Adsorption of Serum Albumin onto Octacalcium Phosphate in Supersaturated Solutions Regarding Calcium Phosphate Phases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of OCP
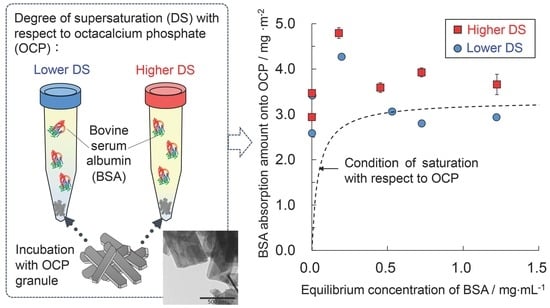
2.2. Adsorption Experiment
2.3. Measurement of Ca2+ and Pi Ion Concentrations and Calculation of DS
2.4. Characterization of OCP before and after Soaking in Supersaturated Solutions
2.5. Immersion Experiment for OCP Granules Pre-adsorbed to BSA in Supersaturated Solution
2.6. Statistical Analysis
3. Results
3.1. Measurement of BSA Adsorption Capacity onto OCP in Solutions with Different DS
3.2. Measurement of Ca2+ and Pi Ion Concentration in Buffer Solutions and Calculation of DS Values
3.3. Characterization of OCP Granules before and after Soaking in Buffer Solutions Containing BSA
3.3.1. XRD Analysis
3.3.2. Raman Spectrometry
3.3.3. TEM Observation
3.4. Estimation of the Mass Ratio of Newly Formed OCP to OCP Granules after Incubation
3.5. Mesurement of Ion Concentrations after the Incubation of OCP Granules with Pre-Adsorption of BSA in Supersaturated Solution
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, W.E. Crystal growth of bone mineral. Clin. Orthop. Relat. Res. 1966, 44, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.E.; Smith, J.P.; Frazier, A.W.; Lehr, J.R. Crystallographic and chemical relations between octacalcium phosphate and hydroxyapatite. Nature 1962, 196, 1050–1055. [Google Scholar] [CrossRef]
- Brown, W.E.; Mathew, M.; Tung, M.S. Crystal chemistry of octacalcium phosphate. Prog. Cryst. Growth Charact. 1981, 4, 59–87. [Google Scholar] [CrossRef]
- Meyer, J.L.; Eanes, E.D. Thermodynamic analysis of secondary transition in spontaneous precipitation of calcium phosphate. Calcif. Tissue Res. 1978, 25, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.C. Next generation calcium phosphate-based biomaterials. Dent. Mater. J. 2009, 28, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Leng, Y. Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials 2005, 26, 1097–1108. [Google Scholar] [CrossRef]
- Suzuki, O.; Nakamura, M.; Miyasaka, Y.; Kagayama, M.; Sakurai, M. Bone formation on synthetic precursors of hydroxyapatite. Tohoku J. Exp. Med. 1991, 164, 37–50. [Google Scholar] [CrossRef]
- Kikawa, T.; Kashimoto, O.; Imaizumi, H.; Kokubun, S.; Suzuki, O. Intramembranous bone tissue response to biodegradable octacalcium phosphate implant. Acta Biomater. 2009, 5, 1756–1766. [Google Scholar] [CrossRef]
- Imaizumi, H.; Sakurai, M.; Kashimoto, O.; Kikawa, T.; Suzuki, O. Comparative study on osteoconductivity by synthetic octacalcium phosphate and sintered hydroxyapatite in rabbit bone marrow. Calcif. Tissue Int. 2006, 78, 45–54. [Google Scholar] [CrossRef]
- Suzuki, O.; Kamakura, S.; Katagiri, T.; Nakamura, M.; Zhao, B.H.; Honda, Y.; Kamijo, R. Bone formation enhanced by implanted octacalcium phosphate involving conversion into Ca-deficient hydroxyapatite. Biomaterials 2006, 27, 2671–2681. [Google Scholar] [CrossRef]
- Anada, T.; Araseki, A.; Matsukawa, S.; Yamasaki, T.; Kamakura, S.; Suzuki, O. Effect of Octacalcium Phosphate Ionic Dissolution Products on Osteoblastic Cell Differentiation; Trans Tech Publications: Zurich, Switzerland, 2008; pp. 31–34. [Google Scholar]
- Takami, M.; Mochizuki, A.; Yamada, A.; Tachi, K.; Zhao, B.; Miyamoto, Y.; Anada, T.; Honda, Y.; Inoue, T.; Nakamura, M.; et al. Osteoclast differentiation induced by synthetic octacalcium phosphate through receptor activator of NF-κ B ligand expression in osteoblasts. Tissue Eng. Part. A 2009, 15, 3991–4000. [Google Scholar] [CrossRef]
- Lotsari, A.; Rajasekharan, A.K.; Halvarsson, M.; Andersson, M. Transformation of amorphous calcium phosphate to bone-like apatite. Nat. Commun. 2018, 9, 11. [Google Scholar] [CrossRef]
- Suzuki, O.; Kamakura, S.; Katagiri, T. Surface chemistry and biological responses to synthetic octacalcium phosphate. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77, 201–212. [Google Scholar] [CrossRef]
- Suzuki, O.; Nakamura, M.; Miyasaka, Y.; Kagayama, M.; Sakurai, M. Maclura pomifera agglutinin-binding glycoconjugates on converted apatite from synthetic octacalcium phosphate implanted into subperiosteal region of mouse calvaria. Bone Miner. 1993, 20, 151–166. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kamakura, S.; Honda, Y.; Anada, T.; Hatori, K.; Sasaki, K.; Suzuki, O. Appositional bone formation by OCP-collagen composite. J. Dent. Res. 2009, 88, 1107–1112. [Google Scholar] [CrossRef]
- Aoba, T.; Shimazu, Y.; Taya, Y.; Soeno, Y.; Sato, K.; Miake, Y. Fluoride and apatite formation in vivo and in vitro. J. Electron. Microsc. 2003, 52, 615–625. [Google Scholar] [CrossRef]
- Kerschnitzki, M.; Akiva, A.; Ben Shoham, A.; Asscher, Y.; Wagermaier, W.; Fratzl, P.; Addadi, L.; Weiner, S. Bone mineralization pathways during the rapid growth of embryonic chicken long bones. J. Struct. Biol. 2016, 195, 82–92. [Google Scholar] [CrossRef]
- Kaneko, H.; Kamiie, J.; Kawakami, H.; Anada, T.; Honda, Y.; Shiraishi, N.; Kamakura, S.; Terasaki, T.; Shimauchi, H.; Suzuki, O. Proteome analysis of rat serum proteins adsorbed onto synthetic octacalcium phosphate crystals. Anal. Biochem. 2011, 418, 276–285. [Google Scholar] [CrossRef]
- Suzuki, O.; Imaizumi, H.; Kamakura, S.; Katagiri, T. Bone regeneration by synthetic octacalcium phosphate and its role in biological mineralization. Curr. Med. Chem. 2008, 15, 305–313. [Google Scholar] [CrossRef]
- Eidelman, N.; Chow, L.C.; Brown, W.E. Calcium phosphate saturation levels in ultrafiltered serum. Calcif. Tissue Int. 1987, 40, 71–78. [Google Scholar] [CrossRef]
- Shibuya, I.; Yoshimura, K.; Miyamoto, Y.; Yamada, A.; Takami, M.; Suzawa, T.; Suzuki, D.; Ikumi, N.; Hiura, F.; Anada, T.; et al. Octacalcium phosphate suppresses chondrogenic differentiation of ATDC5 cells. Cell Tissue Res. 2013, 352, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, B.; Anada, T.; Shiwaku, Y.; Miyatake, N.; Tsuchiya, K.; Nakamura, M.; Takahashi, T.; Suzuki, O. Immune cell response and subsequent bone formation induced by implantation of octacalcium phosphate in a rat tibia defect. RSC Adv. 2016, 6, S7475–S7484. [Google Scholar] [CrossRef]
- Ea, H.K.; Uzan, B.; Rey, C.; Liote, F. Octacalcium phosphate crystals directly stimulate expression of inducible nitric oxide synthase through p38 and JNK mitogen-activated protein kinases in articular chondrocytes. Arthritis Res. Ther. 2005, 7, R915–R926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, O.; Yagishita, H.; Yamazaki, M.; Aoba, T. Adsorption of bovine serum albumin onto octacalcium phosphate and its hydrolyzates. Cells Mater. 1995, 5, 45–54. [Google Scholar]
- Aoba, T.; Moreno, E.C.; Shimoda, S. Competitive adsorption of magnesium and calcium-ions onto synthetic and biological apatites. Calcif. Tissue Int. 1992, 51, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.C.; Kresak, M.; Hay, D.I. Adsorption of molecules of biological interest onto hydroxyapatite. Calcif. Tissue Int. 1984, 36, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Myers, C.; Zaide, L.; Nalam, P.C.; Caporizzo, M.A.; Daep, C.A.; Eckmann, D.M.; Masters, J.G.; Composto, R.J. Competitive adsorption of polyelectrolytes onto and into pellicle-coated hydroxyapatite investigated by QCM-D and force spectroscopy. ACS Appl. Mater. Interfaces 2017, 9, 13079–13091. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.C.; Varughese, K.; Hay, D.I. Effect of human salivary proteins on the precipitation kinetics of calcium phosphate. Calcif. Tissue Int. 1979, 28, 7–16. [Google Scholar] [CrossRef]
- Lamkin, M.S.; Arancillo, A.A.; Oppenheim, F.G. Temporal and compositional characteristics of salivary protein adsorption to hydroxyapatite. J. Dent. Res. 1996, 75, 803–808. [Google Scholar] [CrossRef]
- Komlev, V.S.; Barinov, S.M.; Bozo, I.I.; Deev, R.V.; Eremin, I.I.; Fedotov, A.Y.; Gurin, A.N.; Khromova, N.V.; Kopnin, P.B.; Kuvshinova, E.A.; et al. Bioceramics composed of octacalcium phosphate demonstrate enhanced biological behavior. ACS Appl. Mater. Interfaces 2014, 6, 16610–16620. [Google Scholar] [CrossRef]
- Kawai, T.; Echigo, S.; Matsui, K.; Tanuma, Y.; Takahashi, T.; Suzuki, O.; Kamakura, S. First clinical application of octacalcium phosphate collagen composite in human bone defect. Tissue Eng. Part. A 2014, 20, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Handa, T.; Anada, T.; Honda, Y.; Yamazaki, H.; Kobayashi, K.; Kanda, N.; Kamakura, S.; Echigo, S.; Suzuki, O. The effect of an octacalcium phosphate co-precipitated gelatin composite on the repair of critical-sized rat calvarial defects. Acta Biomater. 2012, 8, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Anada, T.; Suzuki, K.; Saito, K.; Shiwaku, Y.; Miyatake, N.; Baba, K.; Imaizumi, H.; Hosaka, M.; Itoi, E.; et al. Effect of resorption rate and osteoconductivity of biodegradable calcium phosphate materials on the acquisition of natural bone strength in the repaired bone. J. Biomed. Mater. Res. Part. A 2016, 104, 2833–2842. [Google Scholar] [CrossRef] [PubMed]
- Medvecky, L.; Sopcak, T. Preparation and properties of octacalcium phosphate-polyhydroxybutyrate thin film composites. Mater. Lett. 2012, 68, 157–160. [Google Scholar] [CrossRef]
- Heydari, Z.; Mohebbi-Kalhori, D.; Afarani, M.S. Engineered electrospun polycaprolactone (PCL)/octacalcium phosphate (OCP) scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 127–132. [Google Scholar] [CrossRef]
- Birgani, Z.T.; Malhotra, A.; van Blitterswijk, C.A.; Habibovic, P. Human mesenchymal stromal cells response to biomimetic octacalcium phosphate containing strontium. J. Biomed. Mater. Res. Part. A 2016, 104, 1946–1960. [Google Scholar] [CrossRef] [PubMed]
- Hiromoto, S.; Inoue, M.; Taguchi, T.; Yamane, M.; Ohtsu, N. In vitro and in vivo biocompatibility and corrosion behaviour of a bioabsorbable magnesium alloy coated with octacalcium phosphate and hydroxyapatite. Acta Biomater. 2015, 11, 520–530. [Google Scholar] [CrossRef]
- Moreno, E.C.; Margolis, H.C. Composition of human plaque fluid. J. Dent. Res. 1988, 67, 1181–1189. [Google Scholar] [CrossRef]
- Aoba, T.; Moreno, E.C. The enamel fluid in the early secretory stage of porcine amelogenesis: Chemical composition and saturation with respect to enamel mineral. Calcif. Tissue Int. 1987, 41, 86–94. [Google Scholar] [CrossRef]
- Moreno, E.C.; Kresak, M.; Zahradni, R.T. Fluoridated hydroxyapatite solubility and caries formation. Nature 1974, 247, 64–65. [Google Scholar] [CrossRef]
- Tung, M.S.; Eidelman, N.; Sieck, B.; Brown, W.E. Octacalcium phosphate solubility product from 4 to 37 °C. J. Res. Natl. Bur. Stand. 1988, 93, 613–624. [Google Scholar] [CrossRef]
- Moreno, E.C.; Brown, W.E.; Osborn, G. Solubility of dicalcium phosphate dihydrate in aqueous systems I. Soil Sci. Soc. Am. J. 1960, 24, 94–98. [Google Scholar] [CrossRef]
- Fowler, B.O.; Markovic, M.; Brown, W.E. Octacalcium phosphate. 3. infrared and raman vibrational-spectra. Chem. Mater. 1993, 5, 1417–1423. [Google Scholar] [CrossRef]
- Chen, M.C.; Lord, R.C. laser-excited raman-spectroscopy of biomolecules. VIII. conformational study of bovine serum-albumin. J. Am. Chem. Soc. 1976, 98, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Anada, T.; Handa, T.; Kanda, N.; Yoshinari, M.; Takahashi, T.; Suzuki, O. Osteoconductive property of a mechanical mixture of octacalcium phosphate and amorphous calcium phosphate. ACS Appl. Mater. Interfaces 2014, 6, 22602–22611. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Anada, T.; Hamai, R.; Shiwaku, Y.; Tsuchiya, K.; Sakai, S.; Baba, K.; Sasaki, K.; Suzuki, O. Culture of hybrid spheroids composed of calcium phosphate materials and mesenchymal stem cells on an oxygen-permeable culture device to predict in vivo bone forming capability. Acta Biomater. 2019, 88, 477–490. [Google Scholar] [CrossRef]
- Yokoi, T.; Kim, I.Y.; Ohtsuki, C. Mineralization of calcium phosphate on octacalcium phosphate in a solution mimicking in vivo conditions. Phosphorus Res. Bull. 2012, 26, 71–76. [Google Scholar] [CrossRef]
- Ito, N.; Kamitakahara, M.; Yoshimura, M.; Ioku, K. Importance of nucleation in transformation of octacalcium phosphate to hydroxyapatite. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 121–126. [Google Scholar] [CrossRef]
- Sai, Y.; Shiwaku, Y.; Anada, T.; Tsuchiya, K.; Takahashi, T.; Suzuki, O. Capacity of octacalcium phosphate to promote osteoblastic differentiation toward osteocytes in vitro. Acta Biomater. 2018, 69, 362–371. [Google Scholar] [CrossRef]
- Suzuki, O.; Yagishita, H.; Amano, T.; Aoba, T. Reversible structural changes of octacalcium phosphate and labile acid phosphate. J. Dent. Res. 1995, 74, 1764–1769. [Google Scholar] [CrossRef]
- Aoba, T.; Moreno, E.C. Adsorption of phosphoserine onto hydroxyapatite and its inhibitory activity on crystal-growth. J. Colloid Interface Sci. 1985, 106, 110–121. [Google Scholar] [CrossRef]
- Wen, H.B.; de Wijn, J.R.; van Blitterswijk, C.A.; de Groot, K. Incorporation of bovine serum albumin in calcium phosphate coating on titanium. J. Biomed. Mater. Res. 1999, 46, 245–252. [Google Scholar] [CrossRef]
- Oyane, A.; Wang, X.P.; Sogo, Y.; Ito, A.; Tsurushima, H. Calcium phosphate composite layers for surface-mediated gene transfer. Acta Biomater. 2012, 8, 2034–2046. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Oyane, A.; Kim, H.M.; Kokubo, T.; Ito, A. Biomimetic coating of laminin-apatite composite on titanium metal and its excellent cell-adhesive properties. Adv. Mater. 2004, 16, 1071. [Google Scholar] [CrossRef]
- Moradian-Oldak, J. Amelogenins: assembly, processing and control of crystal morphology. Matrix Biol. 2001, 20, 293–305. [Google Scholar] [CrossRef]
- Ishiko-Uzuka, R.; Anada, T.; Kobayashi, K.; Kawai, T.; Tanuma, Y.; Sasaki, K.; Suzuki, O. Oriented bone regenerative capacity of octacalcium phosphate/gelatin composites obtained through two-step crystal preparation method. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2017, 105, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, S.; Anada, T.; Shiwaku, Y.; Tsuchiya, K.; Yamazaki, H.; Suzuki, O. Surface reactivity of octacalcium phosphate-derived fluoride-containing apatite in the presence of polyols and fluoride. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2018, 106, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Crenshaw, M.A.; Linde, A. Induction and inhibition of hydroxyapatite formation by rat dentin phosphoprotein invitro. Arch. Oral Biol. 1988, 33, 685–691. [Google Scholar] [CrossRef]
- Pedersen, K.O. binding of calcium to serum albumin. I. Stoichiometry and intrinsic association constant at physiological ph, ionic strength, and temperature. Scand. J. Clin. Lab. Investig. 1971, 28, 459–469. [Google Scholar] [CrossRef]
- Carr, C.W. Studies on the binding of small ions in protein solutions with the use of membrane electrodes. II. The binding of calcium ions in solutions of bovine serum albumin. Arch. Biochem. Biophys. 1953, 43, 147–156. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C.; Freche, M. In vitro crystallization of octacalcium phosphate on type I collagen: influence of serum albumin. J. Mater. Sci. Mater. Med. 1999, 10, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Combes, C.; Rey, C. Adsorption of proteins and calcium phosphate materials bioactivity. Biomaterials 2002, 23, 2817–2823. [Google Scholar] [CrossRef]
- Iijima, K.; Sakai, A.; Komori, A.; Sakamoto, Y.; Matsuno, H.; Serizawa, T.; Hashizume, M. Control of biomimetic hydroxyapatite deposition on polymer substrates using different protein adsorption abilities. Colloids Surf. B-Biointerfaces 2015, 130, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Heughebaert, J.C.; Nancollas, G.H. Kinetics of crystallization of octacalcium phosphate. J. Phys. Chem. 1984, 88, 2478–2481. [Google Scholar] [CrossRef]









| Sample | Ca2+/mM a | Pi ion/mM a | pH |
|---|---|---|---|
| Original Ca1.5P1.0 | 1.49 | 1.00 | 7.4 |
| Ca1.5P1.0 BSA0 | 1.52 | 1.00 | 7.4 |
| Ca1.5P1.0 BSA0.20 | 1.51 | 0.996 | 7.4 |
| Ca1.5P1.0 BSA 0.25 | 1.51 | 0.955 | 7.4 |
| Ca1.5P1.0 BSA0.50 | 1.48 | 0.975 | 7.4 |
| Ca1.5P1.0 BSA0.75 | 1.47 | 0.975 | 7.4 |
| Ca1.5P1.0 BSA1.0 | 1.50 | 0.991 | 7.4 |
| Ca1.5P1.0 BSA1.5 | 1.47 | 0.981 | 7.4 |
| Original Ca3.0P1.0 | 3.07 | 0.987 | 7.4 |
| Ca3.0P1.0 BSA0 | 3.05 | 0.995 | 7.4 |
| Ca3.0P1.0 BSA0.20 | 3.00 | 1.00 | 7.4 |
| Ca3.0P1.0 BSA0.25 | 3.02 | 0.965 | 7.4 |
| Ca3.0P1.0 BSA0.50 | 3.02 | 0.960 | 7.4 |
| Ca3.0P1.0 BSA0.75 | 3.07 | 0.975 | 7.4 |
| Ca3.0P1.0 BSA1.0 | 3.02 | 0.97 | 7.4 |
| Ca3.0P1.0 BSA1.5 | 3.09 | 0.95 | 7.4 |
| Sample | Ca2+/ mM a | Pi ion/ mM a | pH | DS at 37 °C (150 mM Tris-HCl) | ||
|---|---|---|---|---|---|---|
| HA | OCP | DCPD | ||||
| Original Ca1.5P1.0 | 1.49 | 1.00 | 7.4 | 1.56 × 1011 | 581 | 4.70 × 10−1 |
| Ca1.5P1.0 BSA0 | 0.503 | 0.737 | 7.4 | 3.16 × 108 | 3.45 | 1.22 × 10−1 |
| Ca1.5P1.0 BSA0.20 | 0.512 | 0.722 | 7.4 | 3.25 × 108 | 3.48 | 1.22 × 10−1 |
| Ca1.5P1.0 BSA0.25 | 0.500 | 0.705 | 7.4 | 2.73 × 108 | 2.98 | 1.16 × 10−1 |
| Ca1.5P1.0 BSA0.50 | 0.491 | 0.714 | 7.4 | 2.59 × 108 | 2.88 | 1.16 × 10−1 |
| Ca1.5P1.0 BSA0.75 | 0.511 | 0.726 | 7.4 | 3.27 × 108 | 3.51 | 1.22 × 10−1 |
| Ca1.5P1.0 BSA1.0 | 0.500 | 0.727 | 7.4 | 2.95 × 108 | 3.24 | 1.20 × 10−1 |
| Ca1.5P1.0 BSA1.5 | 0.518 | 0.747 | 7.4 | 3.80 × 108 | 4.02 | 1.27 × 10−1 |
| Original Ca3.0P1.0 | 3.07 | 0.987 | 7.4 | 4.57 × 1012 | 8.30 × 103 | 8.99 × 10−1 |
| Ca3.0P1.0 BSA0 | 1.39 | 0.285 | 7.4 | 2.86 × 109 | 11.1 | 1.28 × 10−1 |
| Ca3.0P1.0 BSA 0.20 | 1.36 | 0.257 | 7.4 | 1.89 × 109 | 7.54 | 1.13 × 10−1 |
| Ca3.0P1.0 BSA0.25 | 1.35 | 0.263 | 7.4 | 1.93 × 109 | 7.76 | 1.15 × 10−1 |
| Ca3.0P1.0 BSA0.50 | 1.38 | 0.259 | 7.4 | 2.03 × 109 | 8.00 | 1.15 × 10−1 |
| Ca3.0P1.0 BSA0.75 | 1.40 | 0.273 | 7.4 | 2.60 × 109 | 10.1 | 1.23 × 10−1 |
| Ca3.0P1.0 BSA1.0 | 1.39 | 0.267 | 7.4 | 2.32 × 109 | 9.08 | 1.20 × 10−1 |
| Ca3.0P1.0 BSA1.5 | 1.41 | 0.270 | 7.4 | 2.64 × 109 | 10.1 | 1.23 × 10−1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamai, R.; Tsuchiya, K.; Suzuki, O. Adsorption of Serum Albumin onto Octacalcium Phosphate in Supersaturated Solutions Regarding Calcium Phosphate Phases. Materials 2019, 12, 2333. https://doi.org/10.3390/ma12142333
Hamai R, Tsuchiya K, Suzuki O. Adsorption of Serum Albumin onto Octacalcium Phosphate in Supersaturated Solutions Regarding Calcium Phosphate Phases. Materials. 2019; 12(14):2333. https://doi.org/10.3390/ma12142333
Chicago/Turabian StyleHamai, Ryo, Kaori Tsuchiya, and Osamu Suzuki. 2019. "Adsorption of Serum Albumin onto Octacalcium Phosphate in Supersaturated Solutions Regarding Calcium Phosphate Phases" Materials 12, no. 14: 2333. https://doi.org/10.3390/ma12142333