Low-Temperature High-Efficiency Preparation of TiB2 Micro-Platelets via Boro/Carbothermal Reduction in Microwave Heated Molten Salt
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Raw Materials
2.2. Methodologies
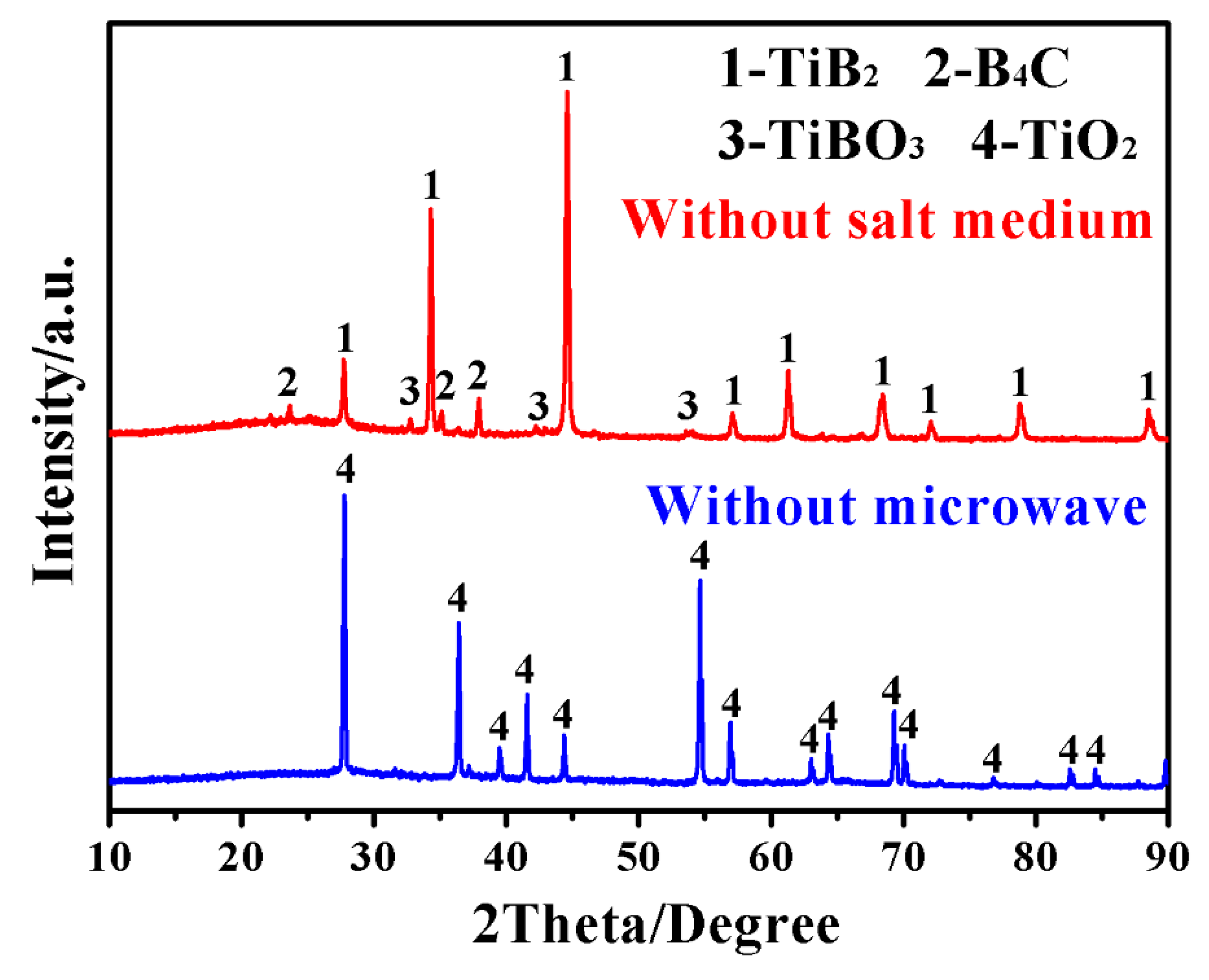
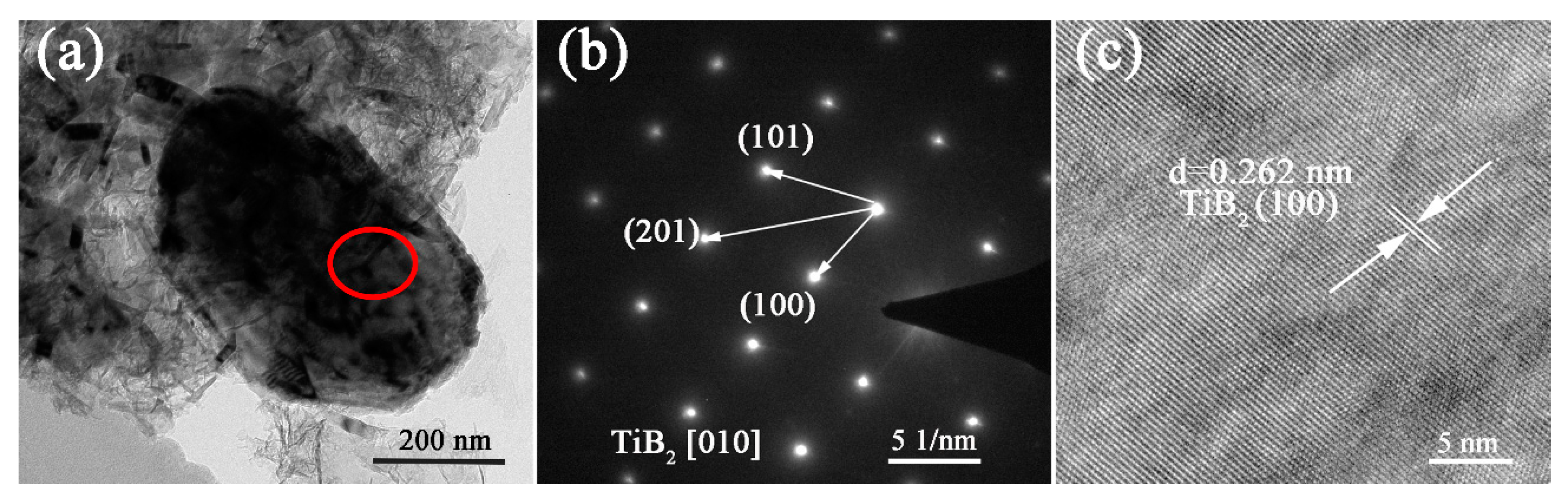
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, W.; Zhang, G.; You, Y.; Wu, S.; Lin, H. TiB2 powders synthesis by borothermal reduction in TiO2 under vacuum. J. Am. Ceram. Soc. 2014, 97, 1359–1362. [Google Scholar] [CrossRef]
- Zhang, G.; Ni, D.; Zou, J.; Liu, H.; Wu, W.; Liu, J.; Suzuki, T.S.; Sakka, Y. Inherent anisotropy in transition metal diborides and microstructure/property tailoring in ultra-high temperature ceramics-A review. J. Eur. Ceram. Soc. 2018, 38, 371–389. [Google Scholar] [CrossRef]
- Fahrenholtz, W.; Hilmas, G.; Talmy, I.; Zaykoski, J. Refractory diborides of Zirconium and Hafnium. J. Am. Ceram. Soc. 2007, 90, 1347–1364. [Google Scholar] [CrossRef]
- Basu, B.; Raju, G.; Suri, A. Processing and properties of monolithic TiB2 based materials. Int. Mater. Rev. 2013, 51, 352–374. [Google Scholar] [CrossRef]
- Chlup, Z.; Bača, Ľ.; Halasová, M.; Neubauer, E.; Hadraba, H.; Stelzer, N.; Roupcová, P. Effect of metallic dopants on the microstructure and mechanical properties of TiB2. J. Eur. Ceram. Soc. 2015, 35, 2745–2754. [Google Scholar] [CrossRef]
- Taranenko, V.I.; Zarutskii, I.V.; Shapoval, V.I.; Matiasovsk, M.; Matiasovsk, K. Mechanism of the cathode process in the electrochemical synthesis of TiB2 in molten salts-II. Chloride-Fluoride Electrolytes. Electrochim. Acta 1992, 37, 263–268. [Google Scholar] [CrossRef]
- Sahoo, S.; Singh, S.K. Synthesis of TiB2 by extended arc thermal plasma. Ceram. Int. 2017, 43, 15561–15566. [Google Scholar] [CrossRef]
- Tabriz, B.N.; Adhami, T.; Kahrizsangi, R.E. Effect of processing parameters on the formation of TiB2 nanopowder by mechanically induced self-sustaining reaction. Ceram. Int. 2014, 40, 7345–7354. [Google Scholar] [CrossRef]
- Kang, S.H.; Kim, D.J. Synthesis of nano-titanium diboride powders by carbothermal reduction. J. Eur. Ceram. Soc. 2007, 27, 715–718. [Google Scholar] [CrossRef]
- Yu, J.; Ma, L.; Zhang, Y.; Gong, H.; Zhou, L. Synthesis of TiB2 powders via carbothermal reduction of TiO2, HBO2 and carbon black. Ceram. Int. 2016, 42, 5512–5516. [Google Scholar] [CrossRef]
- Derin, B.; Kurtoglu, K.; Sahin, F.C.; Yucel, O. Thermochemical modeling and experimental studies on the formation of TiB2 through carbothermic synthesis from TiO2 and B2O3 or B4C. Ceram. Int. 2017, 43, 10975–10982. [Google Scholar] [CrossRef]
- Yu, J.; Ma, L.; Abbas, A.; Zhang, Y.; Gong, H.; Wang, X.; Zhou, L.; Liu, H. Carbothermal reduction synthesis of TiB2 ultrafine powders. Ceram. Int. 2016, 42, 3916–3920. [Google Scholar] [CrossRef]
- Moradi, V.; Nikzad, L.; Mobasherpour, I.; Razavi, M. Low temperature synthesis of titanium diboride by carbothermal method. Ceram. Int. 2018, 44, 19421–19426. [Google Scholar] [CrossRef]
- Liu, D.; Chu, Y.; Jing, S.; Ye, B.; Zhou, X. Low-temperature synthesis of ultrafine TiB2 nanopowders by molten-salt assisted borothermal reduction. J. Am. Ceram. Soc. 2018, 101, 5299–5303. [Google Scholar] [CrossRef]
- Welham, N.J. Formation of nanometric TiB2 from TiO2. J. Am. Ceram. Soc. 2000, 83, 1290–1292. [Google Scholar] [CrossRef]
- Bao, K.; Wen, Y.; Khangkhamano, M.; Zhang, S. Low-temperature preparation of titanium diboride fine powder via magnesiothermic reduction in molten salt. J. Am. Ceram. Soc. 2017, 100, 2266–2272. [Google Scholar] [CrossRef]
- Tan, C.; Liu, J.; Zhang, H.; Wang, J.; Li, S.; Song, J.; Zhang, Y.; Zhang, S. Low-temperature synthesis of 2H-SiC powders via molten-salt-mediated magnesiothermic reduction. Ceram. Int. 2017, 43, 2431–2437. [Google Scholar] [CrossRef]
- Zhang, S.; Khangkhamano, M.; Zhang, H.; Yeprem, H.A. Novel synthesis of ZrB2 powder via molten-salt-mediated magnesiothermic reduction. J. Am. Ceram. Soc. 2014, 97, 1686–1688. [Google Scholar] [CrossRef]
- Subramanian, C.; Murthy, T.S.R.C.; Suri, A.K. Synthesis and consolidation of titanium diboride. Int. J. Refract. Met. Hard Mater. 2007, 25, 345–350. [Google Scholar] [CrossRef]
- Wu, J.; Niu, B.; Zhang, F.; Lei, L.; Zhang, J.; Ren, L.; Wang, W.; Fu, Z. Effect of titanium diboride on the homogeneity of boron carbide ceramic by flash spark plasma sintering. Ceram. Int. 2018, 44, 15323–15330. [Google Scholar] [CrossRef]
- Ma, L.; Yu, J.; Guo, X.; Xie, B.; Gong, H.; Zhang, Y.; Zhai, Y.; Wu, X. Preparation and sintering of ultrafine TiB2 powders. Ceram. Int. 2018, 44, 4491–4495. [Google Scholar] [CrossRef]
- Rabiezadeh, A.; Hadian, A.M.; Ataie, A. Synthesis and sintering of TiB2 nanoparticles. Ceram. Int. 2014, 40, 15775–15782. [Google Scholar] [CrossRef]
- Zhang, H.; Li, F. Preparation and microstructure evolution of diboride ultrafine powder by sol–gel and microwave carbothermal reduction method. J. Sol-Gel Sci. Technol. 2007, 45, 205–211. [Google Scholar] [CrossRef]
- Javadi, A.; Pan, S.; Cao, C.; Yao, G.; Li, X. Facile synthesis of 10 nm surface clean TiB2 nanoparticles. Mater. Lett. 2018, 229, 107–110. [Google Scholar] [CrossRef]
- Wei, T.; Liu, Z.; Ren, D.; Deng, X.; Deng, Q.; Huang, Q.; Ran, S. Low-temperature synthesis of TaB2 nanorods by molten-salt assisted borothermal reduction. J. Am. Ceram. Soc. 2018, 101, 45–49. [Google Scholar] [CrossRef]
- Danko, G.A.; Silberglitt, R.; Colombo, P.; Pippel, E.; Woltersdorf, J. Comparison of microwave hybrid and conventional heating of preceramic polymers to form silicon carbide and silicon oxycarbideceramics. J. Am. Ceram. Soc. 2004, 83, 1617–1625. [Google Scholar] [CrossRef]
- Gordon, J.; Kazemian, H.; Rohani, S. Rapid and efficient crystallization of MIL-53(Fe) by ultrasound and microwave irradiation. Microporous Mesoporous Mater. 2012, 162, 36–43. [Google Scholar] [CrossRef]
- Liu, H.; Xiang, K.; Yang, B.; Yang, S.; Chai, L. Microwave intensified synthesis of regular shaped sodium bisulfate crystal. Chem. Eng. Process. Process Intensif. 2015, 95, 208–213. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Z.; Huo, C.; Li, F.; Zhang, H.; Zhang, S. Low-temperature rapid synthesis of rod-like ZrB2 powders by molten-salt and microwave co-assisted carbothermal reduction. J. Am. Ceram. Soc. 2016, 99, 2895–2898. [Google Scholar] [CrossRef]
- Zeng, Y.; Liang, F.; Liu, J.; Zhang, J.; Zhang, H.; Zhang, S. Highly efficient and low-temperature preparation of plate-like ZrB2-SiC powders by a molten-salt and microwave-modified boro/carbothermal reduction method. Materials 2018, 11, 1811. [Google Scholar] [CrossRef]




| Sample No. | Molar Ratio | Heating Mode | Temperature (°C) | Dwelling Time (min) | Salt Medium | ||
|---|---|---|---|---|---|---|---|
| TiO2 | B4C | C | |||||
| MSMBC-1 | 1.0 | 0.8 | 1.5 | MWH † | 1150 | 20 | NaCl/KCl |
| MSMBC-2 | 1.0 | 0.8 | 1.5 | MWH | 1150 | 60 | NaCl/KCl |
| MSMBC-3 | 1.0 | 0.8 | 1.5 | MWH | 1200 | 0 | NaCl/KCl |
| MSMBC-4 | 1.0 | 0.8 | 1.5 | MWH | 1200 | 20 | NaCl/KCl |
| MSMBC-5 | 1.0 | 0.8 | 1.5 | CH† | 1200 | 20 | NaCl/KCl |
| MSMBC-6 | 1.0 | 0.8 | 1.5 | MWH | 1200 | 20 | – |
| MSMBC-7 | 1.0 | 0.7 | 1.5 | MWH | 1200 | 20 | NaCl/KCl |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Liu, J.; Zeng, Y.; Zhang, H.; Li, Z. Low-Temperature High-Efficiency Preparation of TiB2 Micro-Platelets via Boro/Carbothermal Reduction in Microwave Heated Molten Salt. Materials 2019, 12, 2555. https://doi.org/10.3390/ma12162555
Liu J, Liu J, Zeng Y, Zhang H, Li Z. Low-Temperature High-Efficiency Preparation of TiB2 Micro-Platelets via Boro/Carbothermal Reduction in Microwave Heated Molten Salt. Materials. 2019; 12(16):2555. https://doi.org/10.3390/ma12162555
Chicago/Turabian StyleLiu, Jie, Jianghao Liu, Yuan Zeng, Haijun Zhang, and Zhi Li. 2019. "Low-Temperature High-Efficiency Preparation of TiB2 Micro-Platelets via Boro/Carbothermal Reduction in Microwave Heated Molten Salt" Materials 12, no. 16: 2555. https://doi.org/10.3390/ma12162555





