Optimizing the Properties of InGaZnOx Thin Film Transistors by Adjusting the Adsorbed Degree of Cs+ Ions
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
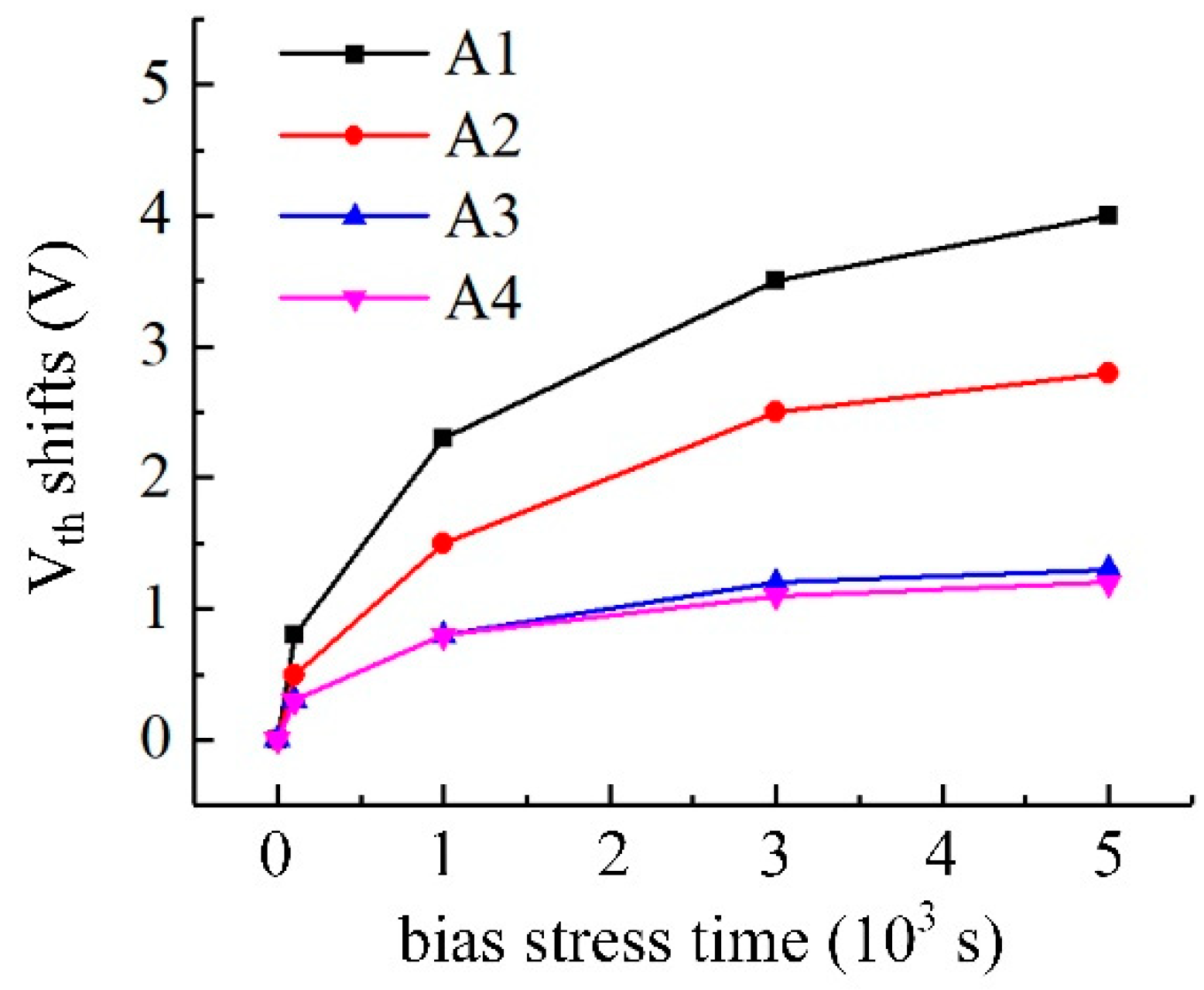
3.1. Electrical Characteristics of Cs-IGZO TFTs
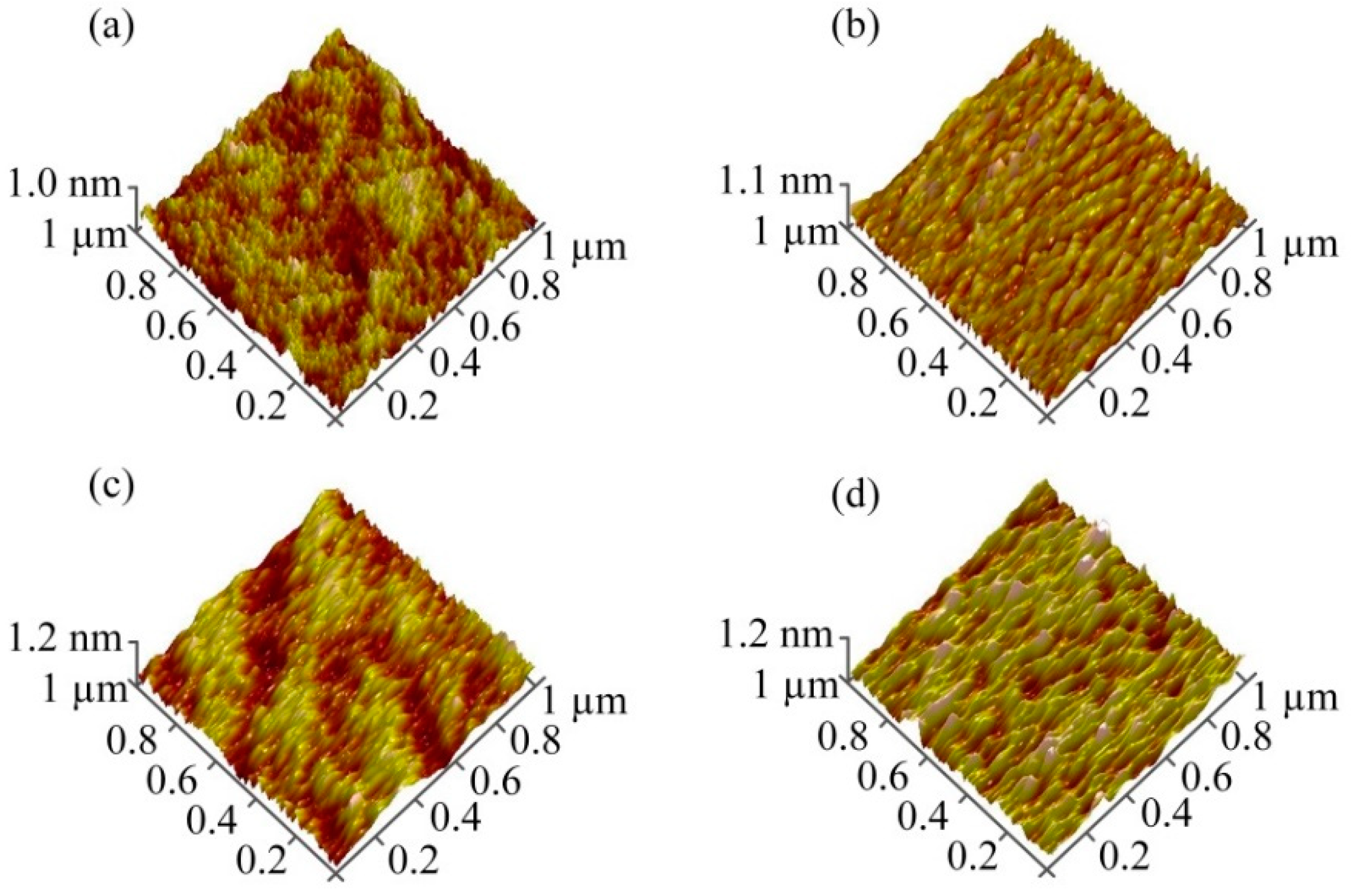
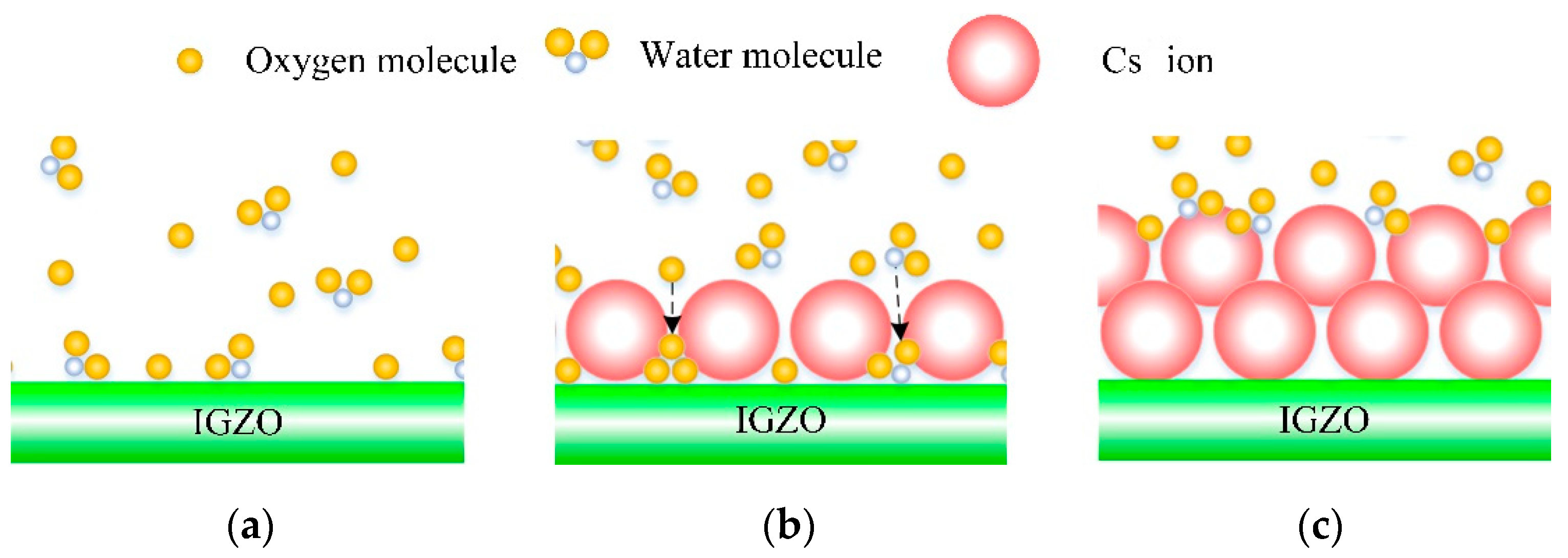
3.2. Surface Structure of Cs-IGZO Films
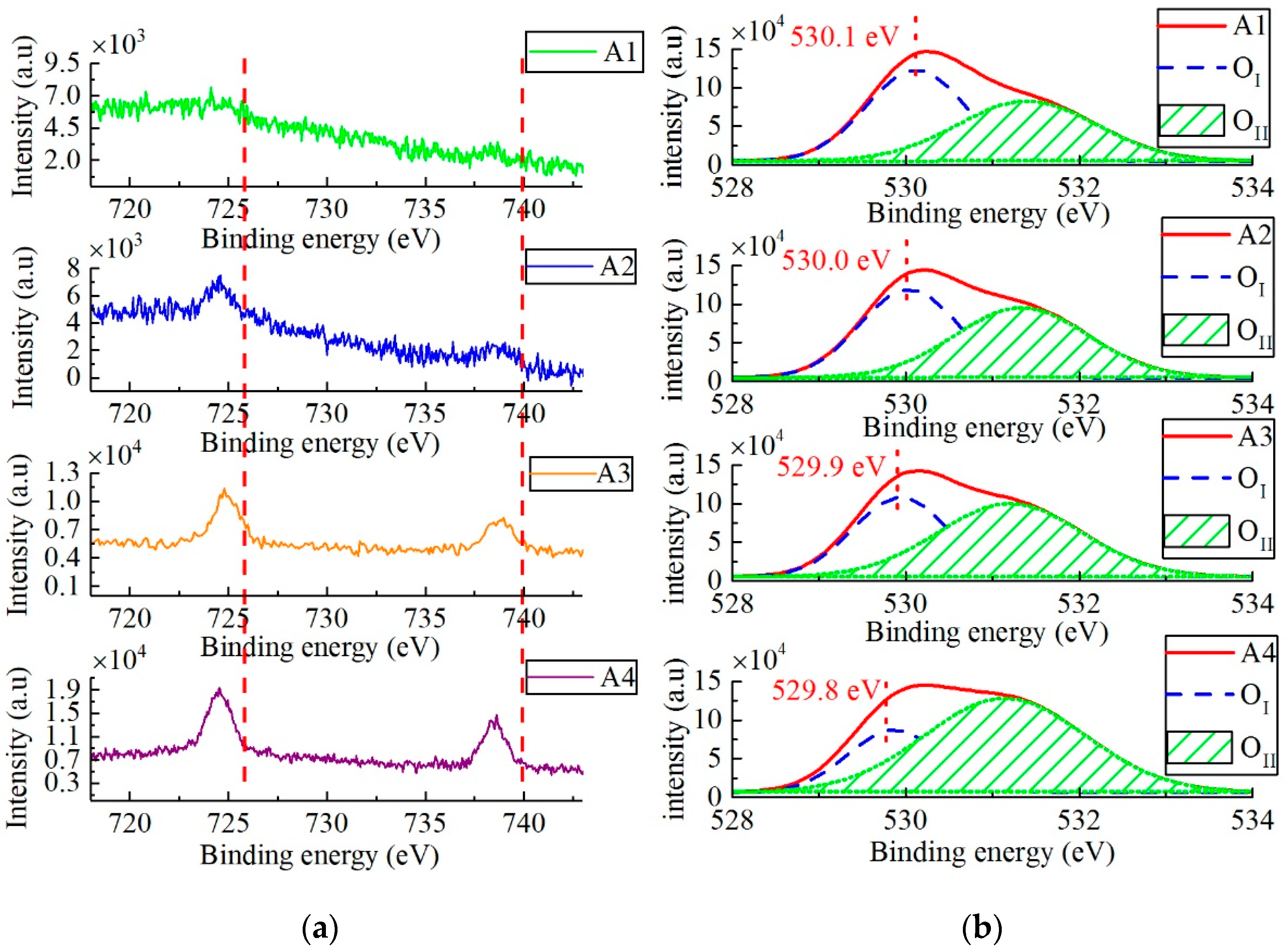
3.3. Chemical Studies on Cs-IGZO Films
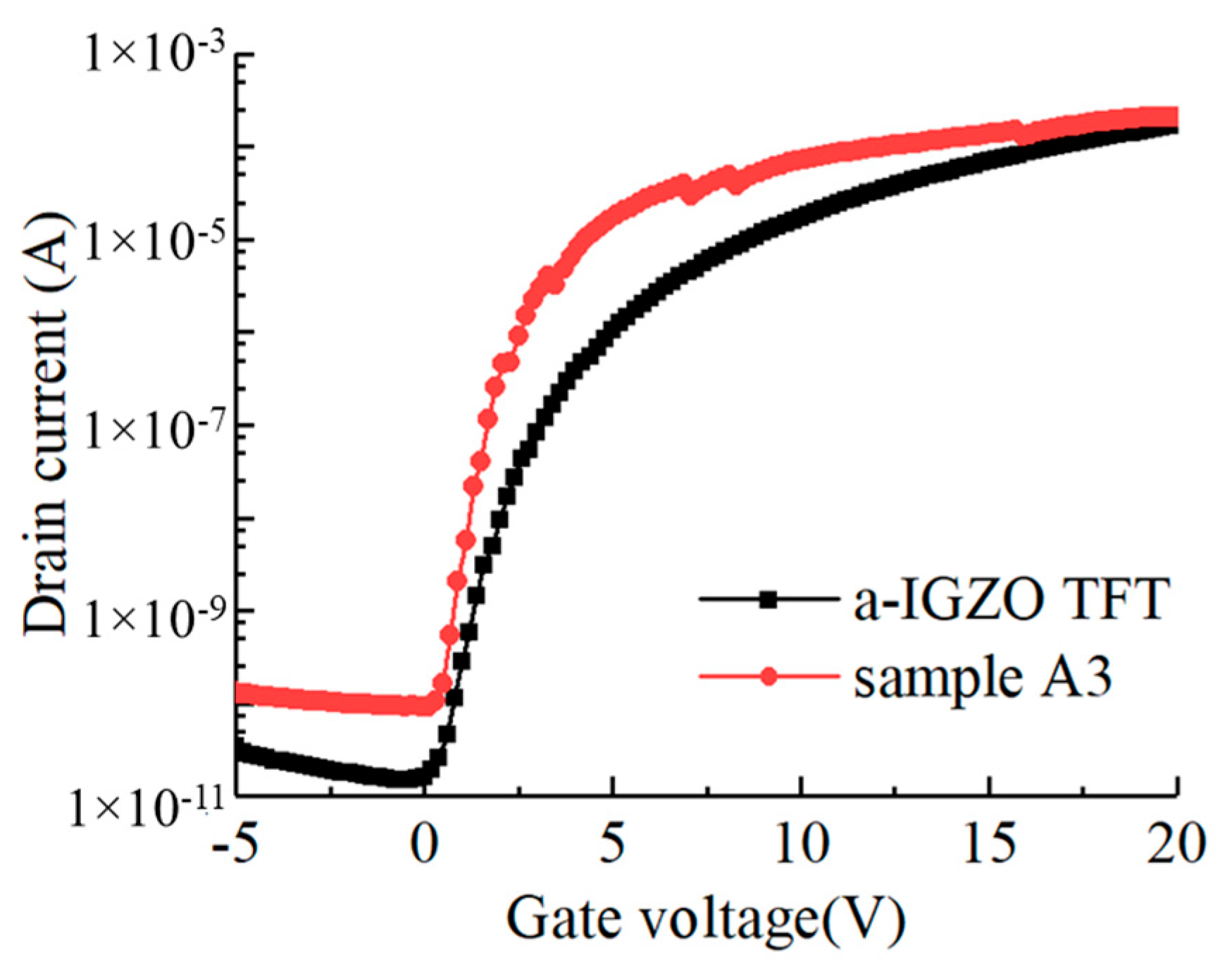
3.4. Comparison between Cs-IGZO TFT and Annealed IGZO TFT
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yabuta, H.; Sano, M.; Abe, K.; Aiba, T.; Den, T.; Kumomi, H.; Nomura, K.; Kamiya, T.; Hosono, H. High-mobility thin-film transistor with amorphous ingazno4 channel fabricated by room temperature rf-magnetron sputtering. Appl. Phys. Lett. 2006, 89, 112123. [Google Scholar] [CrossRef]
- Kamiya, T.; Nomura, K.; Hosono, H. Present status of amorphous In-Ga-Zn-O thin-film transistors. Sci. Technol. Adv. Mater. 2010, 11, 044305. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Lai, Y.C.; Lai, C.C.; Wu, B.S.; Zan, H.W.; Yu, P.; Chueh, Y.L.; Tsai, C.C. Highly effective field-effect mobility amorphous ingazno tft mediated by directional silver nanowire arrays. ACS Appl. Mater. Interfaces 2015, 7, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.M.; Wu, Y.C.; Hsu, Y.J.; Yu, M.J.; Im, J.S.; Lu, P.Y. P-169: A 31-inch 4k2k top-emission oled display using good uniformity and long-term reliability top-gate self-aligned IGZO TFTs. SID Symp. Dig. Tech. Pap. 2018, 49, 1796–1799. [Google Scholar] [CrossRef]
- Mo, Y.G.; Kim, M.; Kang, C.K.; Jeong, J.H.; Park, Y.S.; Choi, C.G.; Kim, H.D.; Kim, S.S. Amorphous-oxide TFT backplane for large-sized AMOLED TVs. J. Soc. Inf. Disp. 2011, 19, 16–20. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, S.; Wu, W.; Zhu, L.; Xiao, H.; Liu, Y.; Zhang, H.; Javaid, K.; Cao, H. Extended-gate-type igzo electric-double-layer tft immunosensor with high sensitivity and low operation voltage. Appl. Phys. Lett. 2016, 109, 173501. [Google Scholar] [CrossRef]
- Zan, H.W.; Li, C.H.; Yeh, C.C.; Dai, M.Z.; Meng, H.F.; Tsai, C.C. Room-temperature-operated sensitive hybrid gas sensor based on amorphous indium gallium zinc oxide thin-film transistors. Appl. Phys. Lett. 2011, 98, 253503. [Google Scholar] [CrossRef] [Green Version]
- Hung, M.F.; Wu, Y.C.; Chang, J.J.; Chang Liao, K.S. Twin thin-film transistor nonvolatile memory with an indium–gallium–zinc–oxide floating gate. IEEE Electron Device Lett. 2013, 34, 75–77. [Google Scholar] [CrossRef]
- Lee, G.G.; Tokumitsu, E.; Yoon, S.M.; Fujisaki, Y.; Yoon, J.W.; Ishiwara, H. The flexible non-volatile memory devices using oxide semiconductors and ferroelectric polymer poly(vinylidene fluoride-trifluoroethylene). Appl. Phys. Lett. 2011, 99, 12901. [Google Scholar] [CrossRef]
- Petti, L.; Münzenrieder, N.; Vogt, C.; Faber, H.; Büthe, L.; Cantarella, G.; Bottacchi, F.; Anthopoulos, T.D.; Tröster, G. Metal oxide semiconductor thin-film transistors for flexible electronics. Appl. Phys. Rev. 2016, 3, 021303. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Shin, D.; Yun, I. Degradation mechanisms of amorphous inGaZnOthin-film transistors used in foldable displays by dynamic mechanical stress. IEEE Trans. Electron Devices 2017, 64, 170–175. [Google Scholar] [CrossRef]
- Ning, H.; Zeng, Y.; Kuang, Y.; Zheng, Z.; Zhou, P.; Yao, R.; Zhang, H.; Bao, W.; Chen, G.; Fang, Z.; et al. Room-temperature fabrication of high-performance amorphous In-Ga-Zn-O/Al2O3 thin-film transistors on ultrasmooth and clear nanopaper. ACS Appl. Mater. Interfaces 2017, 9, 27792–27800. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jang, I.; Jeong, J. Effects of helium annealing in low-temperature and solution-processed amorphous indium-gallium-zinc-oxide thin-film transistors. AIP Adv. 2019, 9, 045228. [Google Scholar] [CrossRef] [Green Version]
- Minari, T.; Kanehara, Y.; Liu, C.; Sakamoto, K.; Yasuda, T.; Yaguchi, A.; Tsukada, S.; Kashizaki, K.; Kanehara, M. Room-temperature printing of organic thin-film transistors with π-junction gold nanoparticles. Adv. Funct. Mater. 2014, 24, 4886–4892. [Google Scholar] [CrossRef]
- Choi, C.; Baek, Y.; Lee, B.M.; Kim, K.H.; Rim, Y.S. Enhanced electrical stability of nitrate ligand-based hexaaqua complexes solution-processed ultrathin a-IGZO transistors. J. Phys. D Appl. Phys. 2017, 50, 485107. [Google Scholar] [CrossRef]
- Kim, Y.R.; Kwon, J.H.; Vincent, P.; Kim, D.K.; Jeong, H.S.; Hahn, J.; Bae, J.H. Effect of UV and water on electrical properties at pre- and post-annealing processes in solution-processed inGaZnO transistors. J. Nanosci. Nanotechnol. 2019, 19, 2240–2246. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Seol, H.; Song, A.; Choi, S.; Song, Y.; Yun, P.S.; Chung, K.-B.; Bae, J.U.; Park, K.S.; Jeong, J.K. Comparative study on performance of igzo transistors with sputtered and atomic layer deposited channel layer. IEEE Trans. Electron Devices 2019, 66, 1783–1788. [Google Scholar] [CrossRef]
- Xu, W.; Xu, M.; Jiang, J.; Xu, S.; Feng, X. Impact of sputtering power on amorphous in-al-zn-o films and thin film transistors prepared by rf magnetron sputtering. IEEE Trans. Electron Devices 2019, 66, 2219–2223. [Google Scholar] [CrossRef]
- Fuh, C.S.; Liu, P.T.; Teng, L.F.; Huang, S.W.; Lee, Y.J.; Shieh, H.P.D.; Sze, S.M. Effects of microwave annealing on nitrogenated amorphous in-ga-zn-o thin-film transistor for low thermal budget process application. IEEE Electron Device Lett. 2013, 34, 1157–1159. [Google Scholar] [CrossRef]
- Lee, Y.S.; Yen, T.W.; Lin, C.I.; Lin, H.C.; Yeh, Y. Electrical characteristics of amorphous in-ga-zn-o thin-film transistors prepared by radio frequency magnetron sputtering with varying oxygen flows. Displays 2014, 35, 165–170. [Google Scholar] [CrossRef]
- Hung, M.P.; Chare, C.; Nag, M.; de Meux, A.D.J.; Genoe, J.; Steudel, S. Effect of high oxygen partial pressure on carrier transport mechanism in a-ingazno tfts. IEEE Trans. Electron Devices 2018, 65, 2833–2837. [Google Scholar] [CrossRef]
- Moon, Y.K.; Lee, S.; Kim, D.H.; Lee, D.H.; Jeong, C.O.; Park, J.W. Application of dc magnetron sputtering to deposition of ingazno films for thin film transistor devices. Jpn. J. Appl. Phys. 2009, 48, 031301. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Zhang, X.; Liu, C. Improvement of electrical characteristics and stability of IGZO TFT through surface single crystallization of IGZO film at room temperature. Semicond. Sci. Technol. 2018, 33, 085015. [Google Scholar] [CrossRef]





| Name of Cs-IGZO Film | A1 | A2 | A3 | A4 |
|---|---|---|---|---|
| Concentration of CsHCO3 Solution (% mol/L) | 0.5 | 1 | 2 | 3 |
| Parameter/Sample | Vth (V) | μFE (cm2 V−1 s−1) | S.S (V/decade) |
|---|---|---|---|
| A1 | 7.9 | 8.6 | 0.28 |
| A2 | 5.7 | 13.1 | 0.25 |
| A3 | 0.2 | 18.7 | 0.23 |
| A4 | −1.7 | 21.5 | 0.22 |
| Samples/Parameter | Vth (V) | μFE (cm2 V−1 s−1) | S.S (V/decade) |
|---|---|---|---|
| Cs-IGZO TFT | 0.2 | 18.7 | 0.23 |
| a-IGZO TFT | 0.2 | 12.6 | 0.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wang, Y.; Wang, R.; Zhang, X.; Liu, C. Optimizing the Properties of InGaZnOx Thin Film Transistors by Adjusting the Adsorbed Degree of Cs+ Ions. Materials 2019, 12, 2300. https://doi.org/10.3390/ma12142300
Zhang H, Wang Y, Wang R, Zhang X, Liu C. Optimizing the Properties of InGaZnOx Thin Film Transistors by Adjusting the Adsorbed Degree of Cs+ Ions. Materials. 2019; 12(14):2300. https://doi.org/10.3390/ma12142300
Chicago/Turabian StyleZhang, He, Yaogong Wang, Ruozheng Wang, Xiaoning Zhang, and Chunliang Liu. 2019. "Optimizing the Properties of InGaZnOx Thin Film Transistors by Adjusting the Adsorbed Degree of Cs+ Ions" Materials 12, no. 14: 2300. https://doi.org/10.3390/ma12142300





