L.; Lu, F.; Ma, A.; Song, D.; Zhang, L.; Yang, D.; Chen, J. Microstructure and corrosion behaviour of Mg-2Gd-1Y-1Zn-0·2Zr (at.%) alloy processed by equal channel angular pressing. Corros. Eng. Sci. Technol. 2014, 49, 316–320. [Google Scholar] [CrossRef]
- Shi, Z.M.; Cao, F.Y.; Song, G.L.; Liu, M.; Atrens, A. Corrosion behaviour in salt spray and in 3.5% NaCl solution saturated with Mg(OH)2 of as-cast and solution heat-treated binary Mg-RE alloys: RE = Ce, La, Nd, Y, Gd = Ce, La, Nd, Y, Gd. Corros. Sci. 2013, 76, 98–118. [Google Scholar] [CrossRef]
- Meng, J.; Sun, W.; Tian, Z.; Qiu, X.; Zhang, D. 2-Corrosion performance of magnesium (Mg) alloys containing rare-earth (RE) elements. Corros. Prev. Magnes. Alloys 2013. [Google Scholar] [CrossRef]
- She, J.; Pan, F.S.; Hu, H. Microstructures and mechanical properties of as-extruded Mg-5Sn-1Zn-xAl (x =1, 3 and 5) alloys. Progre. Natuar. Sci. Mater. Inter. 2015, 25, 267–275. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, B.; Baek, S.M.; Sohn, S.D.; Shin, H.J.; Jeong, H.Y.; Yim, C.D.; You, B.S.; Ha, H.Y.; Park, S.S. Influence of alloyed Al on the microstructure and corrosion properties of extruded Mg-8Sn-1Zn alloys. Corros. Sci. 2015, 95, 133–142. [Google Scholar] [CrossRef]
- Nam, N.D.; Mathesh, M.; Le, T.V.; Nguyen, H.T. Corrosion behavior of Mg-5Al-xZn alloys in 3.5 wt.% NaCl solution. J. Alloys Compd. 2014, 616, 662–668. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, Y.; Zhou, X.N. Study of the corrosion behavior and the corrosion films formed on the surfaces of Mg-xSn alloys in 3.5 wt.% NaCl solution. Appl. Surf. Sci. 2014, 317, 1143–1150. [Google Scholar] [CrossRef]
- Hou, L.D.; Li, Z.; Zhao, H.; Pan, Y.; Pavlinich, S.; Liu, X.W.; Li, X.L.; Zheng, Y.F.; Li, L. Microstructure, Mechanical Properties, Corrosion Behavior and Biocompatibility of As-Extruded Biodegradable Mg-3Sn-1Zn-0.5Mn. J. Mater. Sci. Technol. 2016, 32, 874–882. [Google Scholar] [CrossRef]
- Wang, B.; Pan, F.S.; Chen, X.H.; Guo, W.; Mao, J.J. Microstructure and mechanical properties of as-extruded and as-aged Mg-Zn-Al-Sn alloys. Mater. Sci. Eng. A. 2016, 65, 165–173. [Google Scholar] [CrossRef]
- Liu, C.Q.; Liu, C.L.; Chen, H.W.; Nie, J.F. Heat-treatable Mg-9Al-6Sn-3Zn extrusion alloy. J. Mater. Sci. Technol. 2018, 34, 284–290. [Google Scholar] [CrossRef]
- Kim, B.; Lee, J.G.; Park, S.S. Superplasticity and load relaxation behavior of extruded Mg-8Sn-3Al-1Zn alloy at 250 °C. Mater. Sci. Eng. A 2016, 656, 234–240. [Google Scholar] [CrossRef]
- She, J.; Pan, F.S.; Zhang, J. Microstructure and mechanical properties of Mg-Al-Sn extruded alloys. J. Alloys. Compd. 2016, 657, 893–905. [Google Scholar] [CrossRef]
- Wang, B.; Chen, X.H.; Pan, F.S.; Mao, J.J. Effects of Sn addition on microstructure and mechanical properties of Mg-Zn-Al alloys. Progre. Natuar. Sci. Mater. Inter. 2017, 27, 695–702. [Google Scholar] [CrossRef]
- Zhu, S.Z.; Luo, T.J.; Yang, Y.S. Improving mechanical properties of age-hardenable Mg-6Zn-4Al-1Sn alloy processed by double-aging treatment. J. Mater. Sci. Technol. 2017, 33, 1249–1254. [Google Scholar] [CrossRef]
- Jung, J.G.; Park, S.H.; You, B.S. Effect of aging prior to extrusion on the microstructure and mechanical properties of Mg-7Sn-1Al-1Zn alloy. J. Alloys. Compd. 2015, 627, 324–332. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Xu, J.Y.; Yu, J.; Liu, X.F. Microstructure and mechanical properties of as-cast and as-hot-rolled novel Mg-xSn-2.5Zn-2Al alloys (x = 2, 4 wt%). Mater. Sci. Eng. A 2018, 712, 65–72. [Google Scholar] [CrossRef]
- Park, S.H.; You, B.S. Effect of homogenization temperature on the microstructure and mechanical properties of extruded Mg-7Sn-1Al-1Zn alloy. J. Alloy. Compd. 2015, 637, 332–338. [Google Scholar] [CrossRef]
- Metalnikov, P.; Hamu, G.B.; Eliezer, D.; Shin, K.S. Role of Sn in microstructure and corrosion behavior of new wrought Mg-5Al alloy. J. Alloys. Compd. 2019, 777, 835–849. [Google Scholar] [CrossRef]
- Cain, T.W.; Glover, C.F.; Scully, J.R. The corrosion of solid solution Mg-Sn binary alloys in NaCl solutions. Electrochim. Acta 2019, 297, 564–575. [Google Scholar] [CrossRef]
- Ha, H.Y.; Kang, J.Y.; Yang, J.; Yim, C.D.; You, B.S. Role of Sn in corrosion and passive behavior of extruded Mg-5 wt% Sn alloy. Corros. Sci. 2016, 102, 355–362. [Google Scholar]
- Boby, A.; Srinivasan, A.; Pillai, U.S.; Pai, B.C. Mechanical characterization and corrosion behavior of newly designed Sn and Y added AZ91 alloy. Mater. Des. 2015, 88, 871–879. [Google Scholar]
- Humphreys, F.J.; Hatherly, M. Recrystallization and Related Annealing Phenomena, 2nd ed.; Elsevier: Oxford, UK, 2004; pp. 285–292. [Google Scholar]
- Lin, H.Q.; Wang, J.G.; Wang, H.Y.; Jiang, Q.C. Effect of predeformation on the globular grains in AZ91D alloy during strain induced melt activation (SIMA) process. J. Alloy. Compd. 2007, 431, 141–147. [Google Scholar] [CrossRef]
- Kim, K.; Yoon, J. Effects of starting microstructure and billet orientations on the texture evolution and the mechanical behavior of Mg-3Al-1Zn rolled plate by half channel angular extrusion (HCAE). Mater. Sci. Eng. A 2015, 622, 46–51. [Google Scholar] [CrossRef]
- Song, Y.W.; Han, E.H.; Shan, D.Y.; Yim, C.D.; You, B.S. The effect of Zn concentration on the corrosion behavior of Mg-xZn alloys. Corros. Sci. 2012, 65, 322–330. [Google Scholar] [CrossRef]
- Curioni, M. The behaviour of magnesium during free corrosion and potentiodynamic polarization investigated by real-time hydrogen measurement and optical imaging. Electrochim. Acta. 2014, 120, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Rajabalizadeh, Z.; Seifzadeh, D. Application of electroless Ni-P coating on magnesium alloy via CrO3/HF free titanate pretreatment. Appl. Surf. Sci. 2017, 422, 696–709. [Google Scholar] [CrossRef]
- Ha, H.Y.; Kang, J.Y.; Kim, S.G.; Kim, B.; Park, S.S.; Yim, C.D.; You, B.S. Influences of metallurgical factors on the corrosion behaviour of extruded binary Mg-Sn alloys. Corros. Sci. 2014, 82, 369–379. [Google Scholar] [CrossRef]
- Gheshlaghi, M.G.; Seifzadeh, D.; Shoghi, P.; Yangjeh, A.H. Electroless Ni-P/nano-WO3 coating and its mechanical and corrosion protection properties. J. Alloys. Compd. 2018, 769, 149–160. [Google Scholar] [CrossRef]
- Feng, H.; Liu, S.H.; Du, Y.; Lei, T.; Zeng, R.C.; Yuan, T.C. Effect of the second phases on corrosion behavior of the Mg-Al-Zn alloys. J. Alloys. Compd. 2017, 695, 2330–2338. [Google Scholar] [CrossRef]
- Bai, X. Chemistry of Nuclear Materials; Press of Chemical Industry: Beijng, China, 2007. [Google Scholar]
- Liu, X.B.; Shan, D.Y.; Song, Y.W.; Chen, R.S.; Han, E.N. Influences of the quantity of Mg2Sn phase on the corrosion behavior of Mg-7Sn magnesium alloy. Electrochim. Acta 2011, 56, 2582–2590. [Google Scholar] [CrossRef]
- Zhong, L.P.; Wang, Y.J.; Lu, H.; Cui, X.J.; Zhang, Y.J.; Dou, B.J.; Peng, J. Influence of aging prior to extrusion on the microstructure and corrosion resistance of Mg-8Sn-2Zn-0.2Mn alloy. J. Alloy. Compd. 2019, 780, 783–791. [Google Scholar] [CrossRef]



| Alloy | Nominal Composition | Analyzed Composition (wt %) | |||
|---|---|---|---|---|---|
| Zn | Al | Sn | Mg | ||
| ZAT540 | Mg–5Zn–4Al–0Sn | 5.02 | 3.52 | 0.00 | Bal |
| ZAT540.5 | Mg–5Zn–4Al–0.5Sn | 5.00 | 3.50 | 0.51 | Bal |
| ZAT541 | Mg–5Zn–4Al–1Sn | 5.01 | 3.56 | 1.03 | Bal |
| ZAT542 | Mg–5Zn–4Al–2Sn | 5.00 | 3.51 | 2.00 | Bal |
| ZAT543 | Mg–5Zn–4Al–3Sn | 5.02 | 3.54 | 3.01 | Bal |
| Position | Composition (at. %) | |||
|---|---|---|---|---|
| Mg | Zn | Al | Sn | |
| A | 90.88 | 05.30 | 03.82 | 0 |
| B | 62.55 | 19.17 | 18.28 | 0 |
| C | 73.90 | 0 | 0 | 26.10 |
| Sample | Ecorr (V) | Icorr (A·cm−2) | Bc (mV/dec) |
|---|---|---|---|
| ZAT540 | −1.5173 | 6.6571 × 10−5 | 130 |
| ZAT540.5 | −1.3628 | 9.4157 × 10−6 | 172 |
| ZAT541 | −1.3309 | 6.7071 × 10−6 | 203 |
| ZAT542 | −1.4235 | 1.1387 × 10−5 | 139 |
| ZAT543 | −1.4941 | 2.5085 × 10−5 | 126 |
| Alloys | Rs (Ω cm2) | CPEdl | Rct (Ω cm2) | L (H cm−2) | RL (Ω cm2) | |
|---|---|---|---|---|---|---|
| Q (Sn Ω−1 cm−2) | n | |||||
| ZAT540 | 11.53 | 1.481 × 10−5 | 0.9417 | 317.5 | 153.7 | 183.0 |
| ZAT540.5 | 11.38 | 1.325 × 10−5 | 0.9623 | 647.2 | 167.5 | 261.4 |
| ZAT541 | 11.22 | 1.505 × 10−5 | 0.9653 | 681.6 | 217.9 | 299.7 |
| ZAT542 | 11.71 | 1.64 × 10−5 | 0.9195 | 635.4 | 172.4 | 215.4 |
| ZAT543 | 14.39 | 2.38 × 10−5 | 0.9697 | 345.6 | 143.5 | 243.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Liu, X.; Wang, Y.; Huang, W.; Wang, B.; Wei, S.; Xia, X.; Liang, Y.; Chen, X.; Pan, F.; et al. Effect of Sn Addition on Microstructure and Corrosion Behavior of As-Extruded Mg–5Zn–4Al Alloy. Materials 2019, 12, 2069. https://doi.org/10.3390/ma12132069
Ding J, Liu X, Wang Y, Huang W, Wang B, Wei S, Xia X, Liang Y, Chen X, Pan F, et al. Effect of Sn Addition on Microstructure and Corrosion Behavior of As-Extruded Mg–5Zn–4Al Alloy. Materials. 2019; 12(13):2069. https://doi.org/10.3390/ma12132069
Chicago/Turabian StyleDing, Jian, Xin Liu, Yujiang Wang, Wei Huang, Bo Wang, Shicheng Wei, Xingchuan Xia, Yi Liang, Xianhua Chen, Fusheng Pan, and et al. 2019. "Effect of Sn Addition on Microstructure and Corrosion Behavior of As-Extruded Mg–5Zn–4Al Alloy" Materials 12, no. 13: 2069. https://doi.org/10.3390/ma12132069




