Age Hardening of Extruded AA 6005A Aluminium Alloy Powders
Abstract
:1. Introduction
2. Materials and Experimental Procedures
3. Results and Discussion
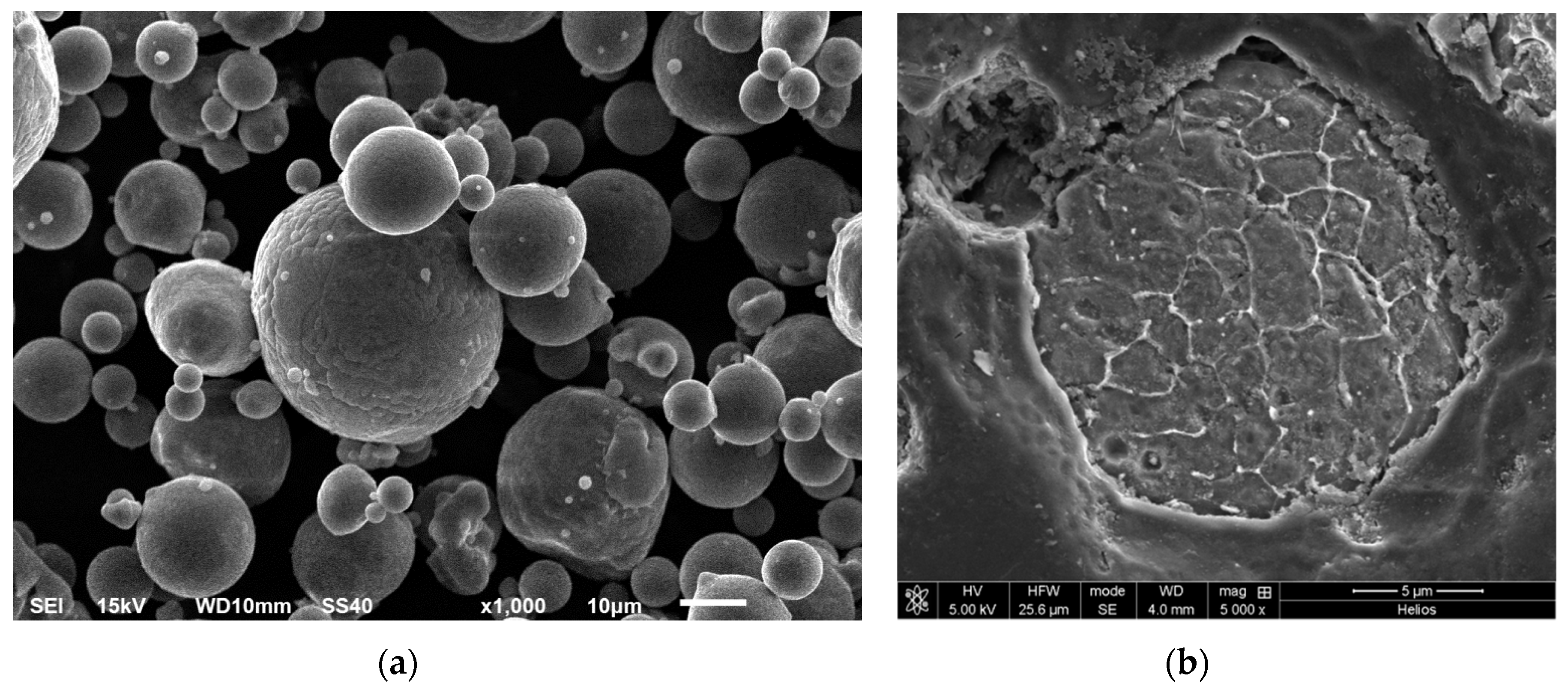
3.1. Characteristics of as Received AA6005A Powder
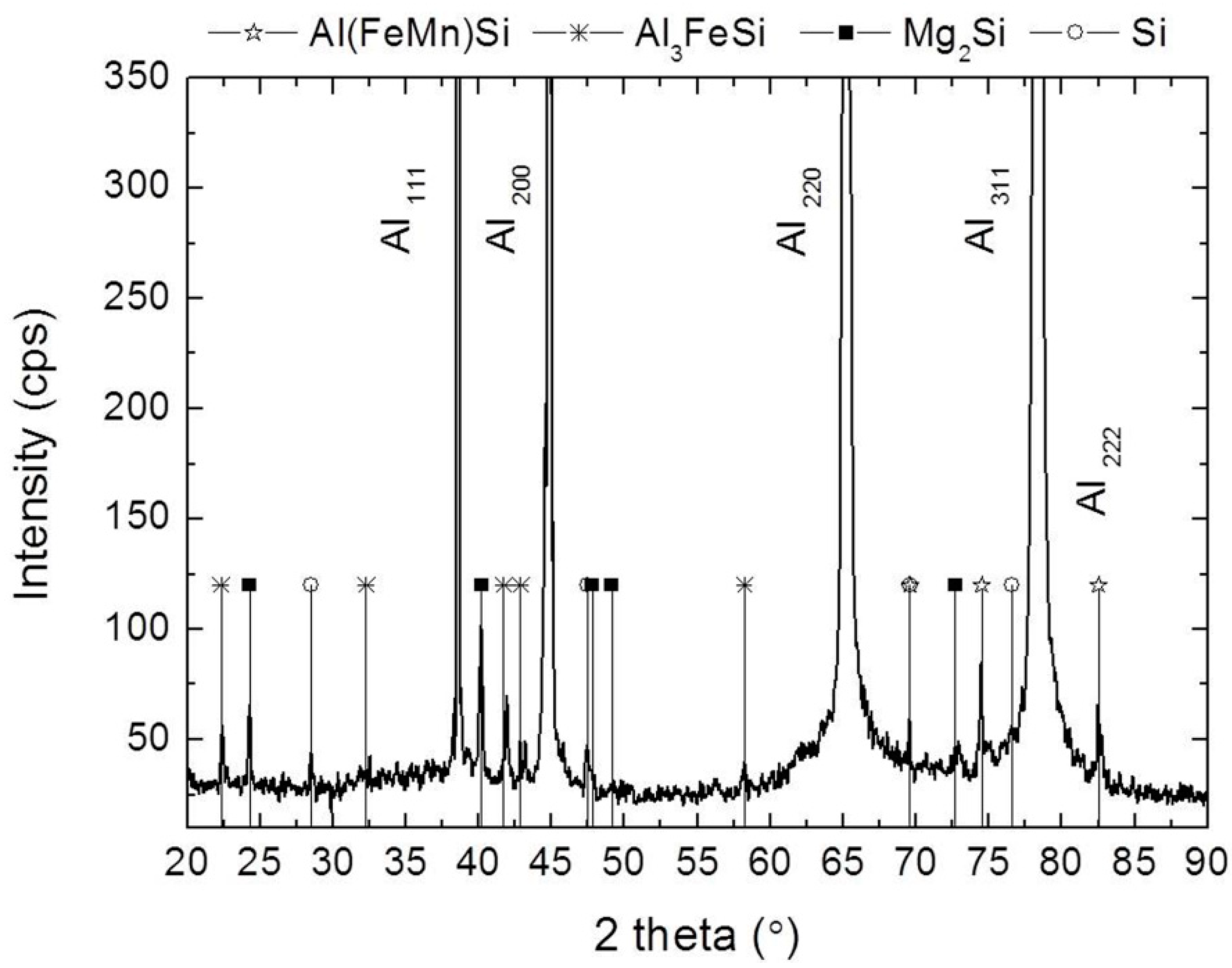
3.2. Characteristics of AA6005A P/M Extruded Alloys
3.3. Age Hardening
3.4. Precipitation Hardening of the Extruded Profiles
4. Conclusions
- -
- The temperature selected temperature for hot extrusion was 500 °C, because the banded structure due to the presence of segregated phases in the pre-alloyed powders is less visible and a more uniform grain size distribution was obtained.
- -
- Peak hardening conditions (T6) for the precipitation hardening of the P/M alloy were: 180 °C and 6 h. Temperature was slightly higher and the time was shorter than those normally used in the conventional route, in good agreement with the published results indicating that high-temperature and short-time aging are more efficient to produce a simultaneous enhancement of strength and ductility.
- -
- The extruded profiles produced by powder metallurgy, hot extruded and aged at peak hardness conditions presented superior mechanical properties than the extruded profiles from conventional ingot metallurgy, achieving a simultaneous improvement of ~40% in strength and ~47% in ductility.
- -
- The increase in both properties can be explained by combined effect of a UFG structure and a high density of nano-sized β″ precipitates.
- -
- This enhancement in properties can open the doors for the use of components extruded from AA 6005A P/M aerospace or military industries where the cost of manufacturing powders can be compensated with best performance of the alloy.
Author Contributions
Funding
Conflicts of Interest
References
- Davis, J.R. ASM Specialty Handbook: Aluminum and Aluminum Alloys; ASM International: Novelty, OH, USA, 1993. [Google Scholar]
- Polmear, I.J. Light Alloys: Metallurgy of the Light Metals, 3rd ed.; J. Wiley & Sons: New York, NY, USA, 1995. [Google Scholar]
- Soboyejo, W.O.; Srivatsan, T.S. Advanced Structural Materials: Properties, Design Optimization, and Applications; CRC Press: New York, NY, USA, 2007. [Google Scholar]
- Froyen, L.; Verlinden, B. TALAT Lecture 1401: Aluminum Powder Metallurgy; European Aluminium Association: Brussels, Belgium, 1994. [Google Scholar]
- Pickens, J.R. High-Strength Aluminum Powder Metallurgy Alloys. In Properties and Selection: Nonferrous Alloys and Special-Purpose Material, 10th ed.; ASM International: Novelty, OH, USA, 1990; Volume 2. [Google Scholar]
- Gökçe, A.; Fehim, F.; Kurt, A.O. Microstructural examination and properties of premixed Al-Cu-Mg powder metallurgy alloy. Mater. Charact. 2011, 62, 730–735. [Google Scholar] [CrossRef]
- Bishop, D.P.; Cahoon, J.R.; Chaturvedi, M.C.; Kipouros, G.J.; Caley, W.F. On enhancing the mechanical properties of aluminum P/M alloys. Mater. Sci. Eng. A 2000, 290, 16–24. [Google Scholar] [CrossRef]
- Capus, J. PM light alloys gaining applications in automotive sector. Met. Powder Rep. 2013, 68, 12–15. [Google Scholar] [CrossRef]
- Ramakrishan, P. Automotive applications of powder metallurgy. Adv. Powder Metall. Prop. Process. Appl. 2013, 493–519. [Google Scholar]
- Loberto, A.; Lopes, H.; Iervolino, F.; Salgado, L. PM Trends for the Automotive Industry. In Proceedings of the SAE 2010 World Congress & Exhibition, Detroit, MI, USA, 13–15 April 2010. [Google Scholar]
- Erdem, O. The Development and Applications of Powder Metallurgy Manufacturing Methods in Automotive Industry. Int. J. Eng. Res. Dev. 2017, 9, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Schubert, T.; Weißgärber, T.; Kieback, B.; Balzer, H.; Neubing, H.-C.; Baum, U.; Braun, R. Aluminium PM ‘is a challenge that industry can overcome’. Met. Powder Rep. 2005, 60, 32–37. [Google Scholar] [CrossRef]
- Schaffer, G.; Sercombe, T.; Lumley, R. Liquid phase sintering of aluminium alloys. Mater. Chem. Phys. 2001, 67, 85–91. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Zhang, Q.; Zhao, G.; Chen, L. Influence of extrusion parameters on microstructure, texture, and second-phase particles in an Al-Mg-Si alloy. J. Mater. Process Technol. 2019, 270, 323–334. [Google Scholar] [CrossRef]
- Reiso, O. Extrusion of AlMgSi Alloys. In Proceedings of the 9th International Conference on Aluminium Alloys (ICAA9), Brisbane, Australia, 2–5 August 2004; pp. 32–46. [Google Scholar]
- Berndt, N.; Frint, P.; Wagner, M.F.-X. Influence of Extrusion Temperature on the Aging Behavior and Mechanical Properties of an AA6060 Aluminum Alloy. Metals 2018, 8, 51. [Google Scholar] [CrossRef]
- Ishikawa, T.; Sano, H.; Yoshida, Y.; Yukawa, N.; Sakamoto, J.; Tozawa, Y. Effect of Extrusion Conditions on Metal Flow and Microstructures of Aluminum Alloys. CIRP Ann. 2006, 55, 275–278. [Google Scholar] [CrossRef]
- Xu, C.; Zheng, R.; Hanada, S.; Xiao, W.; Ma, C. Effect of hot extrusion and subsequent T6 treatment on the microstructure evolution and tensile properties of an Al-6Si-2Cu-0.5Mg alloy. Mater. Sci. Eng. A 2018, 710, 102–110. [Google Scholar] [CrossRef]
- Andersen, S.J.; Zandbergen, H.W.; Jansen, J.; TrÆholt, C.; Tundal, U.; Reiso, O. The crystal structure of the β″ phase in Al-Mg-Si alloys. Acta Mater. 1998, 46, 3283–3298. [Google Scholar] [CrossRef]
- Son, S.K.; Matsumura, S.; Fukui, K.; Takeda, M. The compositions of metastable phase precipitates observed at peak hardness condition in an Al-Mg-Si alloy. J. Alloys Compd. 2011, 509, 241–245. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Abdullah, H.A.; Al-Belushi, K.R. Influence of aging parameters on the mechanical properties of 6063 aluminium alloy. J. Mater. Process. Technol. 2000, 102, 234–240. [Google Scholar] [CrossRef]
- Cuniberti, A.; Tolley, A.; Riglos, M.V.C.; Giovachini, R. Influence of natural aging on the precipitation hardening of an AlMgSi alloy. Mater. Sci. Eng. A 2010, 527, 5307–5311. [Google Scholar] [CrossRef]
- Cai, M.; Cheng, G.J. Microstructure-properties relationship in two Al-Mg-Si alloys through a combination of extrusion and aging. JOM 2007, 59, 58–61. [Google Scholar] [CrossRef]
- Gupta, A.K.; Lloyd, D.J.; Court, S.A. Precipitation hardening in Al-Mg-Si alloys with and without excess Si. Mater. Sci. Eng. A 2001, 316, 11–17. [Google Scholar] [CrossRef]
- Ding, L.; Jia, Z.; Zhang, Z.; Sanders, R.E.; Liu, Q.; Yang, G. The natural aging and precipitation hardening behaviour of Al-Mg-Si-Cu alloys with different Mg/Si ratios and Cu additions. Mater. Sci. Eng. A 2015, 627, 119–126. [Google Scholar] [CrossRef]
- Huang, J.C.; Shin, C.S.; Chan, S.L.I. Effect of temper, specimen orientation and test temperature on tensile and fatigue properties of wrought and PM AA6061-alloys. Int. J. Fatigue 2004, 26, 691–703. [Google Scholar] [CrossRef]
- Durmuş, H.K.; Meric, C. Age-hardening behavior of powder metallurgy AA2014 alloy. Mater. Des. 2007, 28, 982–986. [Google Scholar] [CrossRef]
- Xiang, S.; Matsuki, K.; Takatsuji, N.; Yokote, T.; Kusui, J.; Yokoe, K. An investigation of the age hardening behavior of PM 2024Al-Fe-Ni alloys and the effect of consolidation conditions. J. Mater. Sci. 1999, 34, 1953–1958. [Google Scholar] [CrossRef]
- Gökçe, A.; Fehim, F.; Kurt, A.O. Effects of Mg content on aging behavior of Al4CuXMg PM Alloy. Mater. Des. 2013, 46, 524–531. [Google Scholar] [CrossRef]
- Yildirim, M.; Özyürek, D.; Gürü, M. The Effects of Precipitate Size on the Hardness and Wear Behaviors of Aged 7075 Aluminum Alloys Produced by Powder Metallurgy Route. Arab. J. Sci. Eng. 2016, 41, 4273–4281. [Google Scholar] [CrossRef]
- Cabeza, M.; Feijoo, I.; Merino, P.; Pena, G.; Pérez, M.C.; Cruz, S.; Rey, P. Effect of high energy ball milling on the morphology, microstructure and properties of nano-sized TiC particle-reinforced 6005A aluminium alloy matrix composite. Powder Technol. 2017, 321, 31–43. [Google Scholar] [CrossRef]
- Tian, W.; Li, S.; Liu, J.; Yu, M.; Du, Y. Preparation of bimodal grain size 7075 aviation aluminum alloys and their corrosion properties. Chin. J. Aeronaut. 2017, 30, 1777–1788. [Google Scholar] [CrossRef]
- Jabbari Taleghani, M.A.; Ruiz Navas, E.M.; Torralba, J.M. Microstructural and mechanical characterisation of 7075 aluminium alloy consolidated from a premixed powder by cold compaction and hot extrusion. Mater. Des. 2014, 55, 674–682. [Google Scholar] [CrossRef] [Green Version]
- Zubizarreta, C.; Giménez, S.; Martín, J.M.; Iturriza, I. Effect of the heat treatment prior to extrusion on the direct hot-extrusion of aluminium powder compacts. J. Alloys Compd. 2009, 467, 191–201. [Google Scholar] [CrossRef]
- Asgharzadeh, H.; Simchi, A.; Kim, H.S. Dynamic restoration and microstructural evolution during hot deformation of a P/M Al6063 alloy. Mater. Sci. Eng. A 2012, 542, 56–63. [Google Scholar] [CrossRef]
- Molnárová, O.; Málek, P.; Lukáč, F.; Chráska, T. Spark Plasma Sintering of a Gas Atomized Al7075 Alloy: Microstructure and Properties. Materials 2016, 9, 1004. [Google Scholar] [CrossRef]
- Blum, W.; Zhu, Q.; Merkel, R.; McQueen, H.J. Geometric dynamic recrystallization in hot torsion of Al-5Mg-0.6Mn (AA5083). Mater. Sci. Eng. A 1996, 205, 23–30. [Google Scholar] [CrossRef]
- Huang, K.; Logé, R.E. A review of dynamic recrystallization phenomena in metallic materials. Mater. Des. 2016, 111, 548–574. [Google Scholar] [CrossRef]
- Doherty, R.D.; Hughes, D.A.; Humphreys, F.J.; Jonas, J.J.; Juul Jensen, D.; Kassner, M.E.; King, W.E.; McNelley, T.R.; McQueen, H.J.; Rollett, A.D. Current issues in recrystallization: A review. Mater. Sci. Eng. A 1997, 238, 219–274. [Google Scholar] [CrossRef]
- Gourdet, S.; Montheillet, F. An experimental study of the recrystallization mechanism during hot deformation of aluminium. Mater. Sci. Eng. A 2000, 283, 274–288. [Google Scholar] [CrossRef]
- Dutta, I.; Allen, S.M. A calorimetric study of precipitation in aluminum alloy 6061. J. Mater. Sci. Lett. 1991, 10, 323–326. [Google Scholar] [CrossRef]
- Edwards, G.A.; Stiller, K.; Dunlop, G.L.; Couper, M.J. The precipitation sequence in Al-Mg-Si alloys. Acta Mater. 1998, 46, 3893–3904. [Google Scholar] [CrossRef]
- Yang, W.C.; Wang, M.P.; Zhang, R.R.; Zhang, Q.; Sheng, X.F. The diffraction patterns from β″ precipitates in 12 orientations in Al-Mg-Si alloy. Scr. Mater. 2010, 62, 705–708. [Google Scholar] [CrossRef]
- Ma, E.; Zhu, T. Towards strength–ductility synergy through the design of heterogeneous nanostructures in metals. Mater. Today 2017, 20, 323–331. [Google Scholar] [CrossRef]
- Ovid’ko, I.A.; Valiev, R.Z.; Zhu, Y.T. Review on superior strength and enhanced ductility of metallic nanomaterials. Prog. Mater. Sci. 2018, 94, 462–540. [Google Scholar] [CrossRef]
- Cheng, S.; Zhao, Y.H.; Zhu, Y.T.; Ma, E. Optimizing the strength and ductility of fine structured 2024 Al alloy by nano-precipitation. Acta Mater. 2007, 55, 5822–5832. [Google Scholar] [CrossRef]
- Tawancy, H.M.; Ul-Hamid, A.; Abbas, N.M. Practical Engineering Failure Analysis; CRC Press: New York, NY, USA, 2004. [Google Scholar]


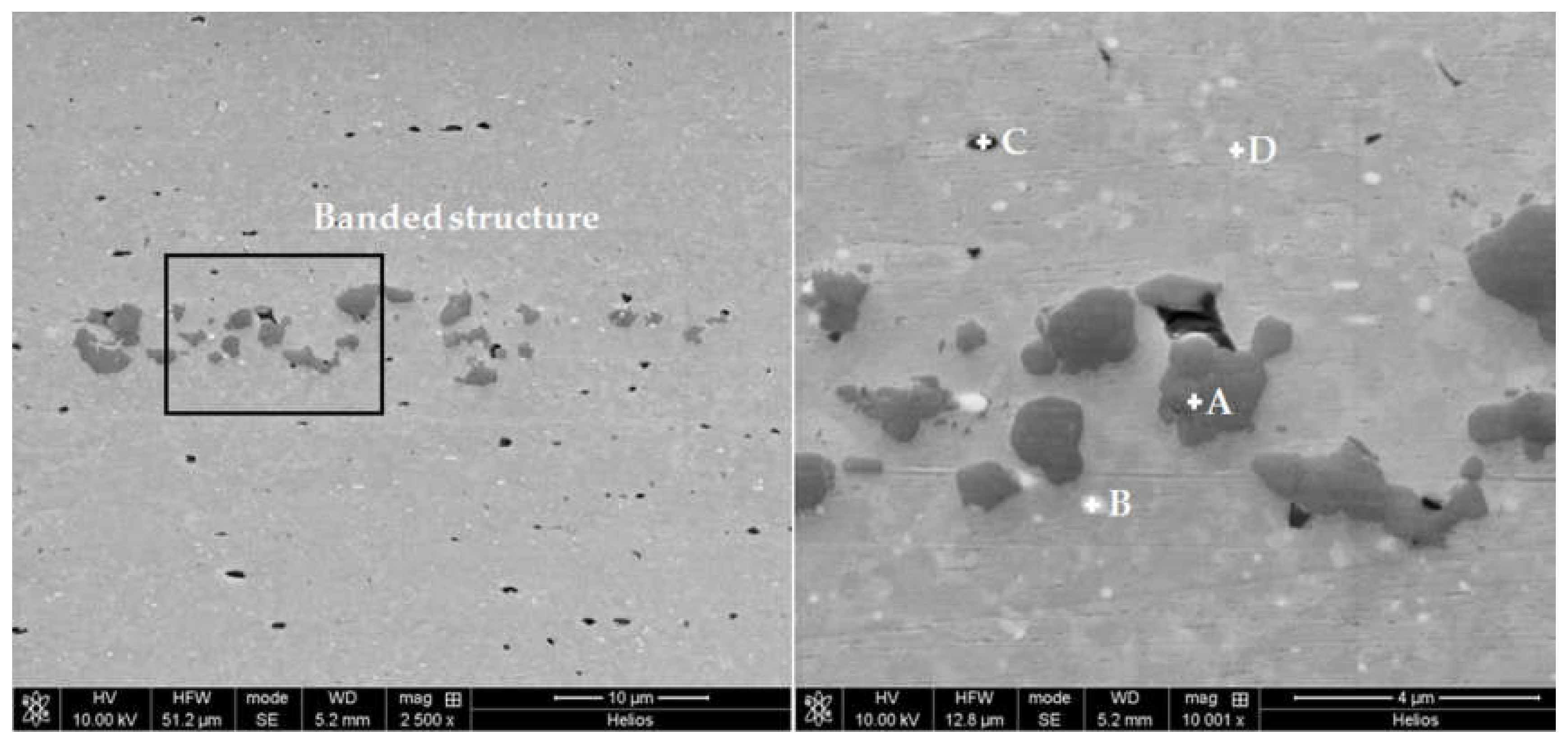
| Spectrum | Element at (%) | |||
|---|---|---|---|---|
| Al | Mg | Si | Fe | |
| A | 58.30 | 0.40 | 41.09 | 0.21 |
| B | 78.81 | 0.57 | 17.84 | 2.78 |
| C | 77.26 | 15.54 | 7.20 | - |
| D | 98.84 | 0.68 | 0.46 | 0.03 |





| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Mn + Cr | Al | Oxygen (ISO 4491/4) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.845 | 0.087 | 0.047 | 0.064 | 0.635 | 0.005 | 0.045 | 0.056 | 0.069 | Bal. | 0.118 |
| Time (h) | Temperature (°C) | ||||
|---|---|---|---|---|---|
| 170 | 175 | 180 | 185 | 190 | |
| 6 | 104 ± 2 | 103 ± 1 | 109 ± 1 | 95 ± 1 | 96 ± 1 |
| 7 | 105 ± 1 | 106 ± 3 | 106 ± 7 | 99 ± 3 | 86 ± 5 |
| 8 | 102 ± 1 | 106 ± 2 | 101 ± 1 | 99 ± 4 | 71 ± 5 |
| Alloy | Aging Treatment | Mechanical Properties | |||
|---|---|---|---|---|---|
| Time (h) | Temperature (°C) | 0, 2%YS (MPa) | UTS (MPa) | Elongation (%) | |
| AA 6005A P/M alloy | 6 | 180 | 318 ± 2 | 359 ± 3 | 21.33 ± 0.76 |
| AA 6005A P/M alloy | 8 | 175 | 313 ± 3 | 354 ± 4 | 19.33 ± 1.75 |
| AA 6005A I/M alloy | 8 | 175 | 228 ± 2 | 257 ± 1 | 14.50 ± 1.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feijoo, I.; Cabeza, M.; Merino, P.; Pena, G.; Rey, P. Age Hardening of Extruded AA 6005A Aluminium Alloy Powders. Materials 2019, 12, 2316. https://doi.org/10.3390/ma12142316
Feijoo I, Cabeza M, Merino P, Pena G, Rey P. Age Hardening of Extruded AA 6005A Aluminium Alloy Powders. Materials. 2019; 12(14):2316. https://doi.org/10.3390/ma12142316
Chicago/Turabian StyleFeijoo, Iria, Marta Cabeza, Pedro Merino, Gloria Pena, and Pilar Rey. 2019. "Age Hardening of Extruded AA 6005A Aluminium Alloy Powders" Materials 12, no. 14: 2316. https://doi.org/10.3390/ma12142316





