Effect of the Iron Reduction Index on the Mechanical and Chemical Properties of Continuous Basalt Fiber
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Sample Preparation
2.3. Characterization Techniques
3. Results
3.1. Iron Redox State
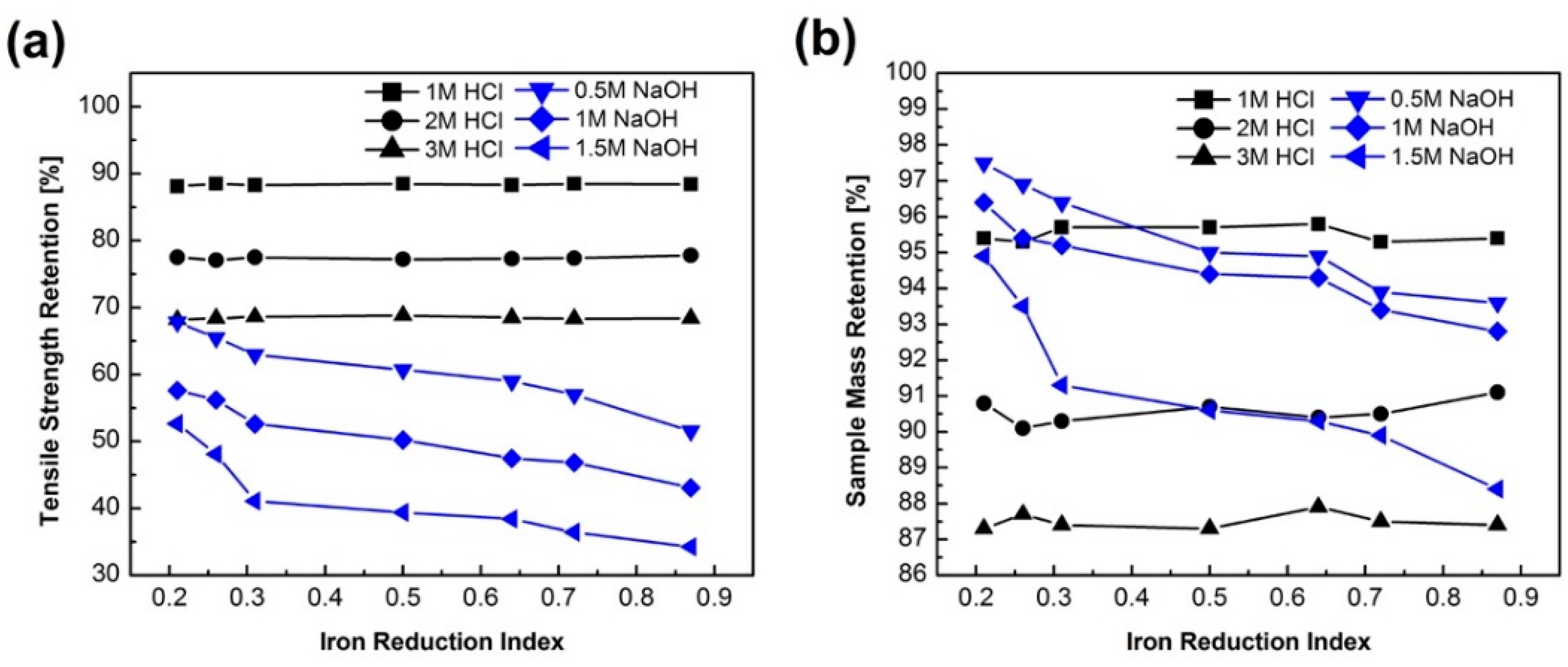
3.2. Tensile Strength and FT-IR
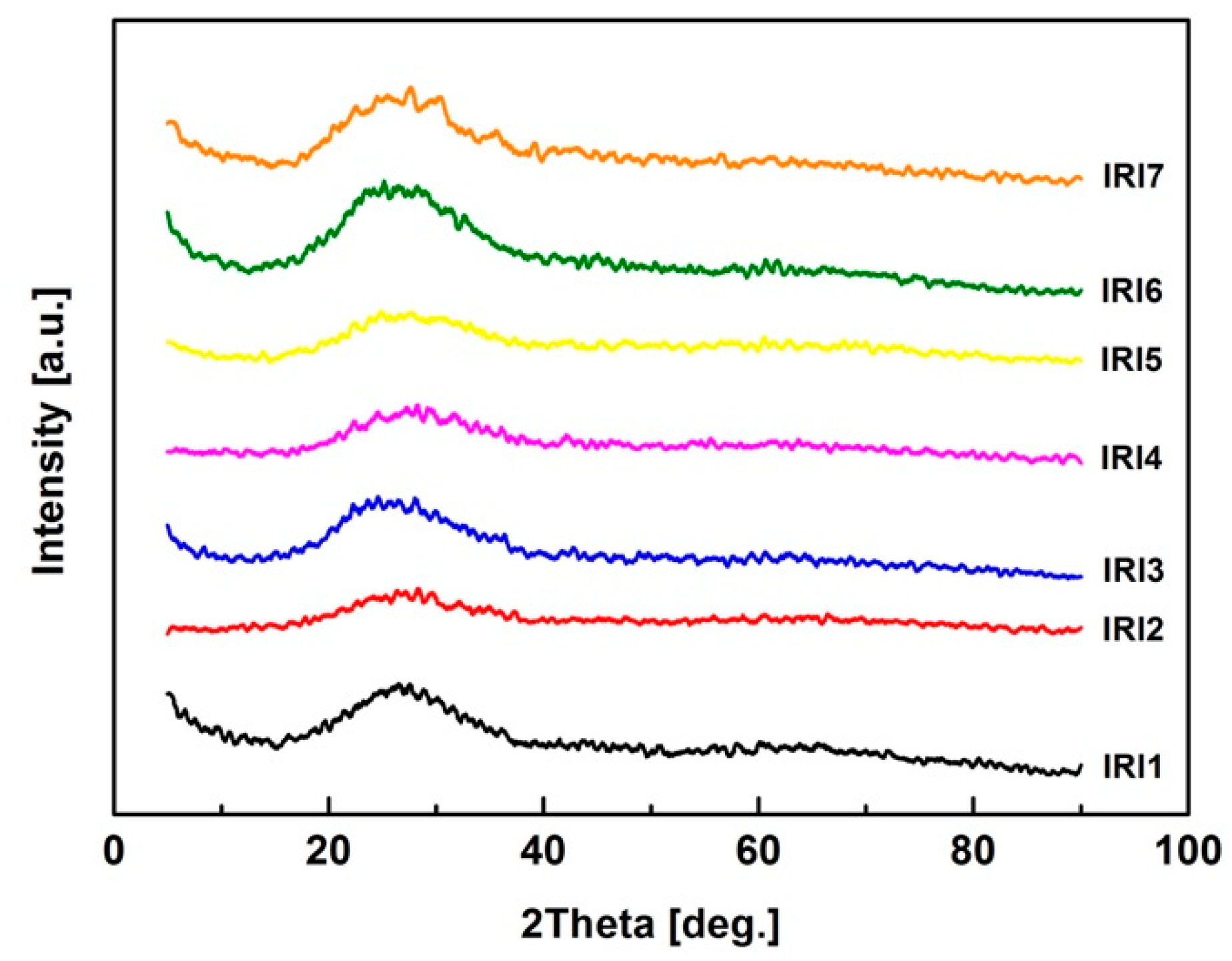
3.3. XRD
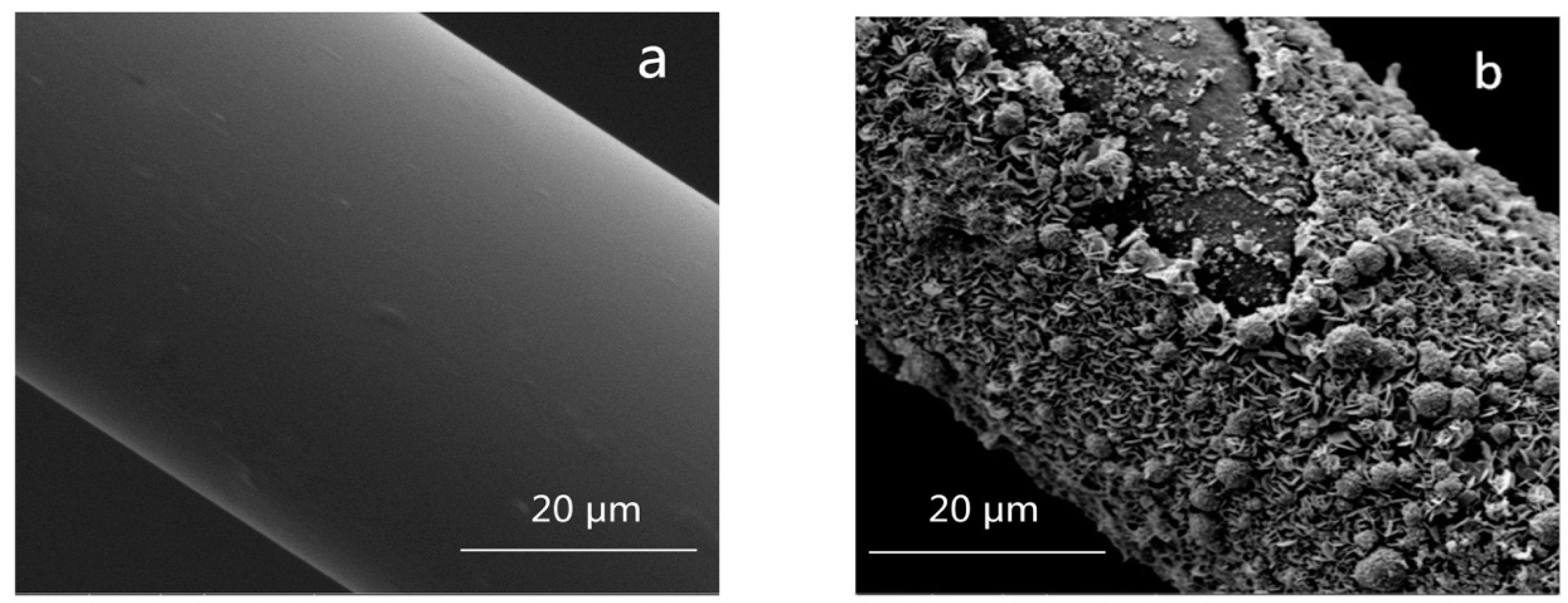
3.4. Chemical Durability
4. Discussion
5. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Kuzmin, K.L.; Gutnikov, S.I.; Zhukovskaya, E.S.; Lazoryak, B.I. Basaltic glass fibers with advanced mechanical properties. J. Non-Cryst. Solids 2017, 476, 144–150. [Google Scholar] [CrossRef]
- Dhand, V.; Mittal, G.; Rhee, K.Y.; Park, S.J.; Hui, D. A short review on basalt fiber reinforced polymer composites. Compos. Part B Eng. 2015, 73, 166–180. [Google Scholar] [CrossRef]
- Gutnikov, S.; Manylov, M.; Lipatov, Y.V.; Lazoryak, B.; Pokholok, K. Effect of the reduction treatment on the basalt continuous fiber crystallization properties. J. Non-Cryst. Solids 2013, 368, 45–50. [Google Scholar] [CrossRef]
- Mysen, B.O.; Richet, P. Silicate Glasses and Melts. Properties and Structure, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Karamanov, A.; Taglieri, G.; Pelino, M. Iron-rich sintered glass-ceramics from industrial wastes. J. Am. Ceram. Soc. 1999, 82, 3012–3016. [Google Scholar] [CrossRef]
- Parmar, R.; Kundu, R.S.; Punia, R.; Aghamkar, P.; Kishore, N. Iron modified structural and optical spectral properties of bismuth silicate glasses. Phys. B Condens. Matter 2014, 450, 39–44. [Google Scholar] [CrossRef]
- Moiseev, E.; Gutnikov, S.; Malakho, A.; Lazoryak, B. Effect of iron oxides on the fabrication and properties of continuous glass fibers. Inorg. Mater. 2008, 44, 1026–1030. [Google Scholar] [CrossRef]
- Karamanov, A.; Pisciella, P.; Cantalini, C.; Pelino, M. Influence of Fe3+/Fe2+ ratio on the crystallization of iron-rich glasses made with industrial wastes. J. Am. Ceram. Soc. 2000, 83, 3153–3157. [Google Scholar] [CrossRef]
- Burkhard, D.J.M.; Scherer, T. The effect of initial oxidation state on crystallization of basaltic glasses. J. Non-Cryst. Solids 2006, 352, 3961–3969. [Google Scholar] [CrossRef]
- Dingwell, D.B. Redox viscometry of some Fe-bearing silicate melts. Am. Mineral. 1991, 76, 1560–1562. [Google Scholar]
- Glebov, L.B.; Boulos, E.N. Absorption of iron and water in the Na2O–CaO–MgO– SiO2 glasses. II. Selection of intrinsic, ferric, and ferrous spectra in the visible and UV regions. J. Non-Cryst. Solids 1998, 242, 49–62. [Google Scholar] [CrossRef]
- Mekki, A.; Holland, D.; McConville, C.F.; Salim, M. An XPS study of iron sodium silicate glass surfaces. J. Non-Cryst. Solids 1996, 208, 267–276. [Google Scholar] [CrossRef]
- Jensen, M.; Zhang, L.; Yue, Y. Probing iron redox state in multicomponent glasses by XPS. Chem. Geol. 2012, 322, 145–150. [Google Scholar] [CrossRef]
- Yue, Y.Z.; Korsgaard, M.; Kirkegaard, L.F.; Heide, G. Formation of nanocrystalline layer on the surface of stone wool fibers. J. Am. Ceram. Soc. 2009, 92, 62–67. [Google Scholar] [CrossRef]
- Magnien, V.; Neuville, D.R.; Cormier, L.; Roux, J.; Hazemann, J.L.; De Ligny, D.; Pascarelli, S.; Vickridge, I.; Pinet, O.; Richet, P. Kinetics and mechanisms of iron redox reactions in silicate melts: The effects of temperature and alkali cations. Geochim. Cosmochim. Acta 2008, 72, 2157–2168. [Google Scholar] [CrossRef]
- Smedskjaer, M.M.; Solvang, M.; Yue, Y. Crystallisation behaviour and high-temperature stability of stone wool fibres. J. Eur. Ceram. Soc. 2010, 30, 1287–1295. [Google Scholar] [CrossRef]
- Sørensen, P.M.; Pind, M.; Yue, Y.Z.; Rawlings, R.D.; Boccaccini, A.R.; Nielsen, E.R. Effect of the redox state and concentration of iron on the crystallization behavior of iron-rich aluminosilicate glasses. J. Non-Cryst. Solids 2005, 351, 1246–1253. [Google Scholar] [CrossRef]
- Wang, Q.W.; Brow, R.K.; Li, H.; Ronchetto, E.A. Effects of iron concentration and redox states on failure of boron-free E-glass fibres under applied stress in different conditions. Bull. Mater. Sci. 2017, 40, 831–839. [Google Scholar] [CrossRef]
- Ma, Q.; Ding, L.; Wang, Q.; Yu, Y.; Luo, L.; Li, H. Preparation and characterization of continuous fly ash derived glass fibers with improved tensile strength. Mater. Lett. 2018, 231, 119–121. [Google Scholar] [CrossRef] [Green Version]
- Partyka, J.; Leśniak, M. Preparation of glass–ceramic glazes in the SiO2–Al2O3–CaO–MgO–K2O–Na2O–ZnO system by variable content of ZnO. Ceram. Int. 2016, 42, 8513–8524. [Google Scholar] [CrossRef]
- Okuno, M.; Zotov, N.; Schmücker, M.; Schneider, H. Structure of SiO2–Al2O3 glasses: Combined X-ray diffraction, IR and Raman studies. J. Non-Cryst. Solids 2005, 351, 1032–1038. [Google Scholar] [CrossRef]
- Wei, B.; Cao, H.L.; Song, S.H. Tensile behavior contrast of basalt and glass fibers after chemical treatment. Mater. Des. 2010, 31, 4244–4250. [Google Scholar] [CrossRef]


| Samples | SiO2 | Al2O3 | Fe2O3 | CaO+MgO | K2O+Na2O | Others | MnO2 | Citric Acid | C | IRI |
|---|---|---|---|---|---|---|---|---|---|---|
| IRI1 | 55.17 | 15.57 | 9.10 | 12.23 | 5.89 | 2.04 | 3.00 | / | / | 0.21 |
| IRI2 | 55.17 | 15.57 | 9.10 | 12.23 | 5.89 | 2.04 | 1.00 | / | / | 0.26 |
| IRI3 | 55.17 | 15.57 | 9.10 | 12.23 | 5.89 | 2.04 | / | / | / | 0.31 |
| IRI4 | 55.17 | 15.57 | 9.10 | 12.23 | 5.89 | 2.04 | / | 0.50 | / | 0.50 |
| IRI5 | 55.17 | 15.57 | 9.10 | 12.23 | 5.89 | 2.04 | / | 1.00 | / | 0.64 |
| IRI6 | 55.17 | 15.57 | 9.10 | 12.23 | 5.89 | 2.04 | / | / | 0.50 | 0.72 |
| IRI7 | 55.17 | 15.57 | 9.10 | 12.23 | 5.89 | 2.04 | / | / | 1.50 | 0.87 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Zhang, Q.; Wang, Q.; Xiao, J.; Liu, J.; Ding, L.; Jiang, W. Effect of the Iron Reduction Index on the Mechanical and Chemical Properties of Continuous Basalt Fiber. Materials 2019, 12, 2472. https://doi.org/10.3390/ma12152472
Luo L, Zhang Q, Wang Q, Xiao J, Liu J, Ding L, Jiang W. Effect of the Iron Reduction Index on the Mechanical and Chemical Properties of Continuous Basalt Fiber. Materials. 2019; 12(15):2472. https://doi.org/10.3390/ma12152472
Chicago/Turabian StyleLuo, Lida, Qichang Zhang, Qingwei Wang, Jiwen Xiao, Jin Liu, Linfeng Ding, and Weizhong Jiang. 2019. "Effect of the Iron Reduction Index on the Mechanical and Chemical Properties of Continuous Basalt Fiber" Materials 12, no. 15: 2472. https://doi.org/10.3390/ma12152472





