Effect of Addition of Ca2+ and CO32− Ions with Temperature Control on Self-Healing of Hardened Cement Paste
Abstract
:1. Introduction
2. Materials and Methods
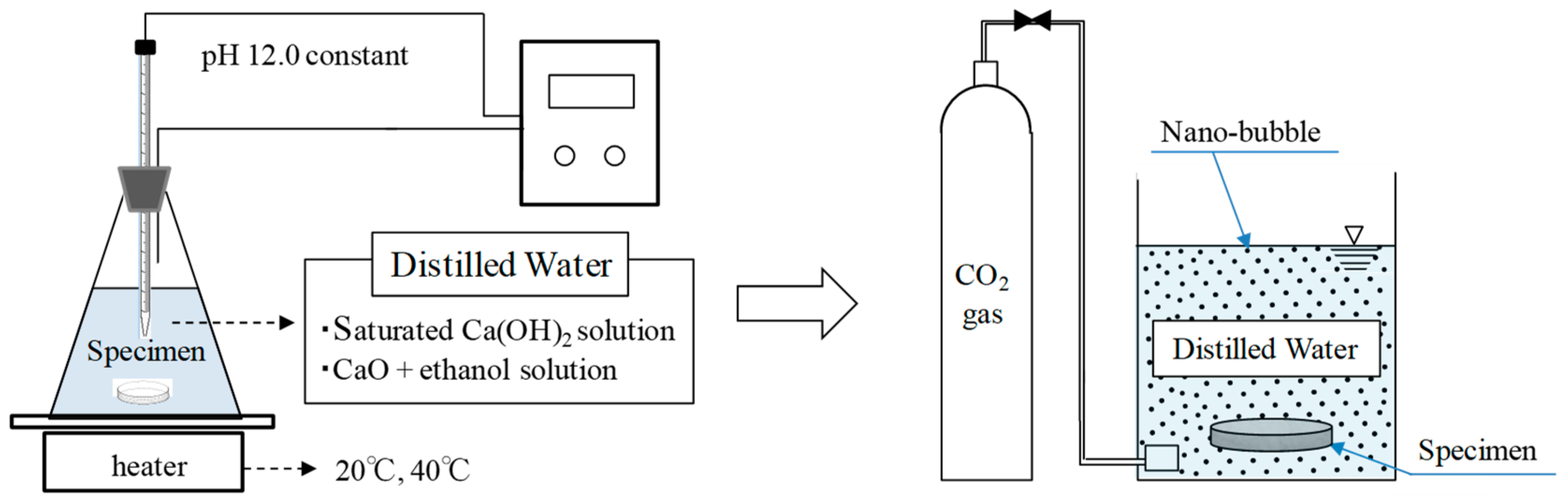
2.1. Materials and Specimen Overview
2.2. Experimental Methods
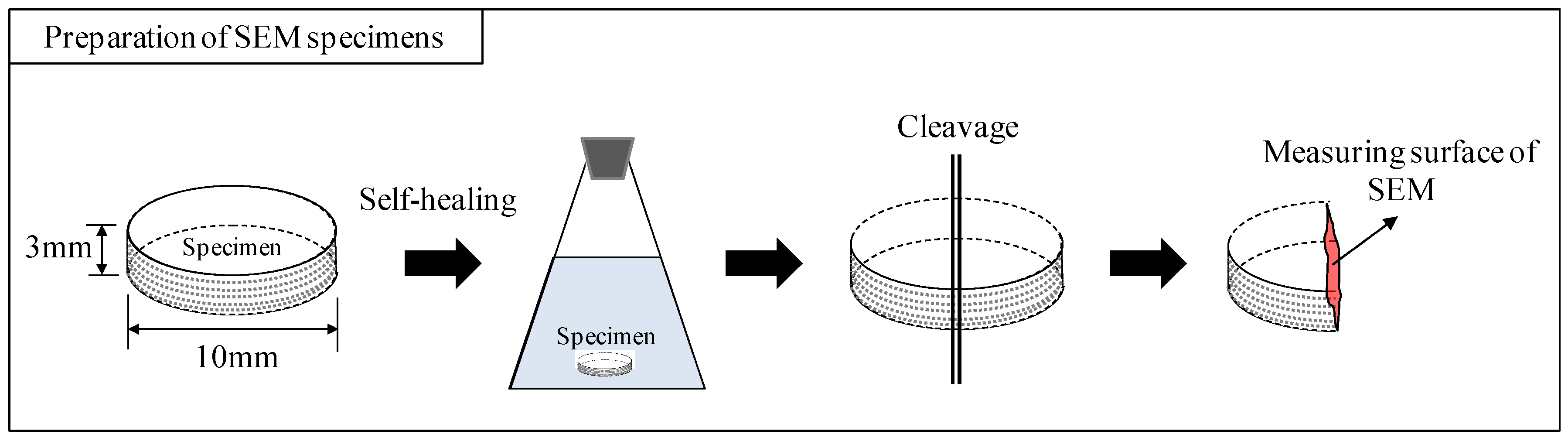
2.3. Characterization
3. Results and Discussion
3.1. Absolute Dry Weight Ratio Change by Self-Healing
3.2. Change in Porosity Reduction by Pore Filling of Self-Healing
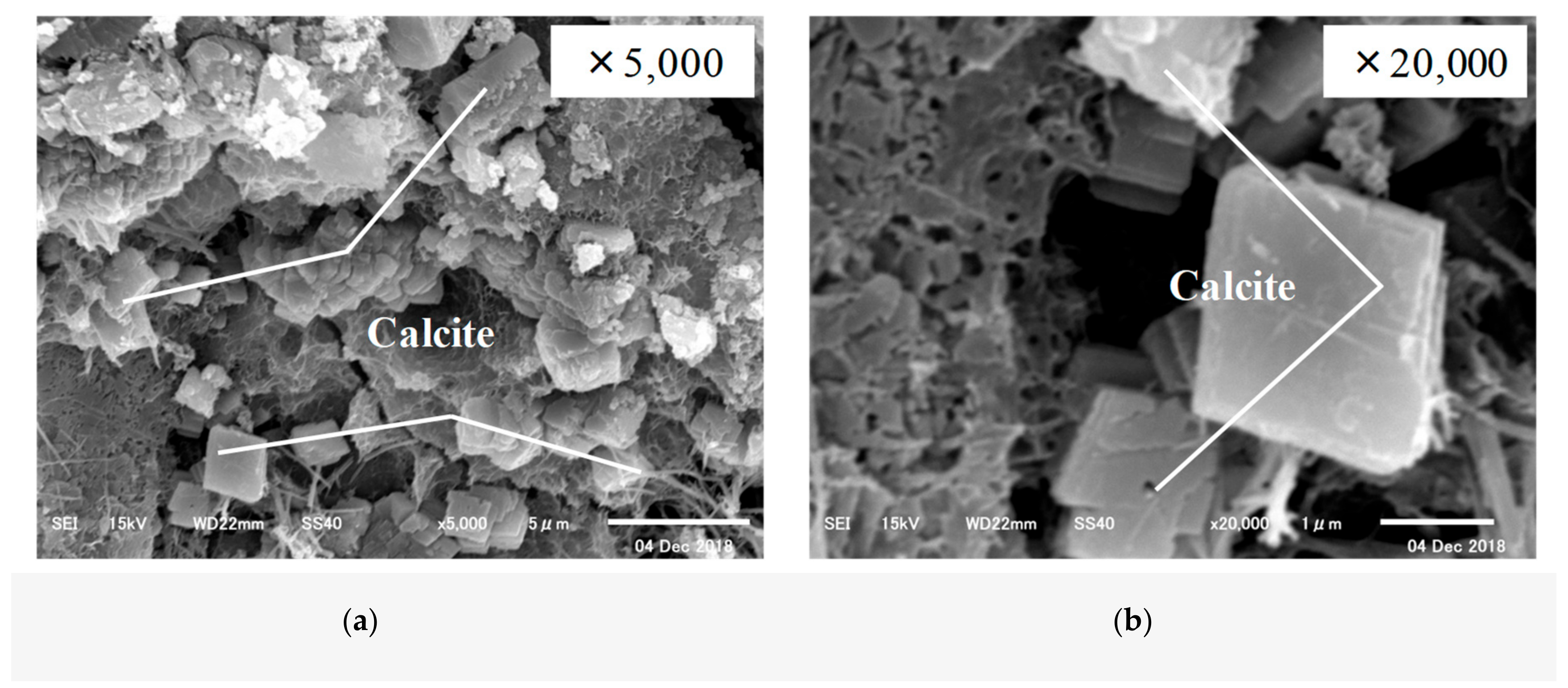
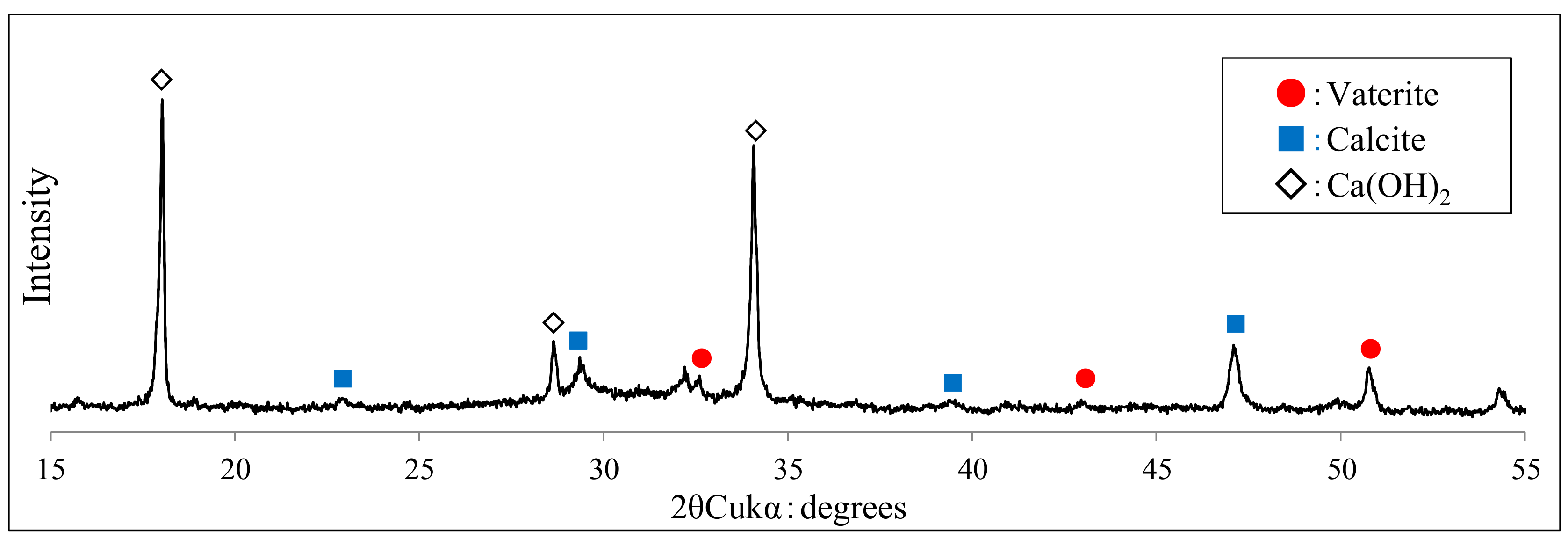
3.3. Crystallographic Change in Calcium Carbonate (CaCO3) by Scanning Electron Microscopy (SEM) and X-Ray Diffraction (XRD)
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Choi, H.S.; Inoue, M.; Kwon, S.M.; Choi, H.G.; Lim, M.K. Effective crack control of concrete by self-healing of cementitious composites using synthetic fiber. J. Mater. 2016, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S. Effect of cracking and healing on chloride transport in OPC concrete. Cem. Concr. Res. 1996, 26, 869–881. [Google Scholar] [CrossRef]
- Wang, J.; Basheer, P.A.M.; Nanukuttan, S.V.; Adrian, E.L.; Yun, B. Influence of service loading and the resulting micro-cracks on chloride resistance of concrete. Constr. Build. Mater. 2016, 108, 56–66. [Google Scholar] [Green Version]
- Wang, J.; Basheer, P.A.M.; Nanukuttan, S.V.; Yun, B. Influence of cracking caused by structural loading on chloride-induced corrosion process in reinforced concrete elements: A review. In Durability of Reinforced Concrete from Composition to Protection; Andrade, C., Gulikers, J., Polder, R., Eds.; Springer: Basel, Switzerland, 2015; pp. 99–113. [Google Scholar]
- Japan Concrete Institute. Practical Guideline For Investigation, Repair and Strengthening of Cracked Concrete Structure; Japan Concrete Institute: Tokyo, Japan, 2013. (In Japanese) [Google Scholar]
- Romildo, D.; Filho, T. Free, restrained and drying shrinkage of cement mortar composites reinforced with vegetable fibers. Cem. Concr. Compos. 2005, 27, 537–546. [Google Scholar]
- Wang, K.; Jansen, D.C.; Shah, S.P. Permeability study of cracked concrete. Cem. Concr. Res. 1997, 27, 381–393. [Google Scholar] [CrossRef] [Green Version]
- Khatri, R.P.; Sirivivatnanon, V. Role of permeability in sulfate attack. Cem. Concr. Res. 1997, 27, 1179–1189. [Google Scholar] [CrossRef]
- Neville, A.M. Properties Concrete; Longman London: Harlow, UK, 1995. [Google Scholar]
- Sanjun, M.A. Effectiveness of crack control at early age on the corrosion of steel bars in low modulus sisal and coconut fiber-reinforced mortars. Cem. Concr. Res. 1998, 28, 555–565. [Google Scholar] [CrossRef]
- Jacobsen, S. SEM Observations of the microstructure of frost deteriorated and self-healed concrete. Cem. Concr. Res. 1995, 25, 1781–1790. [Google Scholar] [CrossRef]
- Edvardsen, C. Water permeability and autogenous healing of cracks in concrete. Mater. J. 1999, 96, 448–454. [Google Scholar]
- Wang, Y.; He, F.; Wang, J.; Hu, Q. Comparison of effects of sodium bicarbonate and sodium carbonate on the hydration and properties of Portland cement paste. J. Mater. 2019, 12, 1033. [Google Scholar]
- Wang, J.; Liu, E.; Li, L. Characterization on the recycling of waste seashells with Portland cement towards sustainable cementitious materials. J. Clean. Prod. 2019, 220, 235–252. [Google Scholar]
- Nishiwaki, T.; Koda, M.; Yamada, M.; Mihashi, H.; Kikuta, T. Experimental study on self-healing capability of FRCC using different types of synthetic fibers. J. Adv. Concr. Technol. 2012, 10, 195–206. [Google Scholar] [CrossRef]
- Homma, D.; Mihashi, H.; Nishiwaki, T. Self-healing capability of fiber reinforced cementitious composites. J. Adv. Concr. Technol. 2009, 7, 217–228. [Google Scholar] [CrossRef]
- Gruyaert, E.; Van Tittelboom, K.; Sucaet, J.; Anrijs, J.; Van Vlierberghe, S.; Dubruel, P.; De Geest, B.G.; Remon, J.P.; De Belie, N. Capsules with evolving brittleness to resist the preparation of self-healing concrete. J. Mater. Const. 2016, 66, 092. [Google Scholar]
- Hilloulin, B.; Van Tittelboom, K.; Gruyaert, E.; De Belie, N.; Loukili, A. Design of polymeric capsules for self-healing concrete. Cem. Concr. Compos. 2015, 55, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Jonkers, H.M. Bacteria-based self-healing concrete. Heron 2011, 56, 1–12. [Google Scholar]
- Fukuda, D.; Nara, Y.; Kobayashi, Y.; Maruyama, M.; Koketsu, M.; Hayashi, D.; Ogawa, H.; Kaneko, K. Investigation of self-sealing in high-strength and ultra-low-permeability concrete in water using micro-focus X-ray CT. Cem. Concr. Res. 2012, 42, 1494–1500. [Google Scholar] [CrossRef] [Green Version]
- Hilloulin, B.; Legland, J.-B.; Lys, E.; Abraham, O.; Loukili, A.; Grondin, F.; Durand, O.; Tournat, V. Monitoring of autogenous crack healing in cementitious materials by the nonlinear modulation of ultrasonic coda waves, 3D microscopy and X-ray microtomography. Constr. Build. Mater. 2016, 123, 143–152. [Google Scholar] [Green Version]
- Kishi, T.; Ahn, T.-H.; Hosoda, A.; Suzuki, S.; Takaoka, H. Self-healing behavior by cementitious recrystallization of cracked concrete incorporating expansive agent. In Proceedings of the First International Conference on Self-Healing Materials, Noordwijk aan Zee, The Netherlands, 18–20 April 2007. [Google Scholar]
- Snoeck, D.; Belie, D.N. Repeated autogenous healing in strain-hardening cementitious composites by using superabsorbent polymers. J. Mater. Civ. Eng. 2015, 28, 1–11. [Google Scholar] [CrossRef]
- Snoeck, D.; Dewanckele, J.; Cnudde, V.; de Belie, N. X-ray computed microtomography to study autogenous healing of cementitious materials promoted by superabsorbent polymers. Cem. Concr. Compos. 2016, 65, 83–93. [Google Scholar] [CrossRef]
- Yang, Y.; Lepech, M.D.; Yang, E.-H.; Li, V.C. Autogenous healing of engineered cementitious composites under wet-dry cycles. Cem. Concr. Res. 2009, 39, 382–390. [Google Scholar] [CrossRef]
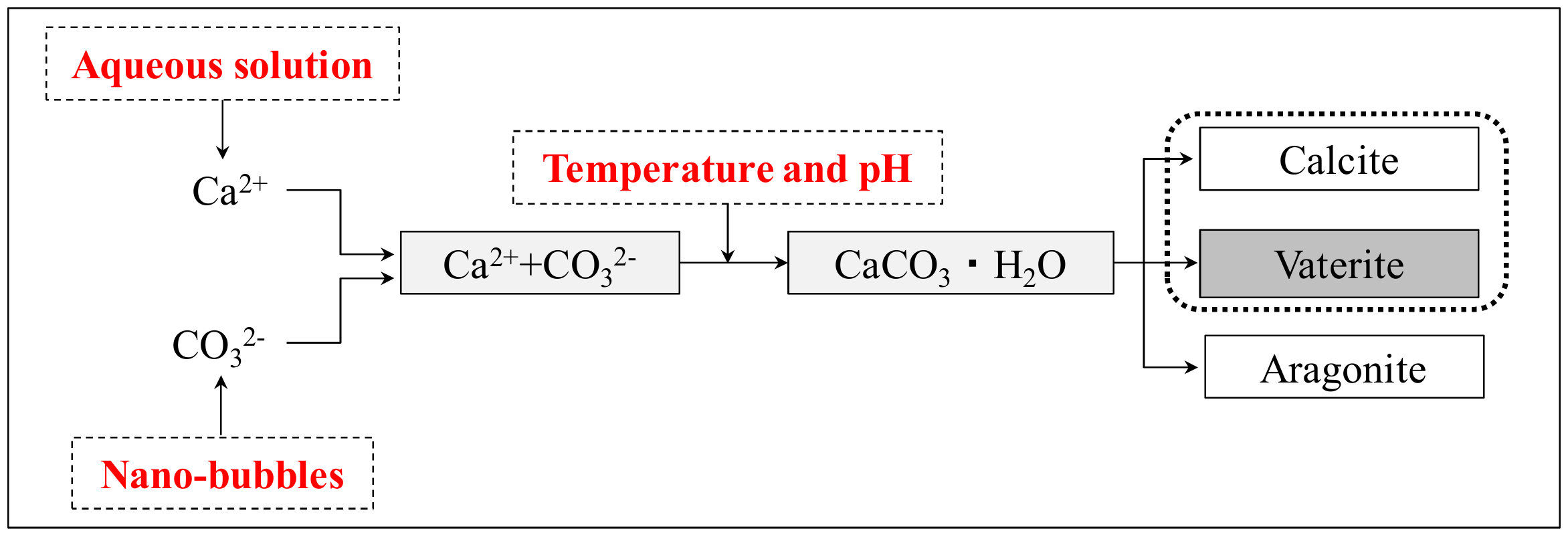
- Choi, H.-S.; Inoue, M.; Sengoku, R. Change in crystal polymorphism of CaCO3 generated in cementitious material under various pH conditions. Constr. Build. Mater. 2018, 188, 1–8. [Google Scholar] [CrossRef]
- Choi, H.-S.; Inoue, M.; Choi, H.-G.; Lim, M.-K.; Nishiwaki, T.; Kawajiri, S. The fundamental study of the crack control by self-healing of PVA fiber reinforced cementitious composites. J. Civ. Eng. Archit. Res. 2016, 9, 1680–1688. [Google Scholar]
- Choi, H.-S.; Inoue, M.; Sengoku, R.; Choi, H.-G. Control of polymorphism of calcium carbonate compounds produced in cracked part of cementitious materials by self-healing. J. Appl. Sci. 2017, 7, 1–16. [Google Scholar]
- Matsumoto, M. Polymorph control of calcium carbonate by reactive crystallization using microbubble technique. Chem. Eng. Res. Des. 2010, 88, 1624–1630. [Google Scholar] [CrossRef]
- Kojima, Y. Controls of polymorphism and morphology of calcium carbonate compounds formed by crystallizing amorphous calcium carbonate hydrate. J. Ceram. Soc. Jpn. 1994, 12, 1128–1136. (In Japanese) [Google Scholar] [CrossRef]
- Wada, N.; Takao, U. Effect of cation (Sr, Pb and Ba) on calcium carbonate polymorphs under diffusional conditions. Gypsum Lime 1993, 245, 211–219. (In Japanese) [Google Scholar]
- ASTM C150/C150M-19a. Standard Specification for Portland Cement; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Nakayama, K.; Shoji, T.; Makino, K. Influence of alcohol addiction on the specific surface area of calcium hydroxide powder prepared from quicklime -In case of the alcohol/water successive treatment. J. Assoc. Mater. Eng. Resour. Jpn. 2001, 14, 29–34. (In Japanese) [Google Scholar] [CrossRef]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Physical and Chemical Characteristics Affecting the Durability of Concrete. ACI Mater. J. 1991, 88, 186–196. [Google Scholar]
- Kim, J.-H.; Kitakaki, R.; Warita, S. A study on the effect of packing voids by carbonation reaction using CO2 nano-bubbles. J. JCI 2015, 37, 1543–1548. (In Japanese) [Google Scholar]
- Brunauer, S.; Copeland, L.-E. Chemistry of Cement of Concrete. J. Sci. Am. 1986, 210, 82–110. [Google Scholar]
- Taylor, H.-F.-W. Chemistry of Cement Hydration. In Proceedings of the 8th International Congress on the Chemistry of Cement, Rio de Janeiro, Brazil, 22–27 September 1986. [Google Scholar]
- Tai, C.Y.; Chen, C. Particle morphology, habit, and size control of using reverse microemulsion technique. J. Chem. Eng. Sci. 2008, 63, 3632–3642. [Google Scholar] [CrossRef]
- Saraya, M.E.-S.I.; Rokbaa, H.H.A.L. Preparation of Vaterite Calcium Carbonate in the Form of Spherical Nano-size Particles with the Aid of Polycarboxylate Superplasticizer as a Capping Agent. Am. J. Nanomater. 2016, 4, 44–51. [Google Scholar]









| Specimen: Hardened Cement Paste (Water/Cement Ratio: 0.4) | |||
|---|---|---|---|
| Self-Healing Condition [29,30] | Temperature: 20 °C and 40 °C (Constant pH of 12) | Ca(OH)2 + CO2 Nano-bubble (CH) CaO + Ethanol + CO2 Nano-bubble (CE) | |
| Self-healing period | Prior to self-healing | ||
| After self-healing | CH, CE (5 h) + CO2 nano-bubble (4 h) | Ⅰ | |
| CH, CE (10 h) + CO2 nano-bubble (4 h) | Ⅱ | ||
| CH, CE (15 h) + CO2 nano-bubble (4 h) | Ⅲ | ||
| CH, CE (20 h) + CO2 nano-bubble (4 h) | Ⅳ | ||
| Step | Experimental Sequence | Subject and Method of Evaluation | |
|---|---|---|---|
| Physical Property Change | Self-Healing Substances | ||
| A | Prior to self-healing | -Absolute dry weight -Absolute dry weight ratio -Porosity reduction by carbonation | -SEM -XRD |
| B | After self-healing | ||
| Temp. | Type | Increased Absolute Dry Weight (g) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | |||||||||||
| Ⅰ | Ⅱ | Ⅲ | Ⅳ | Ⅰ | Ⅱ | Ⅲ | Ⅳ | Ⅰ | Ⅱ | Ⅲ | Ⅳ | ||
| 20 °C | CH20 | 0.018 | 0.026 | 0.046 | 0.076 | 0.016 | 0.023 | 0.044 | 0.076 | 0.018 | 0.024 | 0.047 | 0.077 |
| CE20 | 0.025 | 0.060 | 0.078 | 0.090 | 0.021 | 0.058 | 0.074 | 0.088 | 0.024 | 0.059 | 0.080 | 0.089 | |
| 40 °C | CH40 | 0.024 | 0.046 | 0.078 | 0.088 | 0.025 | 0.045 | 0.080 | 0.087 | 0.026 | 0.047 | 0.077 | 0.091 |
| CE40 | 0.035 | 0.072 | 0.096 | 0.123 | 0.033 | 0.070 | 0.095 | 0.119 | 0.033 | 0.069 | 0.101 | 0.124 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Inoue, M.; Kim, D.; Choi, H.; Sengoku, R. Effect of Addition of Ca2+ and CO32− Ions with Temperature Control on Self-Healing of Hardened Cement Paste. Materials 2019, 12, 2456. https://doi.org/10.3390/ma12152456
Choi H, Inoue M, Kim D, Choi H, Sengoku R. Effect of Addition of Ca2+ and CO32− Ions with Temperature Control on Self-Healing of Hardened Cement Paste. Materials. 2019; 12(15):2456. https://doi.org/10.3390/ma12152456
Chicago/Turabian StyleChoi, Heesup, Masumi Inoue, Dongmin Kim, Hyeonggil Choi, and Risa Sengoku. 2019. "Effect of Addition of Ca2+ and CO32− Ions with Temperature Control on Self-Healing of Hardened Cement Paste" Materials 12, no. 15: 2456. https://doi.org/10.3390/ma12152456





