1. Introduction
Reinforced concrete (RC) is one of the most commonly used modern construction materials. The corrosion of the interface between concrete and steel rebar affects the service life of RC seriously. In the past decades, in order to increase the lifespan of RC, researchers have dedicated efforts towards the enhancement of bond strength and corrosion resistance in RC [
1,
2]. Among all the anti-corrosion technology, a most effective way to improve the property of corrosion resistance is to cover the rebar with a durable and adhesive coating [
3], which can be divided into organic coatings and inorganic coatings. Epoxy [
4,
5] and modified resin [
6] have been generally adopted as organic coatings, while inorganic coatings include galvanizing metal coatings [
7], chemically reactive enamel (CRE) coatings [
8], and chemically bonded phosphate ceramic (CBPC) coatings [
9]. In the application of the epoxy coating in reinforced concrete, Brown MC [
10] arrived at the conclusion that the durability of the steel with the epoxy coating was not ideal due to the moisture that penetrates beneath the coating, which means that the epoxy-coated steel has a higher corrosion rate than bare steel. What’s more, the bone strength loss between the epoxy-coated steel and the concrete reached 20% compared to bare rebar [
11]. For the inorganic coatings, a weakening effect on the bond strength between the galvanized reinforcing steel and the concrete was observed due to hydrogen evolution at the interface [
12]. A high temperature of 800 °C needs to be applied to the chemically reactive enamel-coated steel, which can lead to a reduction in the creep limit and strength [
1].
In recent years, more and more studies have focused on the utilization of CBPCs, which are derived from the reactions between base and acid, such as the metal oxide (Al
2O
3, MgO) and the soluble acid phosphate (KH
2PO
4, Al(H
2PO
4)
3) [
13,
14]. Due to their corrosion resistance, mechanical resistance, thermal conductivity and low temperature used in their processing, CBPC coatings have considerable technological importance [
15]. CBPC coatings can be seen to use the phosphate matrixes as the binding phases and the metal oxides as suitable fillers. Researchers [
16,
17,
18] have investigated the influence of ceramic oxides (AlN, MgO, and ZrO
2) on the improvement of the thermal properties of CBPC coatings. The abrasive filler of Al
2O
3-SiC in aluminum phosphate has been produced to enhance the abrasion resistance of coatings [
19]. The ceramic coatings of aluminum phosphate formed by the reaction between soluble acid phosphates and alumina and alumina–sol–gel systems was studied [
20]. H.M. Hawthorne et al. [
21] prepared phosphate ceramic coatings on stainless steel substrates and analyzed the mechanical performance and electrochemical property. The application by thermal spraying for binders was carried out, and the microstructure defects of the coatings were improved [
22]. A series of CBPC coatings were applied to steel surfaces to reduce or eliminate corrosion [
23]. Da Bian et al. [
24] paid attention to graphene-reinforced CBPC coatings. Zhu Ding et al. [
25] studied the mechanical characterization and microstructure of CBPC composites reinforced with fiber, prepared at room temperature.
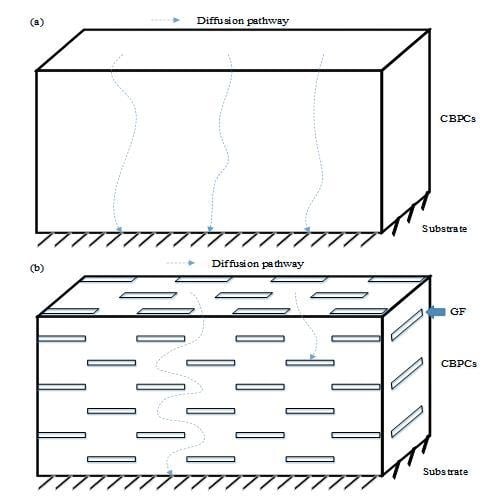
Glass flake (GF) particles are inorganic platelets with excellent resistance to chemicals and aging. Many efforts have been focused towards corrosion-resistant coatings with the advanced barrier properties of GF [
26,
27,
28,
29,
30], and the parallel and overlapped arrangement of GF particles could form a compact impermeable layer in organic coatings. There are microstructure defects between GF particles and the organic coating matrix due to the coating matrix belonging to organic material, while GF belongs to inorganic materials; thus, we can only effectively improve the permeability resistance of organic coatings by surface treatment and functionalization of GF particles.
In all cases cited, there have been few reports on the application of GF particles as fillers in CBPC coatings. Meanwhile, the anticorrosion mechanism of the GF-reinforced CBPC coatings also needs to be illustrated. In this study, CBPC coatings reinforced with GF particles were prepared in order to protect the round steel. The crystalline phase, curing behavior, and micromorphology of the CBPC-based ceramic materials were analyzed, and the electrochemical characterization of the CBPC coatings was carried out in 3.5 wt.% NaCl solution using electrochemical measurement. Furthermore, the anticorrosion mechanism of the coating was also investigated.
2. Materials and Methods
2.1. Materials
The CBPC coatings reinforced with GF were applied onto round steel 8 mm in diameter and 20 mm in length by mixing raw materials of phosphate ceramic materials as shown in
Table 1.
The preparation of monoaluminium phosphate (MAP) binder is based on the reaction between aluminum hydroxide and phosphoric acid at 120 °C under constant stirring for 60 min. The quantities were calculated according to Equation (1). For the preparation of MAP binder, 135.1 g of pure water were used to dilute 345.9 g of 85% phosphoric acid to 60%, and then 78.0 g of aluminum hydroxide were added.
The mixture proportion of phosphate ceramic coating materials are shown in
Table 2. The coated samples were prepared by brush coating with a clean bristle brush. Before preparing coating, 800 grit silicon carbide abrasive papers were used to polish the surface of the round steel, the round steel was degreased in acetone for 15 min (in ultrasonic bath) and then rinsed with deionized water. The coated round steel was placed at room temperature (25 ± 1 °C) for 10 h and then heated in an electric oven according to the curing process as shown in
Figure 1.
2.2. Characterization
Laser scanning confocal microscopy (LSCM, manufacture, Oberkochen, Germany) was used to characterize the thickness and surface topography of GF. The thickness of the coating was tested using a QNIX4500 coating thickness gauge measurement (QNIX, Oberkochen, Germany) at a precision of 1 μm. The crystalline phases of phosphate ceramic coatings after curing were measured by X-ray diffractometer (PANalytical Empyrean, Almelo, The Netherlands) using a CuKα source scanning from 5° to 70° in 2θ. A differential scanning calorimeter (STA instruments, Selb, Germany) was used to analyze the curing behavior of phosphate ceramic coating materials under N2 atmosphere with gas flow of 30 mL/min, heat velocity of 10 °C/min, start temperature 25 °C and end temperature 400 °C. Furthermore, the SEM micrographs of the surface and cross-section of the coated samples were investigated on a JSM-IT300 (JEOL, Tokyo, Japan) under secondary electron mode, test voltage of 10 kV and surface treatment with platinum.
The potentiodynamic polarization was conducted using a workstation CS350 (Wuhan Corrtest Instrument Co., Ltd., Wuhan, China), as well as the electrochemical impedance spectroscopy (EIS) measurement, with a three-electrode system, containing the reference electrode (saturated calomel electrode), the counter electrode (platinum electrode), and the working electrode (sample). The electrochemical experiment was conducted in 3.5 wt.% NaCl solution at 25 ± 1 °C. The exposed surface area was around 2.5 cm2. After the samples were immersed in the NaCl solution for 10 h, the potentiodynamic polarization was performed at a speed of 2 mV·s−1, from −100 mV to 100mV. EIS measurements were carried out with AC signals of 5 mV peak-to-peak amplitude in the frequency start at 100 kHz and end at 0.01 Hz. Z-View software was used to evaluate the EIS data. At least three repeated electrochemical tests were carried out to confirm the reliability of the measurement.