Influence of Carbon Nanoparticle Addition (and Impurities) on Selective Laser Melting of Pure Copper
Abstract
:1. Introduction
2. Materials and Methods
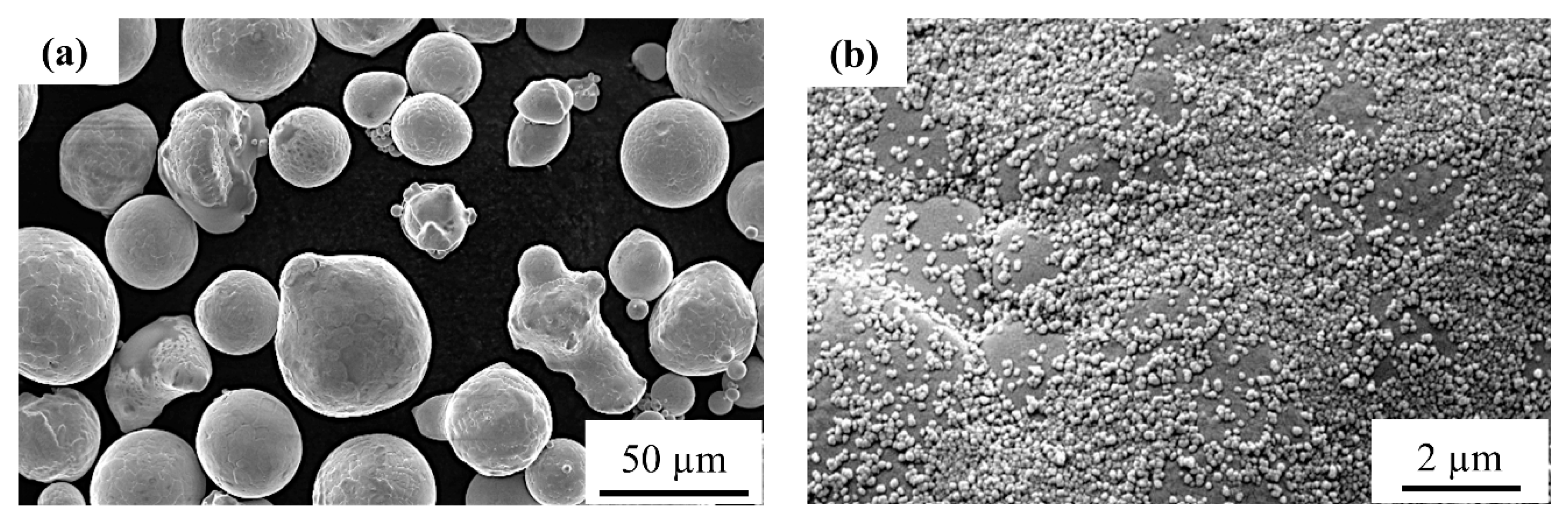
2.1. Starting Powders and Their Characteristics
2.2. Selective Laser Melting
2.3. Microstructural Characterization
2.4. Electrical Conductivity and Mechanical Property Characterization
3. Results
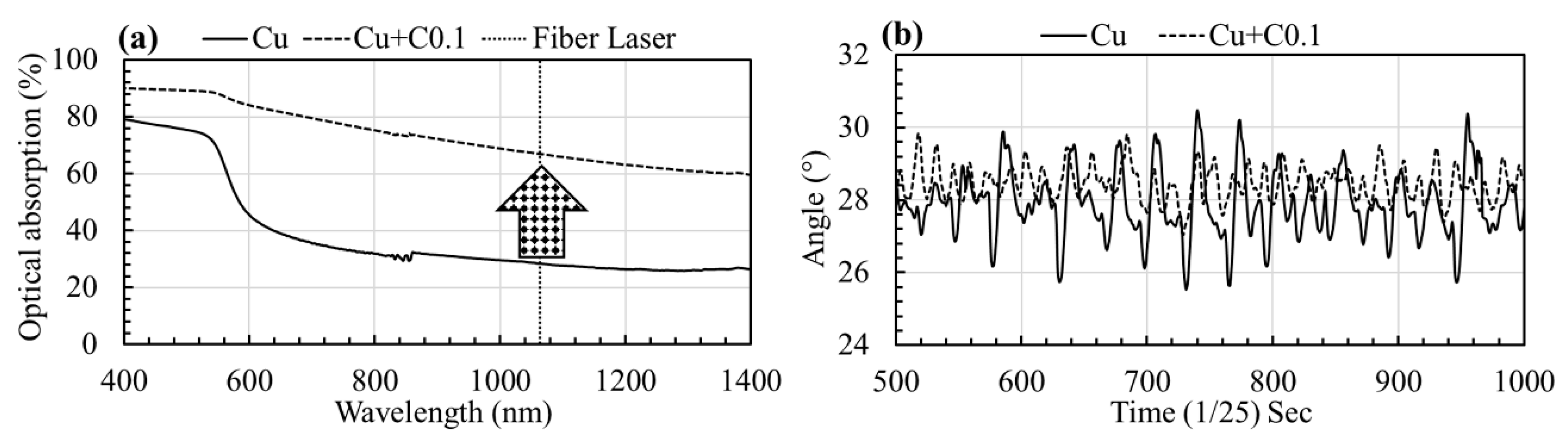
3.1. Influence of Carbon Nanoparticle Addition on the Optical Absorption and Flowability of Copper Powder
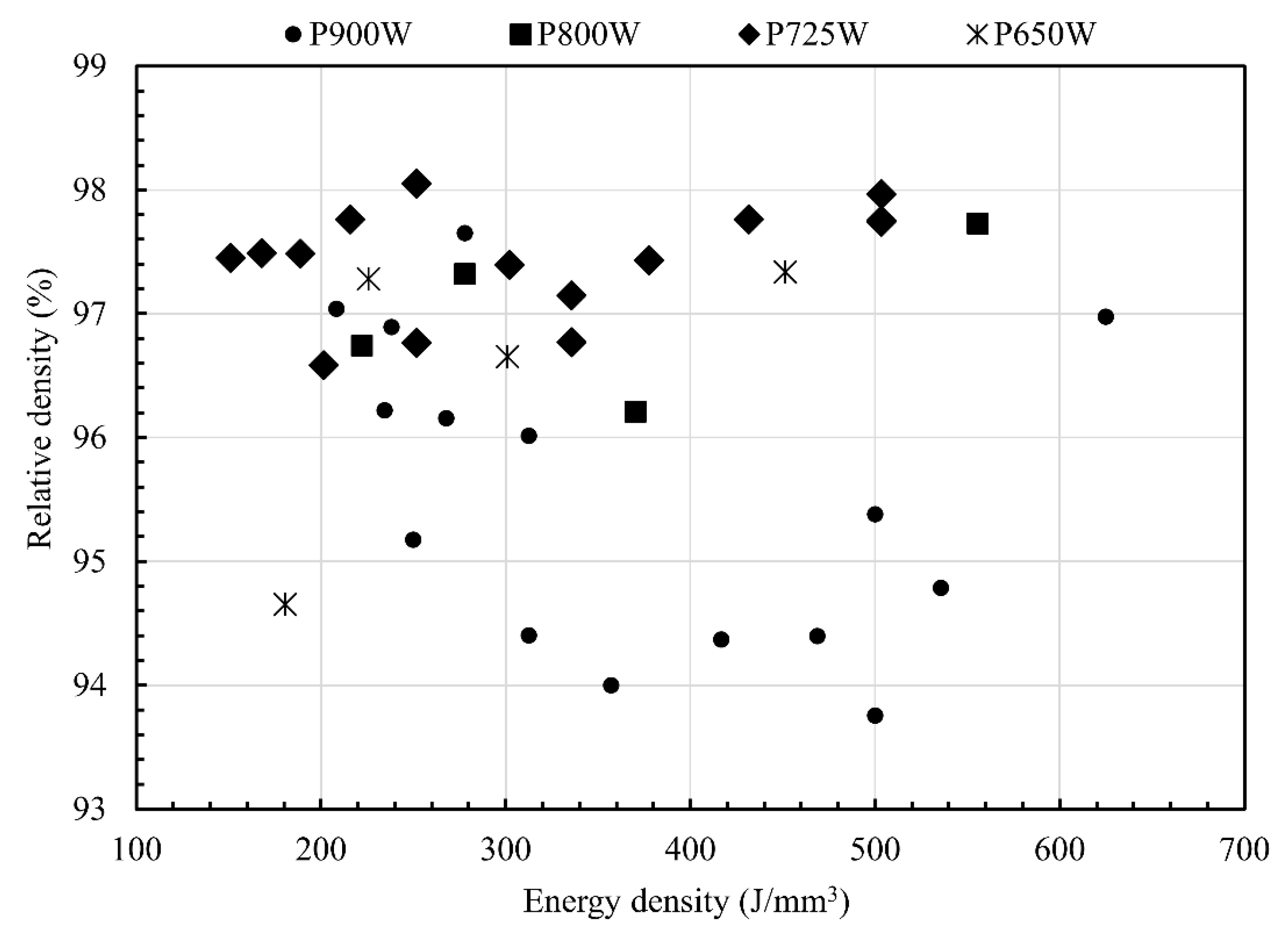
3.2. SLM Parameter Optimization and Carbon and Oxygen Content Evolution
3.3. Microstructural and EDS Analysis
3.3.1. Microstructural Analysis of As-Built (Unpolished) and Polished Top Surfaces
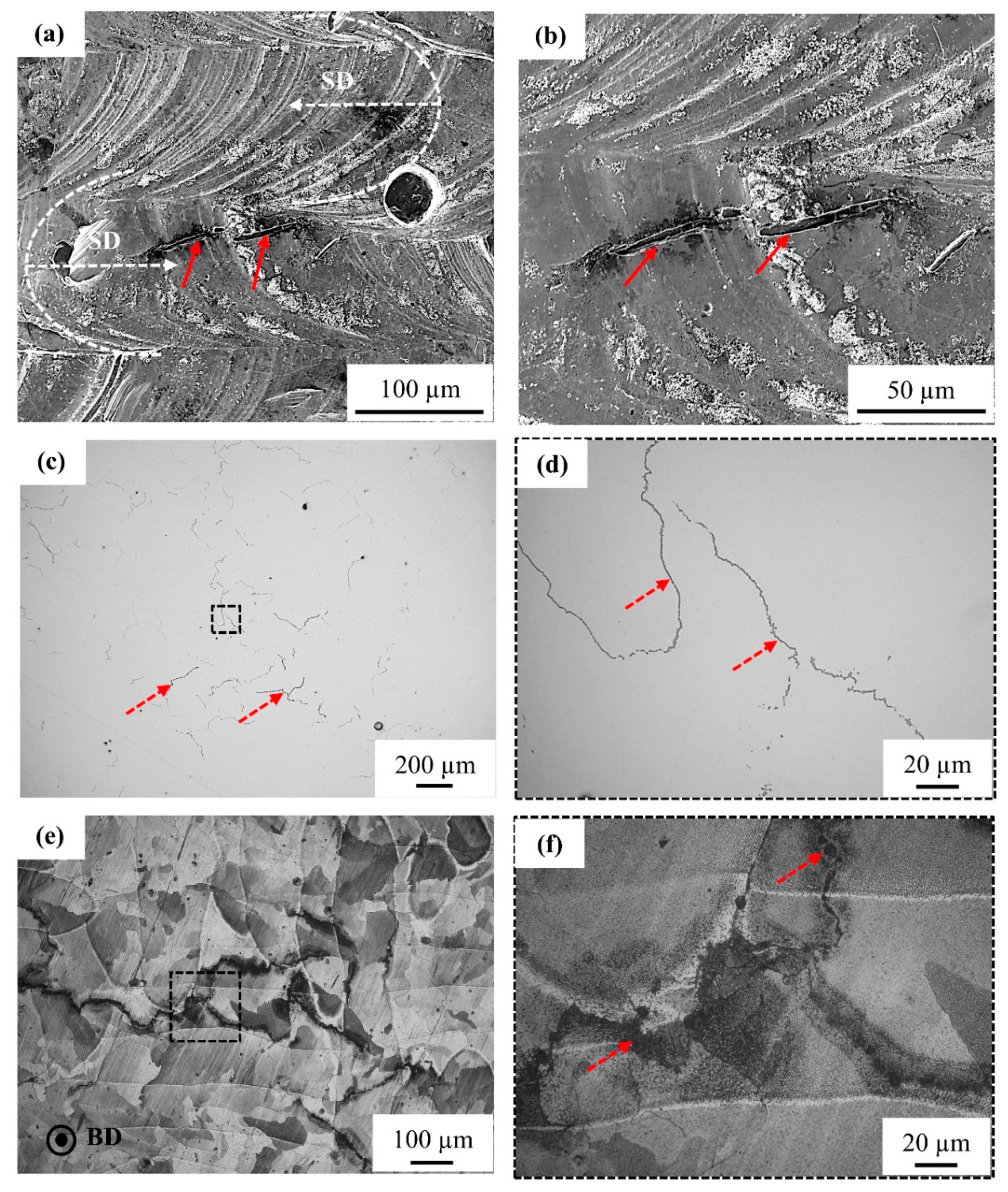
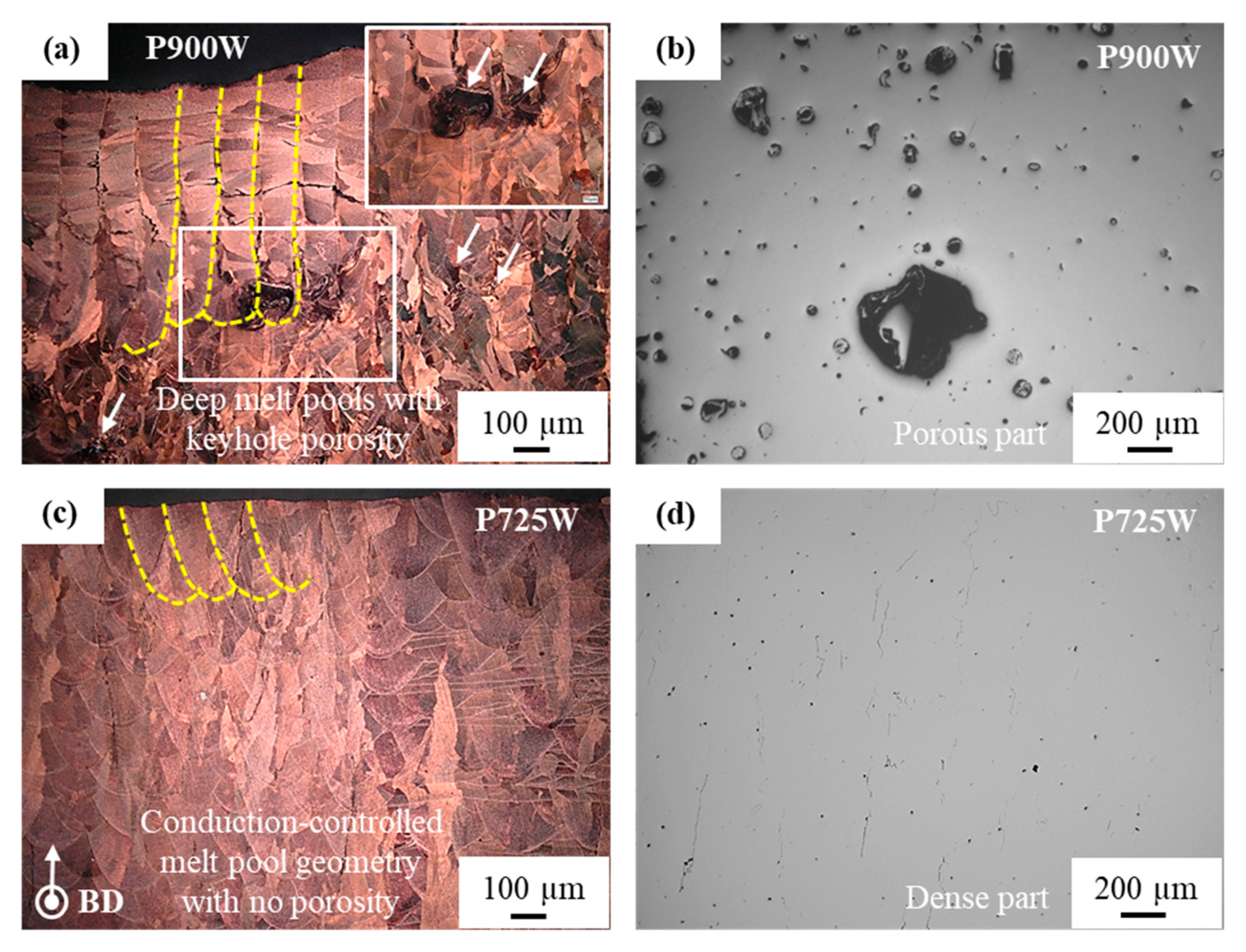
3.3.2. Microstructural Analysis of Cross-Section Plane Parallel to Building Direction
3.4. Mechanical and Electrical Properties
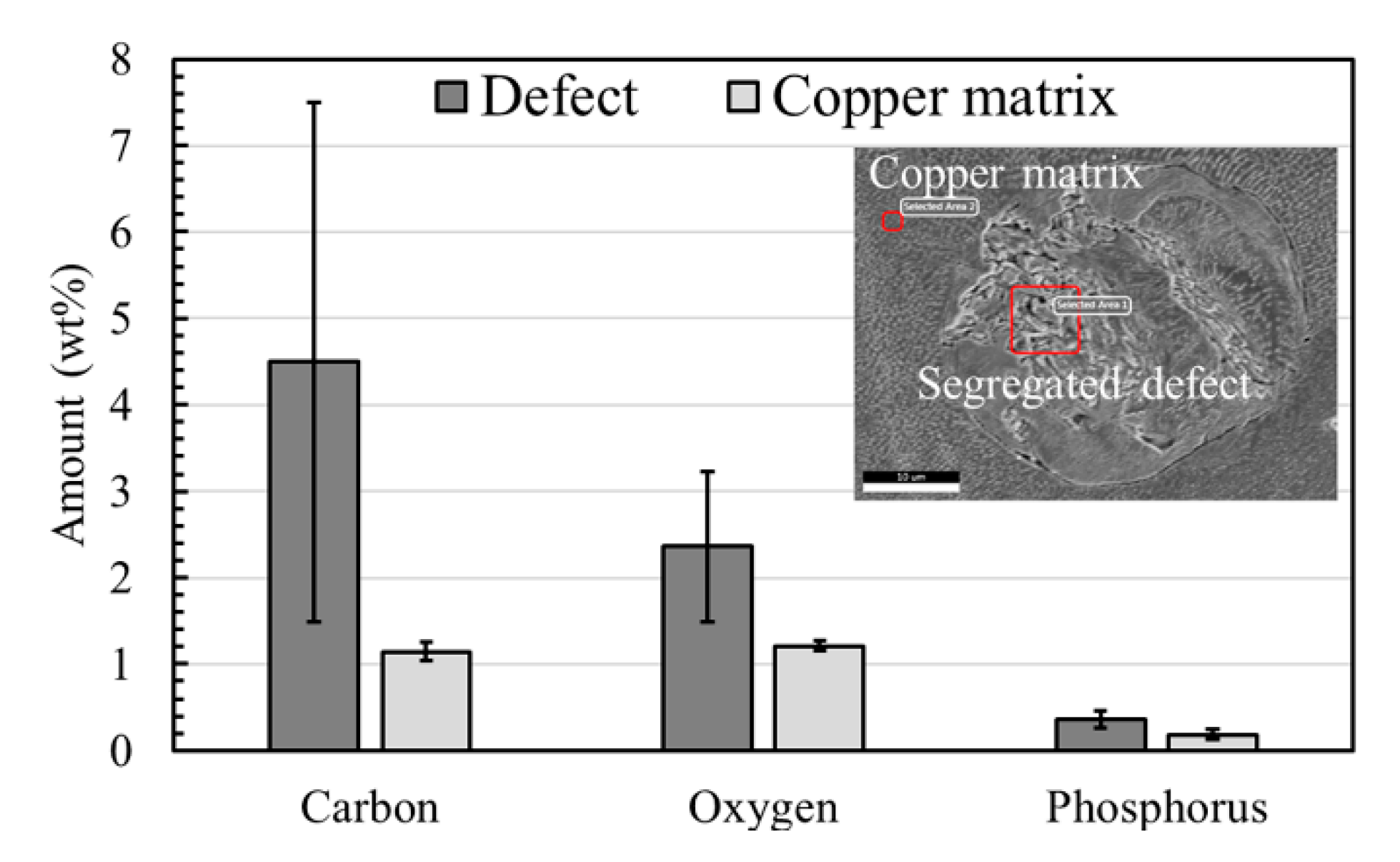
EDS Analysis of the Segregation Defects
3.5. Fracture Analysis of the Tensile Bars
4. Discussion
4.1. Influence of Carbon Nanoparticle Addition on Powder Characteristics and SLM Processability
4.2. Segregation Defects and Their Effect on Mechanical and Electrical Properties
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raab, S.J.; Guschlbauer, R.; Lodes, M.A.; Körner, C. Thermal and Electrical Conductivity of 99.9% Pure Copper Processed via Selective Electron Beam Melting. Adv. Eng. Mater. 2016, 18, 1661–1666. [Google Scholar] [CrossRef]
- Aurubis. Facts & Figures Aurubis Heat Exchanger Material. In Heat Exchanger Material; Aurubis: Hamburg, Germany, 2013; pp. 1–12. [Google Scholar]
- Wu, T.; Lee, P.S.; Mathew, J.; Sun, C.; Aw, B.L. Pool Boiling Heat Transfer Enhancement with Porous Fin Arrays Manufactured by Selective Laser Melting. In Proceedings of the 2019 18th IEEE Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (ITherm), Las Vegas, NV, USA, 29–31 May 2019; pp. 1107–1114. [Google Scholar]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Neugebauer, R.; Müller, B.; Gebauer, M.; Töppel, T. Additive manufacturing boosts efficiency of heat transfer components. Assem. Autom. 2011, 31, 344–347. [Google Scholar] [CrossRef]
- Lykov, P.A.; Safonov, E.V.; Akhmedianov, A.M. Selective Laser Melting of Copper. Mater. Sci. Forum 2016, 843, 284–288. [Google Scholar] [CrossRef]
- Trevisan, F.; Flaviana, C.; Diego, M.; Paolo, F. Selective laser melting of chemical pure copper powders. In Proceedings of the Euro PM2017 Congress & Exhibition, Milan, Italy, 1–5 October 2017; pp. 1–6. [Google Scholar]
- Jadhav, S.D.; Dadbakhsh, S.; Goossens, L.; Kruth, J.P.; Van Humbeeck, J.; Vanmeensel, K. Influence of selective laser melting process parameters on texture evolution in pure copper. J. Mater. Process. Technol. 2019, 270, 47–58. [Google Scholar] [CrossRef]
- Tran, T.Q.; Chinnappan, A.; Lee, J.K.Y.; Loc, N.H.; Tran, L.T.; Wang, G.; Kumar, V.V.; Jayathilaka, W.A.D.M.; Ji, D.; Doddamani, M.; et al. 3D Printing of Highly Pure Copper. Metals 2019, 9, 756. [Google Scholar] [CrossRef]
- Colopi, M.; Caprio, L.; Demir, A.G.; Previtali, B. Selective laser melting of pure Cu with a 1 kW single mode fiber laser. Procedia CIRP 2018, 74, 59–63. [Google Scholar] [CrossRef]
- Ikeshoji, T.T.; Nakamura, K.; Yonehara, M.; Imai, K.; Kyogoku, H. Selective Laser Melting of Pure Copper. JOM 2018, 70, 396–400. [Google Scholar] [CrossRef]
- Colopi, M.; Demir, A.G.; Caprio, L.; Previtali, B. Limits and solutions in processing pure Cu via selective laser melting using a high-power single-mode fiber laser. Int. J. Adv. Manuf. Technol. 2019, 1–14. [Google Scholar] [CrossRef]
- Heussen, D.; Meiners, W. Green Light for New 3D Printing Process. 2017. Available online: https://www.ilt.fraunhofer.de/en/press/press-releases/press-release-2017/press-release-2017-08-30.html (accessed on 30 July 2017).
- Hönl, R. World Premiere at Formnext: Green Laser from TRUMPF Prints Copper and Gold. 2018. Available online: https://www.trumpf.com/en_CN/company/press/global-press-releases/press-release-detail-page/release/world-premiere-at-formnext-green-laser-from-trumpf-prints-copper-and-gold/ (accessed on 21 January 2019).
- Lang, M. Formalloy Making New Wave(length)s in 3D Printing, Repair and Cladding. 2017. Available online: https://additivemanufacturing.com/2017/09/12/formalloy-making-new-wavelengths-in-3d-printing-repair-and-cladding/ (accessed on 15 April 2019).
- Ehrenreich, H.; Philipp, H.R. Optical properties of Ag and Cu. Phys. Rev. 1962, 128, 1622–1629. [Google Scholar] [CrossRef]
- Uchida, S.; Kimura, T.; Nakamoto, T.; Ozaki, T.; Miki, T.; Takemura, M.; Oka, Y.; Tsubota, R. Characteristics of Cu-Cr Alloys Fabricated Using Selective Laser Melting. In Proceedings of the Euro PM2018 Congress & Exhibition, Bilbao, Spain, 14–18 October 2018; pp. 1–6. [Google Scholar]
- Tsubota, R.; Oka, Y.; Okamoto, A.; Nakamoto, T.; Sugahara, T.; Shinomia, N.; Takemura, M.; Uchida, S. Copper Alloy Powder, Method of Producing Additively-Manufactured Article, and Additvely-Manufactured Article. U.S. Patent EP-3315229-A1, 2 May 2018. [Google Scholar]
- Zhang, S.; Zhu, H.; Zhang, L.; Zhang, W.; Yang, H.; Zeng, X. Microstructure and properties of high strength and high conductivity Cu-Cr alloy components fabricated by high power selective laser melting. Mater. Lett. 2019, 237, 306–309. [Google Scholar] [CrossRef]
- Wallis, C.; Buchmayr, B. Effect of heat treatments on microstructure and properties of CuCrZr produced by laser-powder bed fusion. Mater. Sci. Eng. A 2019, 744, 215–223. [Google Scholar] [CrossRef]
- Buchmayr, B.; Panzl, G.; Walzl, A.; Wallis, C. Laser Powder Bed Fusion-Materials Issues and Optimized Processing Parameters for Tool steels, AlSiMg- and CuCrZr-Alloys. Adv. Eng. Mater. 2017, 19, 1600667. [Google Scholar] [CrossRef]
- Popovich, A.; Sufiiarov, V.; Polozov, I.; Borisov, E.; Masaylo, D.; Orlov, A. Microstructure and mechanical properties of additive manufactured copper alloy. Mater. Lett. 2016, 179, 38–41. [Google Scholar] [CrossRef]
- Sabelle, M.; Walczak, M.; Ramos-Grez, J. Scanning pattern angle effect on the resulting properties of selective laser sintered monolayers of Cu-Sn-Ni powder. Opt. Lasers Eng. 2018, 100, 1–8. [Google Scholar] [CrossRef]
- Ventura, A.P.; Wade, C.A.; Pawlikowski, G.; Bayes, M.; Watanabe, M.; Misiolek, W.Z. Mechanical Properties and Microstructural Characterization of Cu-4.3 Pct Sn Fabricated by Selective Laser Melting. Metall. Mater. Trans. A 2017, 48, 178–187. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, H.; Hu, Z.; Zeng, X.; Zhong, F. Selective Laser Melting of Cu 10Zn alloy powder using high laser power. Powder Technol. 2019, 342, 613–620. [Google Scholar] [CrossRef]
- Jadhav, S.D.; Vleugels, J.; Kruth, J.; Van Humbeeck, J.; Vanmeensel, K. Mechanical and electrical properties of selective laser melted parts produced from surface oxidized copper powder. Mater. Des. Process. Commun. 2019, e94. [Google Scholar] [CrossRef]
- Sharon, J.A.; She, Y.; Ei-Wardany, T.I.; Schmidt, W.R. Particulates for Additive Manufacturing Techniques. U.S. Patent US/2017/0044416 A1, 16 February 2017. [Google Scholar]
- Van den Eynde, M.; Strobbe, D.; Verkinderen, O.; Verbelen, L.; Goderis, B.; Kruth, J.P.; Van Puyvelde, P. Effect of thermal treatments on the laser sinterability of cryogenically milled polybutene-1. Mater. Des. 2018, 153, 15–23. [Google Scholar] [CrossRef]
- Clijsters, S.; Craeghs, T.; Buls, S.; Kempen, K.; Kruth, J.P. In situ quality control of the selective laser melting process using a high-speed, real-time melt pool monitoring system. Int. J. Adv. Manuf. Technol. 2014, 75, 1089–1101. [Google Scholar] [CrossRef]
- Kruth, J.P.; Dadbakhsh, S.; Vrancken, B.; Kempen, K.; Vleugels, J.; Van Humbeeck, J. Additive Manufacturing of Metals via Selective Laser Melting: Process Aspects and Material Developments. In Additive Manufacturing: Innovations, Advances, and Applications; Srivatsan, T., Sudarshan, T., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 69–99. ISBN 978-1-4987-1477-8. [Google Scholar]
- Buls, S. A Smart Machine for Selective Laser Melting, KU Leuven. 2018. Available online: https://limo.libis.be/primo-explore/fulldisplay?docid=LIRIAS1748859&context=L&vid=Lirias&search_scope=Lirias&tab=default_tab&lang=en_US&fromSitemap=1 (accessed on 20 May 2019).
- Aurubis Technical Data Sheet-C10200 (Cu-OF). 2018. Available online: https://www.aurubis.com/binaries/content/assets/aurubisrelaunch/files/datenblatter/cu-of/c10200-cu-of-us.pdf (accessed on 10 July 2018).
- William, J.; March, N.H. Theoretical Solid State Physics; Courier Dover Publications: Mineola, NY, USA, 1985; ISBN 0-486-65016-2. [Google Scholar]
- Jallo, L.J.; Schoenitz, M.; Dreizin, E.L.; Dave, R.N.; Johnson, C.E. The effect of surface modification of aluminum powder on its flowability, combustion and reactivity. Powder Technol. 2010, 204, 63–70. [Google Scholar] [CrossRef]
- Castellanos, A. The relationship between attractive interparticle forces and bulk behaviour in dry and uncharged fine powders. Adv. Phys. 2005, 54, 263–376. [Google Scholar] [CrossRef]
- Jafari, D.; Wits, W.W. The utilization of selective laser melting technology on heat transfer devices for thermal energy conversion applications: A review. Renew. Sustain. Energy Rev. 2018, 91, 420–442. [Google Scholar] [CrossRef]
- Moratalla Martinez, P.; Rodriguez Lara, J.C.; Cases Sanchis, C.; Magraner Caceres, J.M.; Mezquida Gisbert, M. Magnetic Inductor and Production Method. U.S. Patent WO/2016/079360, 26 May 2016. [Google Scholar]
- Matthews, M.J.; Guss, G.; Khairallah, S.A.; Rubenchik, A.M.; Depond, P.J.; King, W.E. Denudation of metal powder layers in laser powder bed fusion processes. Acta Mater. 2016, 114, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Whittaker, A.G. The controversial carbon solid–liquid–vapour triple point. Nature 1978, 276, 695–696. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components–Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Dai, D.; Gu, D. Thermal behavior and densification mechanism during selective laser melting of copper matrix composites: Simulation and experiments. Mater. Des. 2014, 55, 482–491. [Google Scholar] [CrossRef]
- AlMangour, B.; Grzesiak, D.; Borkar, T.; Yang, J.M. Densification behavior, microstructural evolution, and mechanical properties of TiC/316L stainless steel nanocomposites fabricated by selective laser melting. Mater. Des. 2018, 138, 119–128. [Google Scholar] [CrossRef]
- AlMangour, B.; Grzesiak, D.; Yang, J.M. In-situ formation of novel TiC-particle-reinforced 316L stainless steel bulk-form composites by selective laser melting. J. Alloy. Compd. 2017, 706, 409–418. [Google Scholar] [CrossRef]
- McLellan, R.B. The solubility of carbon in solid gold, copper, and silver. Scr. Metall. 1969, 3, 389–391. [Google Scholar] [CrossRef]
- Silvain, J.F.; Coupard, D.; Le Petitcorps, Y.; Lahaye, M.; Onillon, M.; Goni, X. Interface characterisation and wettability properties of carbon particle reinforced copper alloy. J. Mater. Chem. 2000, 10, 2213–2218. [Google Scholar] [CrossRef]
- San Francisco Maritime National Park Association Foundry Manual-Part 4. 2018. Available online: https://maritime.org/doc/foundry/part4.htm (accessed on 16 February 2019).
- Caron, R.N. Copper: Alloying. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–9. ISBN 9780128035818. [Google Scholar]
- Zhang, S.; Zhu, H.; Zhang, L.; Zhang, W.; Yang, H.; Zeng, X. Microstructure and properties in QCr0.8 alloy produced by selective laser melting with different heat treatment. J. Alloy. Compd. 2019, 800, 286–293. [Google Scholar] [CrossRef]


| Element | Amount (wt %) |
|---|---|
| Phosphorus | 0.17 |
| Tin | 0.06 |
| Oxygen | 0.0925 |
| Carbon | 0.1 |
| Copper | Balance |
| Element | Virgin Powder (Before SLM) | Powder (After SLM) | SLM Part |
|---|---|---|---|
| Carbon (wt %) | 0.1 | 0.088 | 0.055 |
| Oxygen (wt %) | 0.0925 | 0.0515 | 0.0146 |
| Property/Alloy | P (wt %) | O (wt %) | Hardness (GPa) | E (GPa) | Rp0.2 (MPa) | σ (MPa) | ε (%) | κ (S/m) |
|---|---|---|---|---|---|---|---|---|
| Cu + C0.1 (AB) | 0.17 | 0.0146 | 0.637 ± 0.02 | 105 ± 2 | 64 ± 7 | 125 ± 11 | 3 ± 1 | 22.7 × 106 |
| Reference Cu-OF [32] | - | 0.001 | - | 117 | 69 | 179–262 | 35 | 58 × 106 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadhav, S.D.; Dadbakhsh, S.; Vleugels, J.; Hofkens, J.; Van Puyvelde, P.; Yang, S.; Kruth, J.-P.; Van Humbeeck, J.; Vanmeensel, K. Influence of Carbon Nanoparticle Addition (and Impurities) on Selective Laser Melting of Pure Copper. Materials 2019, 12, 2469. https://doi.org/10.3390/ma12152469
Jadhav SD, Dadbakhsh S, Vleugels J, Hofkens J, Van Puyvelde P, Yang S, Kruth J-P, Van Humbeeck J, Vanmeensel K. Influence of Carbon Nanoparticle Addition (and Impurities) on Selective Laser Melting of Pure Copper. Materials. 2019; 12(15):2469. https://doi.org/10.3390/ma12152469
Chicago/Turabian StyleJadhav, Suraj Dinkar, Sasan Dadbakhsh, Jozef Vleugels, Johan Hofkens, Peter Van Puyvelde, Shoufeng Yang, Jean-Pierre Kruth, Jan Van Humbeeck, and Kim Vanmeensel. 2019. "Influence of Carbon Nanoparticle Addition (and Impurities) on Selective Laser Melting of Pure Copper" Materials 12, no. 15: 2469. https://doi.org/10.3390/ma12152469






