A New Reversible Phase Transformation of Intermetallic Ti3Sn
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Samples
2.2. DSC and Thermal Expansion Measurement
2.3. Transmission Electron Microscopy (TEM) Characterization
2.4. XRD Analysis
2.5. First Principle Calculation
3. Results and Discussion
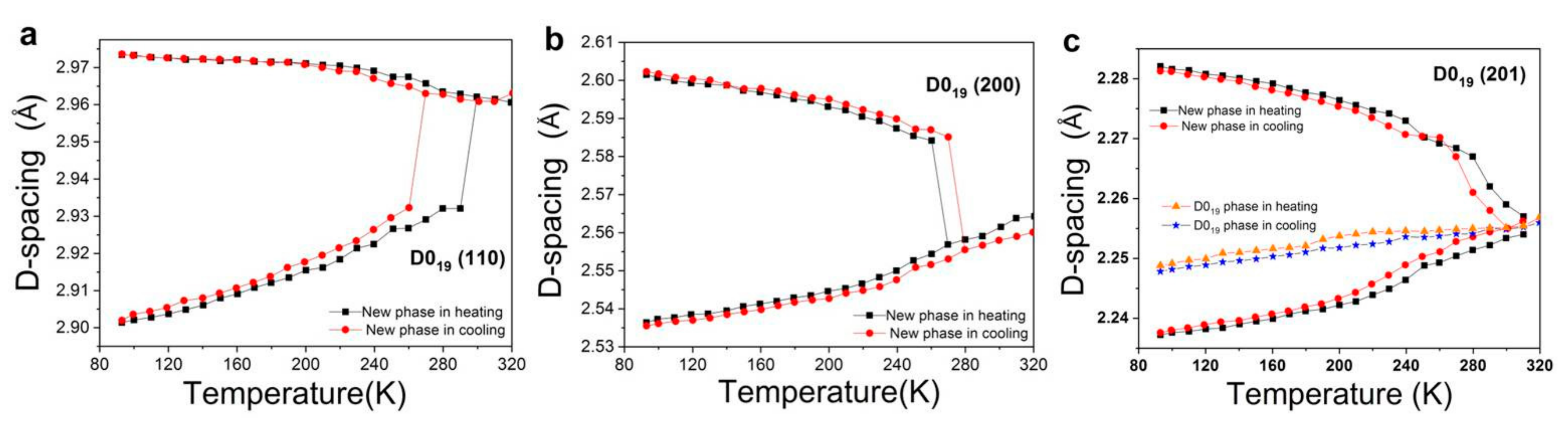
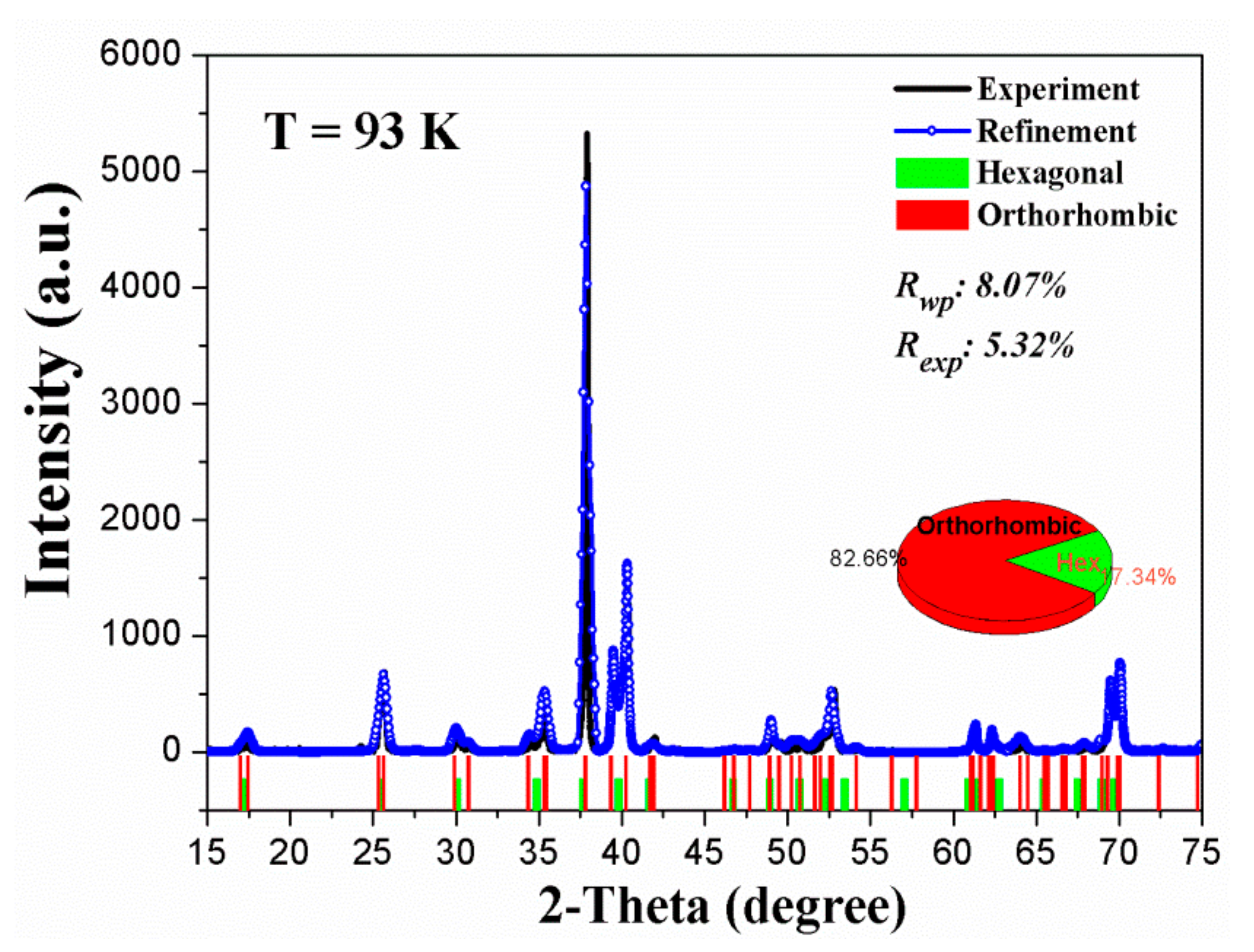
3.1. Detection and Characterization of Phase Transformation of Ti3Sn
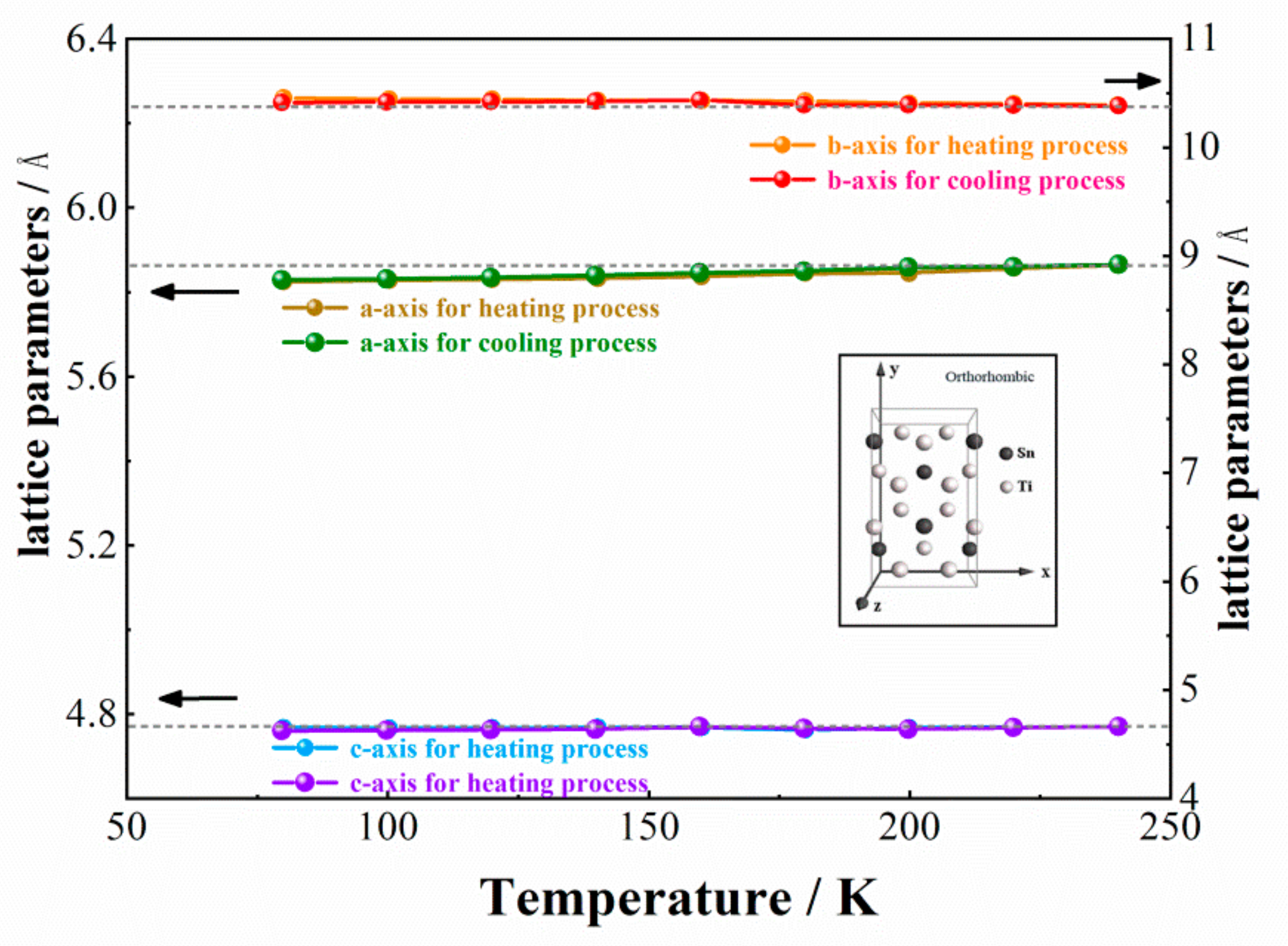
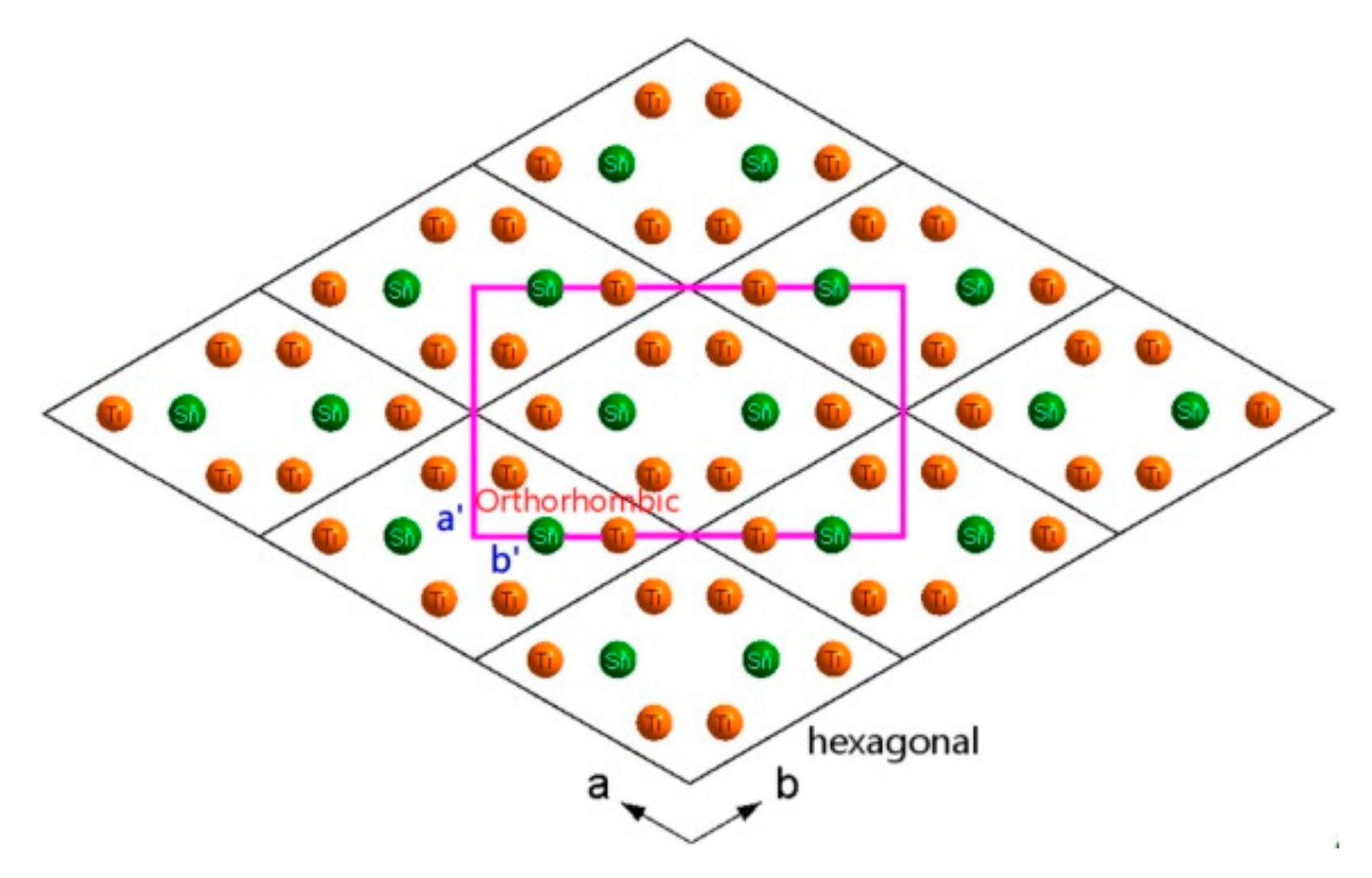
3.2. Crystal Structure of a New Phase of Ti3Sn
3.3. Discussion on the Thermodynamic Possibility of Phase Transformation
4. Conclusions
- The reversible thermal induced phase transformation of Ti3Sn is demonstrated by the means of XRD and thermal expansion experiment;
- The temperature of forward phase transformation during cooling is 270–300 K, and this phase transformation is reversible with a hysteresis of 10–30 K according to XRD results;
- The phase transformation is determined to be a hexagonal → orthorhombic transition, the lattice parameters of the new orthorhombic phase are: a = 5.87 Å, b = 10.37 Å, c = 4.76 Å respectively, α = β = γ = 90°, the space group is Cmcm (No. 63). The atomic Wyckoff positions for orthorhombic Ti3Sn are also provided;
- The orientation relationships between orthorhombic and hexagonal phases are suggested as: (110)orthorhombic//(100)hexagonal, <001>orthorhombic//<001>hexagonal;
- The hexagonal → orthorhombic phase transformation is calculated to be reasonable according to thermodynamics theory.
Author Contributions
Funding
Conflicts of Interest
References
- Yang, R.; Gu, Y.; Li, Y.; Zheng, J.; Li, X. Self-assembled 3-D flower-shaped SnO2 nanostructures with improved electrochemical performance for lithium storage. Acta Mater. 2010, 58, 866. [Google Scholar] [CrossRef]
- Derrien, G.; Hassoun, J.; Panero, S.; Scrosati, B. Nanostructured Sn-C composite as an advanced anode material in high-performance Lithium-ion batteries. Adv. Mater. 2007, 19, 2336. [Google Scholar] [CrossRef]
- Sougrati, M.T.; Fullenwarth, J.; Debenedetti, A.; Fraisse, B.; Jumas, J.C.; Monconduit, L. TiSnSb a new efficient negative electrode for Li-ion batteries: mechanism investigations by operando-XRD and Mössbauer techniques. J. Mater. Chem. 2011, 21, 10069. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Campos, S.; Foster, D.; Read, J.; Behl, W.K. Nano-scale Cu6Sn5 anodes. J. Power Sources 2002, 109, 230. [Google Scholar] [CrossRef]
- Jacobs, J.K.; Dasgupta, S. Negative Electrode for a Rechargeable Lithium Battery Comprising a Solid Solution of Titanium Dioxide and Tin Dioxide. U.S. Patent Application No. 6,007,945, 28 December 1999. [Google Scholar]
- Todd, A.D.W.; Mar, R.E.; Dahn, J.R. Combinatorial study of tin-transition metal alloys as negative electrodes for lithium-ion batteries. J. Electrochem. Soc. 2006, 153, 1998. [Google Scholar] [CrossRef]
- Vdovychenko, O.V.; Bulanova, M.V.; Fartushna, Y.u.V.; Shcheresky, A.A. Dynamic mechanical behavior of intermetallic Ti3Sn. Scr. Mater. 2010, 62, 761. [Google Scholar] [CrossRef]
- Zhang, J.; Perez, R.J.; Lavernia, E.J. Documentation of damping capacity of metallic, ceramic and metal-matrix composite materials. J. Mater. Sci. 1993, 28, 2399. [Google Scholar] [CrossRef]
- Yin, F.; Tedenac, J.C.; Gascoin, F. Thermodynamic modeling of the Ti-Sn system and calculation of the Co-Ti-Sn system. Calphad 2007, 31, 370. [Google Scholar] [CrossRef]
- Cech, R.G. Trans. vidence of solidification of a metastable phase in Fe-Ni alloys. AIME 1956, 206, 585. [Google Scholar]
- Colin, M.C.; Denise, S.M.; Carlos, G.L. Phase selection in undercooled Ti3Sn. Metall. Trans. A 1993, 24, 1481. [Google Scholar]
- Ivanova, O.; Karpets, M.; Yavari, A.R.; Georgarakis, K.; Podrezov, Y. In situ X-ray diffraction study of the phase transformation in the non-stoichiometric intermetallic compound Ti3Sn. J. Alloy. Compd. 2014, 582, 360. [Google Scholar] [CrossRef]
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon Jean-Marie Schalkwijk, W.V. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 367. [Google Scholar] [CrossRef] [PubMed]
- Fransson, L.M.L.; Vaughey, J.T.; Benedek, R.; Edström, K.; Thomas, J.O.; Thackeray, M.M. Phase transitions in lithiated Cu2Sb anodes for lithium batteries: an in situ X-ray diffraction study. Electrochem. Commun. 2001, 3, 317. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef]
- Wolverton, C.; Ozoliš, V. First-principles aluminum database: Energetics of binary Al alloys and compounds. Phys. Rev. B 2006, 73, 144104. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Wang, S.B.; Yuan, D.W.; Yin, M.J.; Wu, C.L. The structure and the propeties of S-phase in AlCuMg alloys. Acta Mater. 2011, 59, 7396. [Google Scholar] [CrossRef]
- Wong, C.R.; Fleischer, R.L. Low frequency damping and ultrasonic attenuation in Ti3Sn-based alloys. J. Mater. Res. 1994, 9, 1441. [Google Scholar] [CrossRef]
- Miller, W.; Smith, C.W.; Mackenzie, D.S.; Evans, K.E. Negative thermal expansion: A review. J. Mater. Sci. 2009, 44, 5441. [Google Scholar] [CrossRef]
- Grima, J.N.; Zammit, V.; Gatt, R. Negative thermal expansion. Xjenza 2006, 11, 17. [Google Scholar]
- Hahn, T. International Tables for Crystallography; Dordrecht Reidel: Dordrecht, The Netherlands, 1992; p. 41. [Google Scholar]
- Lábár, J.L. Consistent indexing of a (set of) single crystal SAED pattern(s) with the ProcessDiffraction program. Ultramicroscopy 2005, 103, 237. [Google Scholar] [CrossRef] [PubMed]




| Phase: Orthorhombic | ||
|---|---|---|
| Space Group: Cmcm (No. 63). 16 Atoms Per Unit Cell | ||
| Lattice Parameters | Atomic Wyckoff Positions | |
| a = 5.87 Å | Ti (8g) Ti (4c) Sn (4c) | x: 0.231 y: 0.904 z: 0.250 x: 0 y: 0.636 z: 0.250 x: 0 y: 0.163 z: 0.250 |
| b = 10.37 Å | ||
| c = 4.76 Å | ||
| α = β = γ = 90° | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, M.; Cui, L.; Liu, F. A New Reversible Phase Transformation of Intermetallic Ti3Sn. Materials 2019, 12, 2484. https://doi.org/10.3390/ma12152484
Du M, Cui L, Liu F. A New Reversible Phase Transformation of Intermetallic Ti3Sn. Materials. 2019; 12(15):2484. https://doi.org/10.3390/ma12152484
Chicago/Turabian StyleDu, Minshu, Lishan Cui, and Feng Liu. 2019. "A New Reversible Phase Transformation of Intermetallic Ti3Sn" Materials 12, no. 15: 2484. https://doi.org/10.3390/ma12152484





