The Effect of Polyol Composition on the Structural and Magnetic Properties of Magnetite Nanoparticles for Magnetic Particle Hyperthermia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Materials Preparation
2.3. Characterization Techniques
3. Results
3.1. Structural Characterization
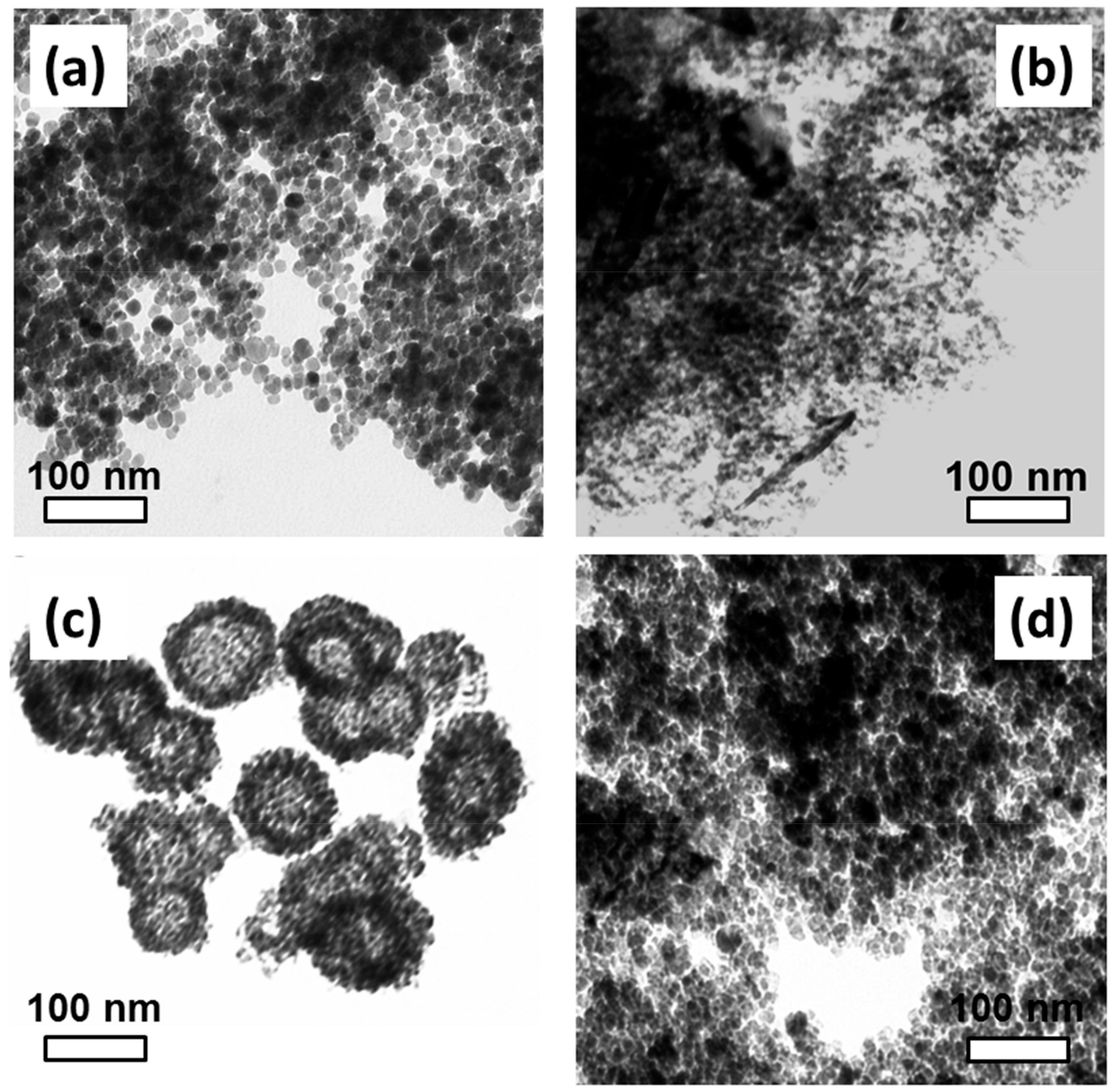
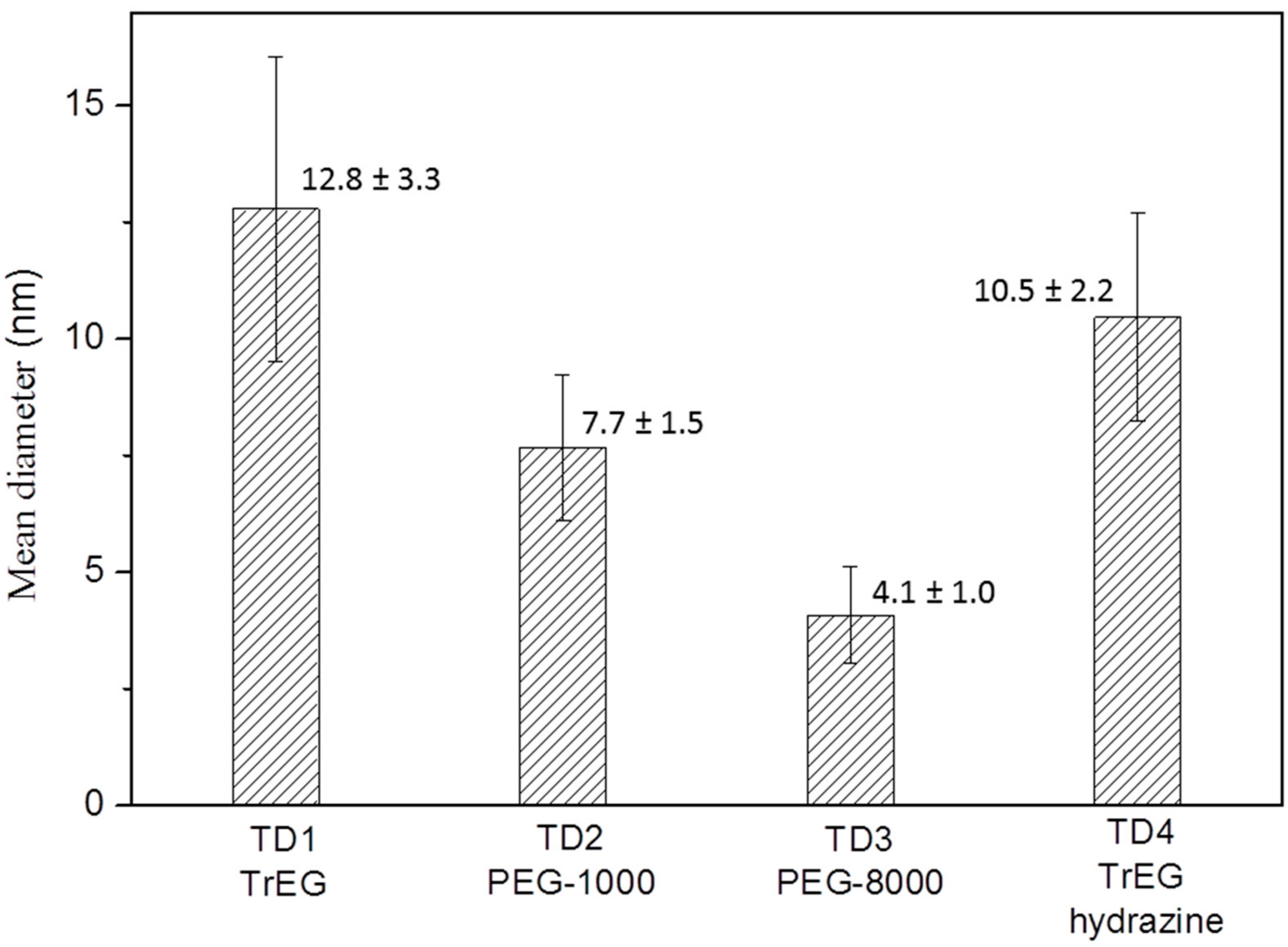
3.1.1. Transmission Electron Microscopy
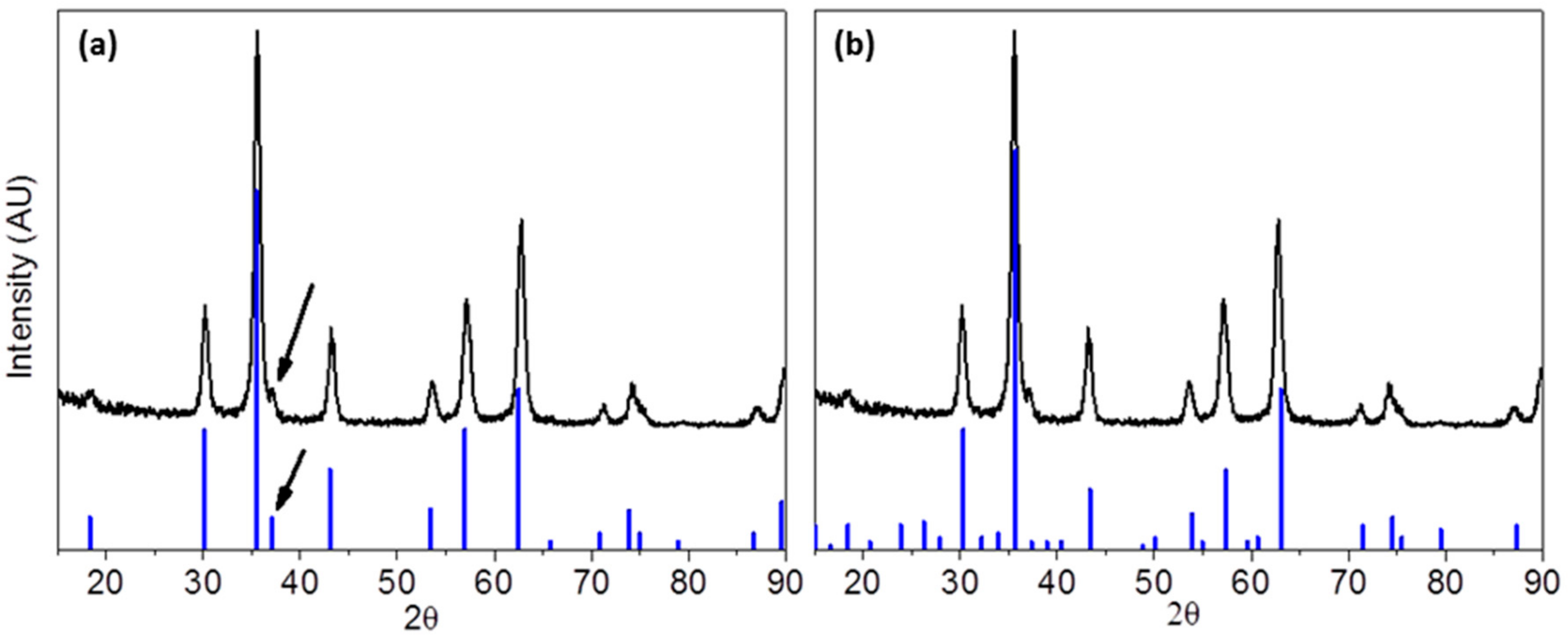
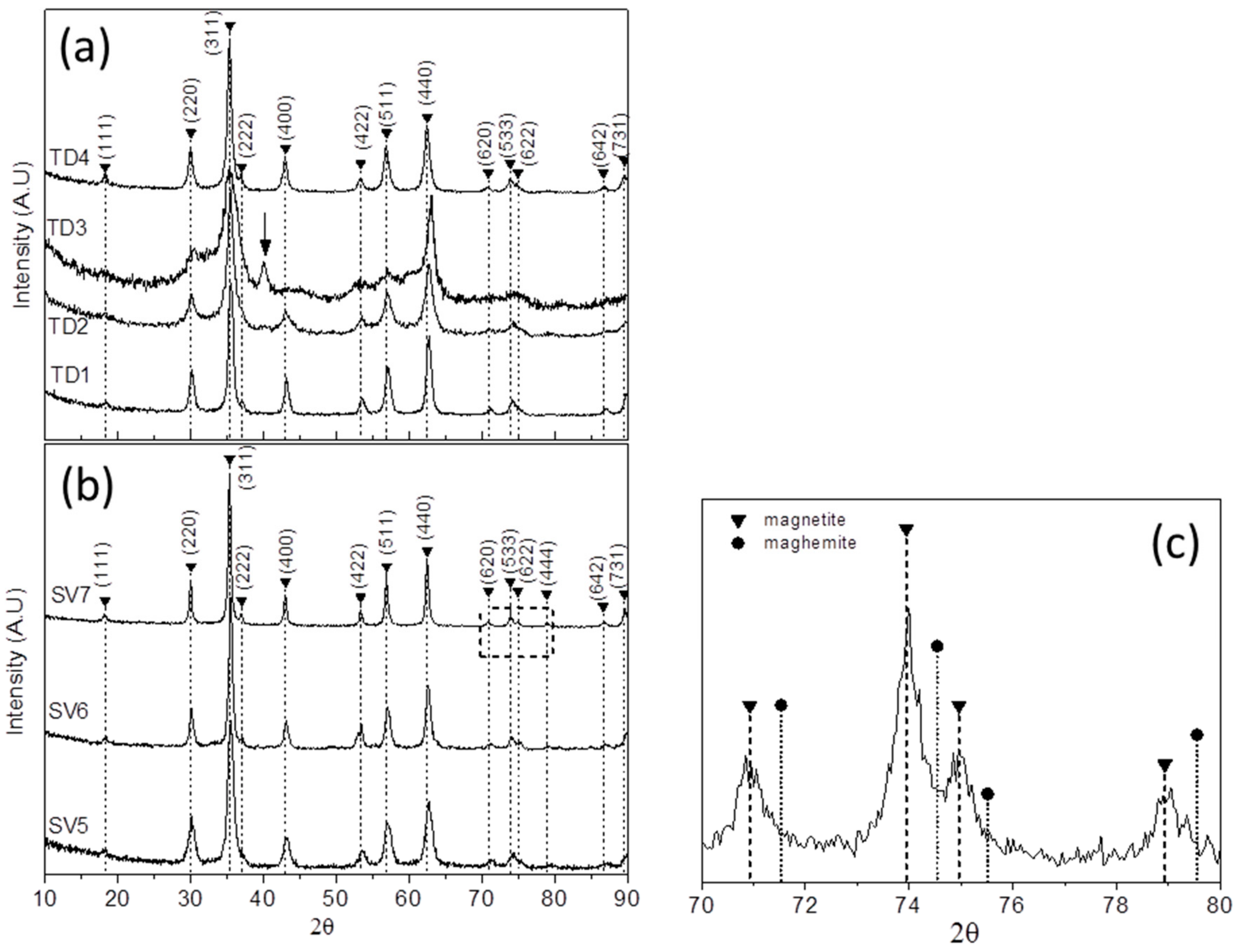
3.1.2. XRD Measurements
3.1.3. Infra-Red Spectroscopy
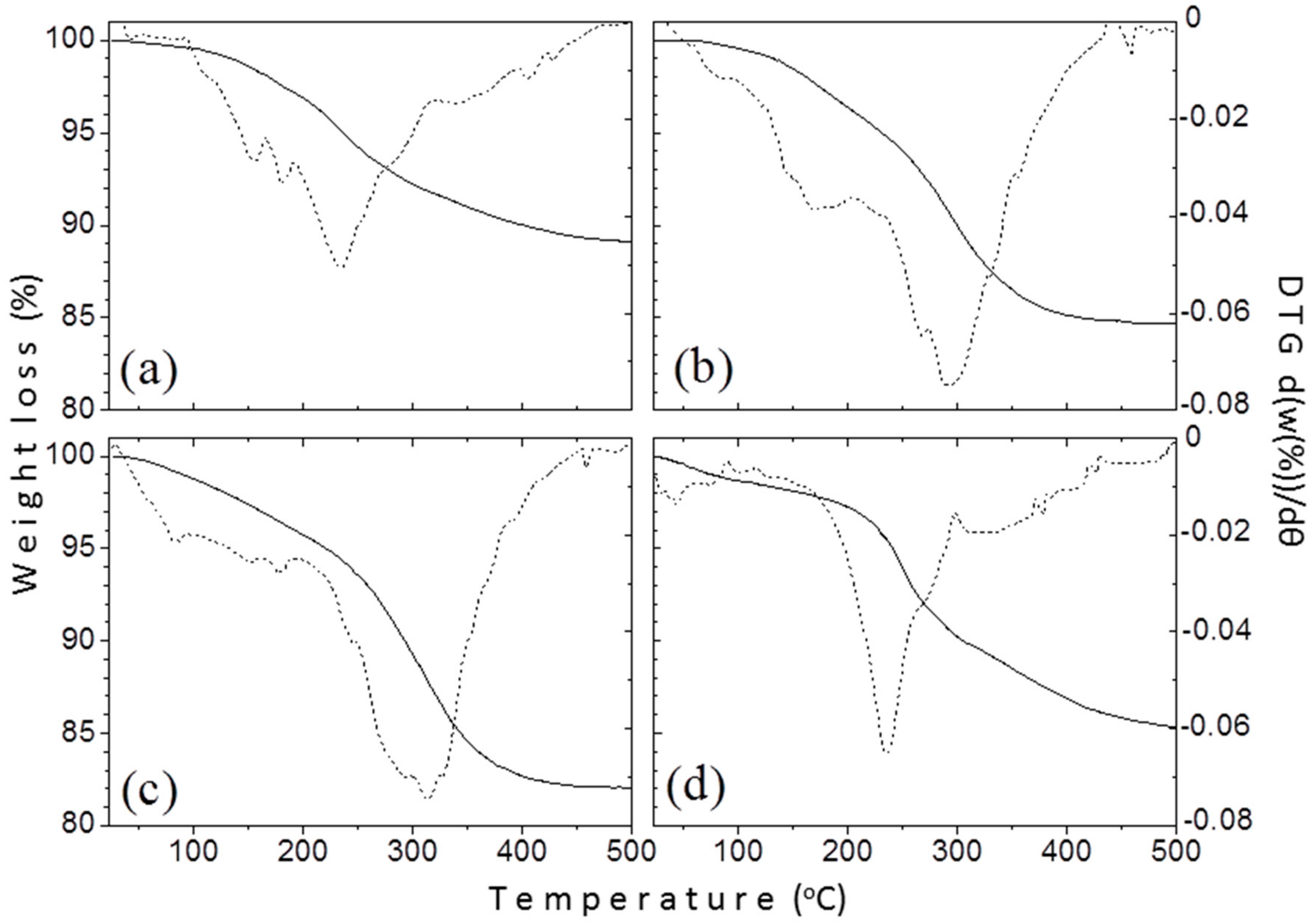
3.1.4. Thermogravimetric Analysis
3.2. Magnetic Characterization and Hyperthermia
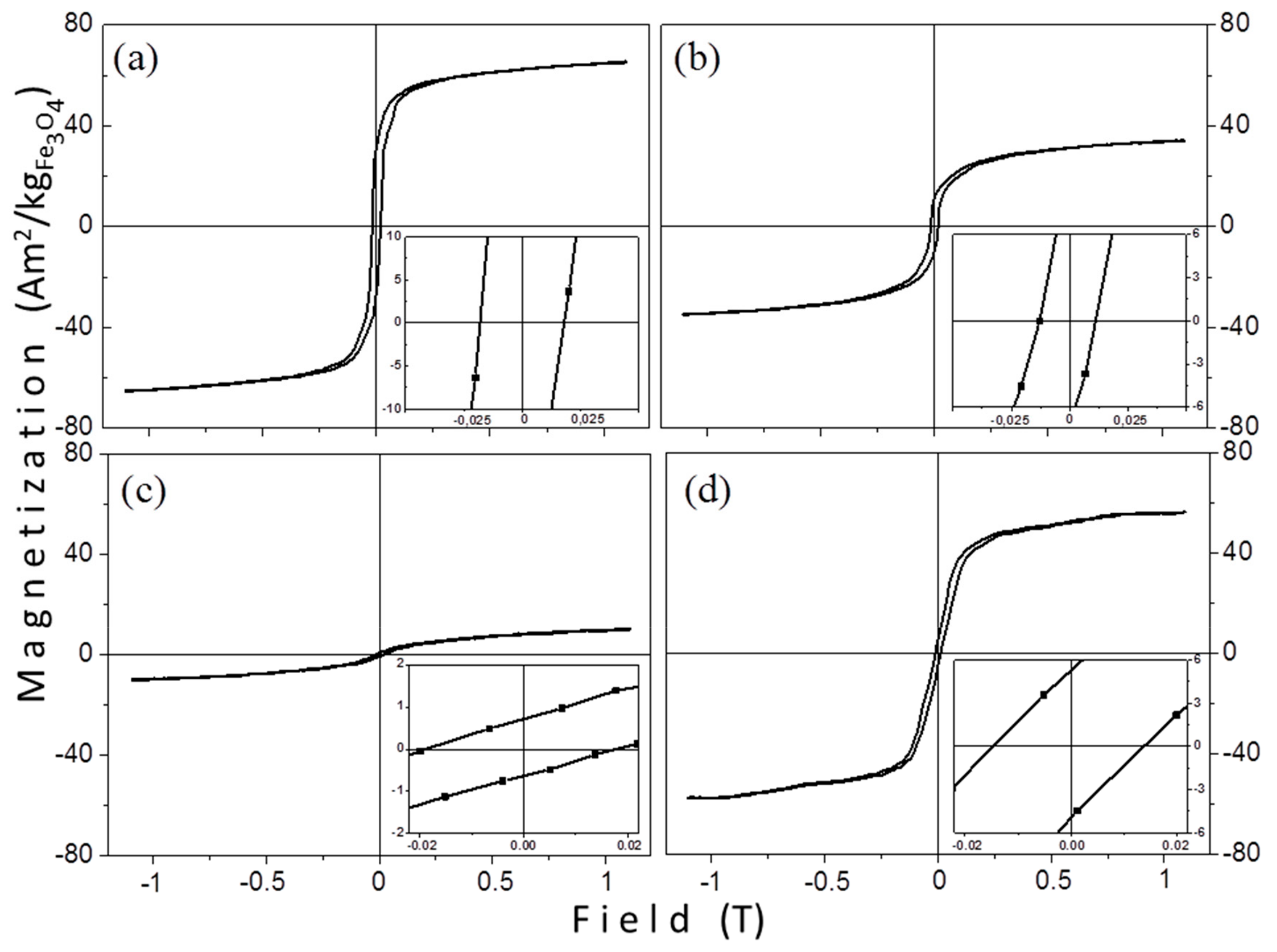
3.2.1. VSM Measurements
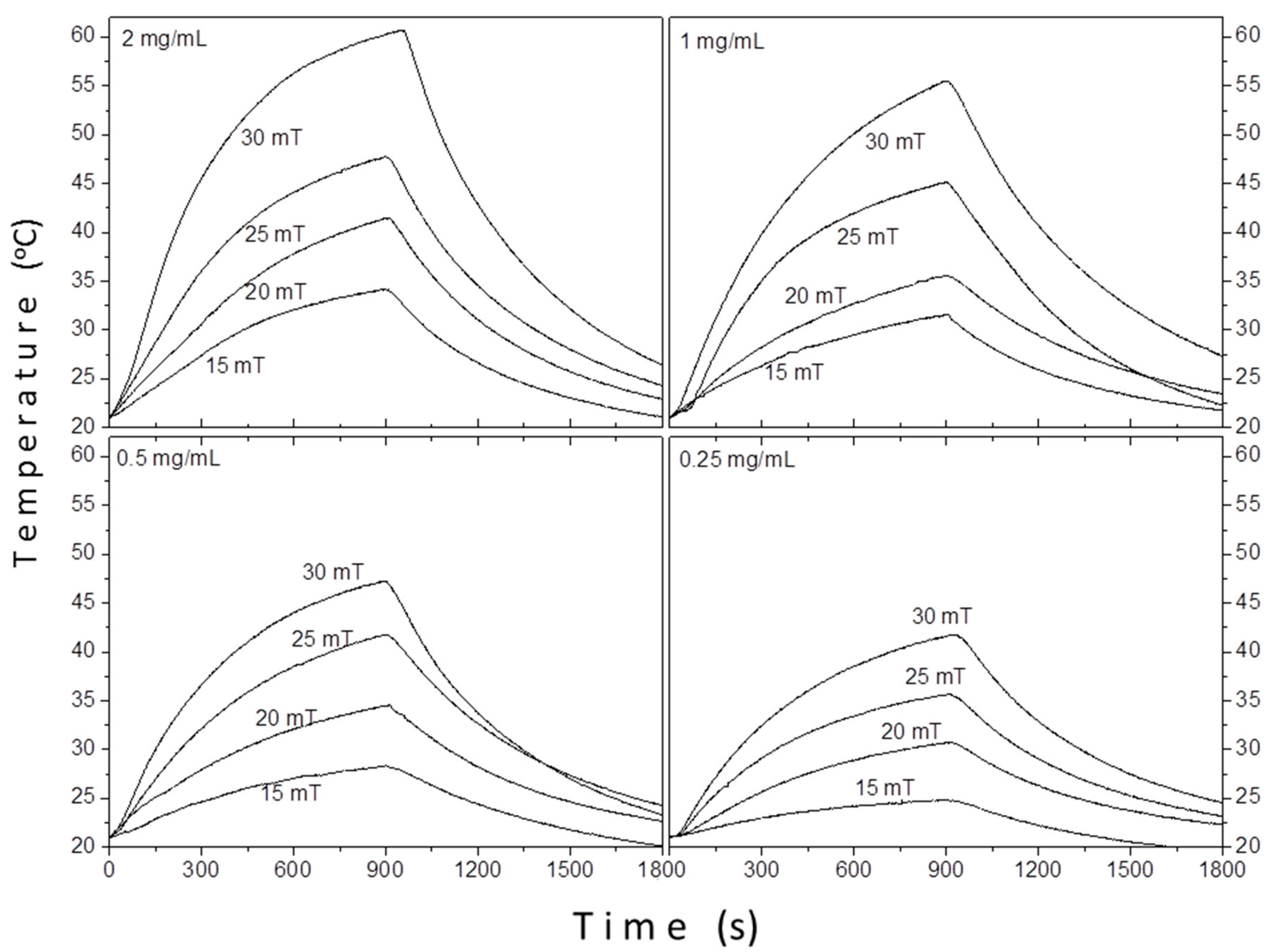
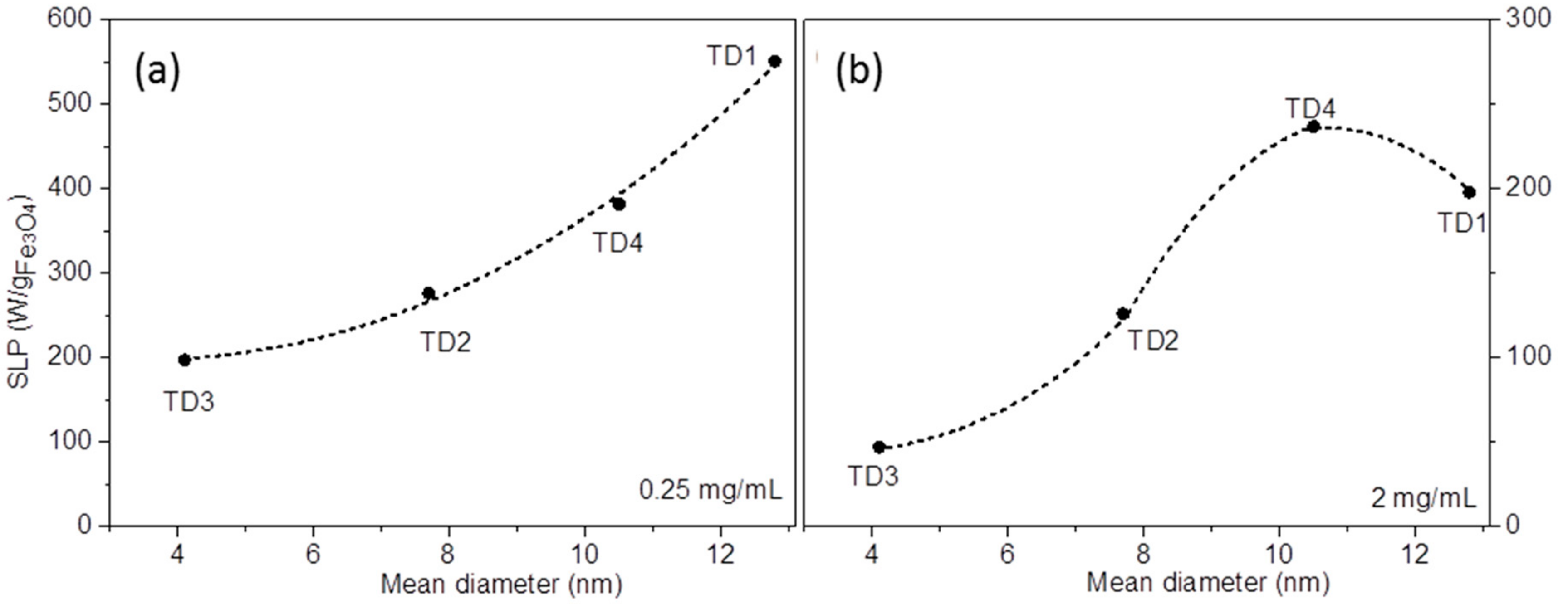
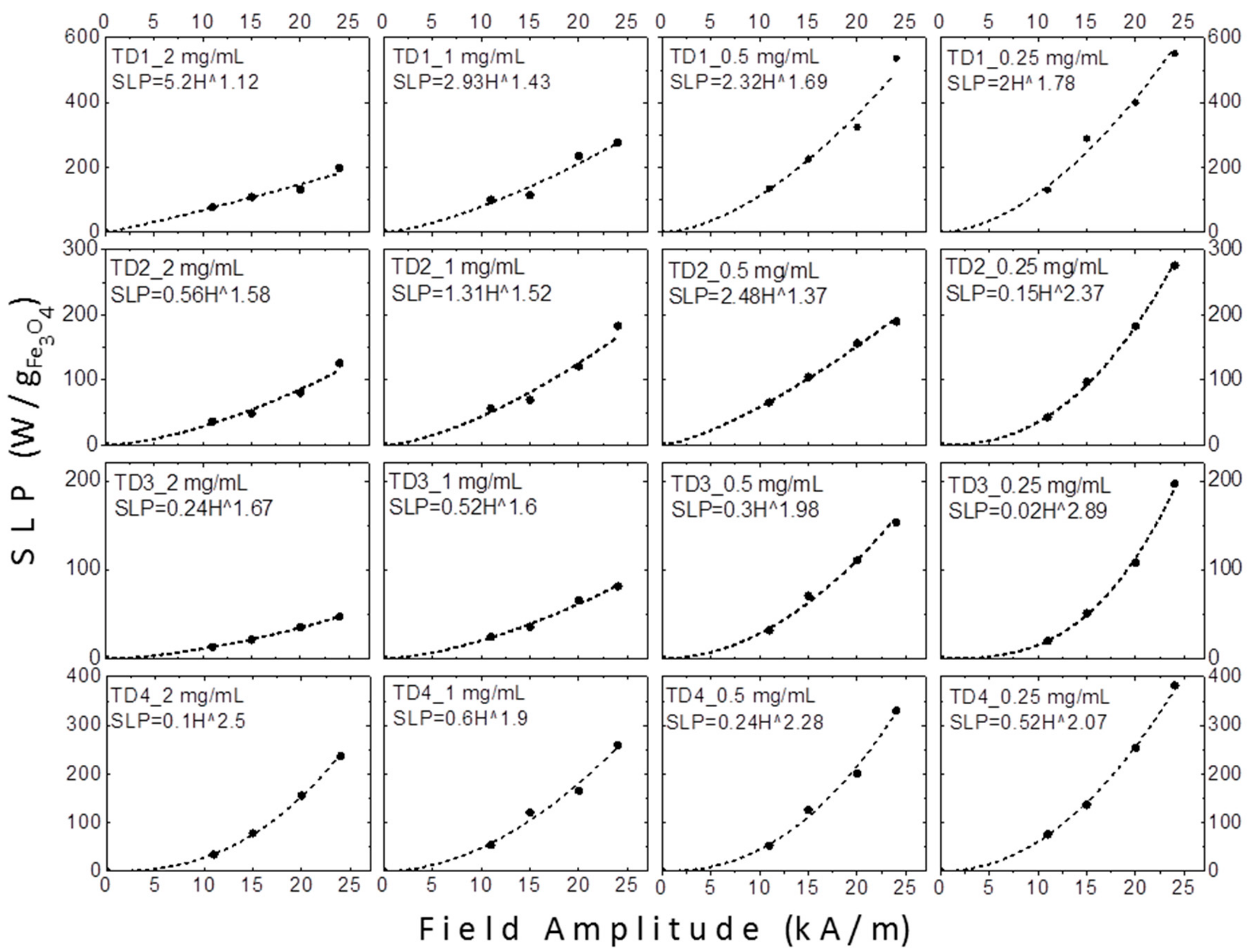
3.2.2. Magnetic Particle Hyperthermia
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stevens, P.D.; Fan, J.; Gardimalla, H.M.R.; Yen, M.; Gao, Y. Superparamagnetic Nanoparticle-Supported Catalysis of Suzuki Cross-Coupling Reactions. Org. Lett. 2005, 7, 2085–2088. [Google Scholar] [CrossRef]
- Li, L.; Fan, M.; Brown, R.C.; Van Leeuven, J.; Wang, J.; Wang, W.; Song, Y.; Zhang, P. Synthesis, Properties, and Environmental Applications of Nanoscale Iron-Based Materials: A Review. Crit. Rev. Environ. Sci. Technol. 2006, 36, 405–431. [Google Scholar] [CrossRef]
- Patel, D.; Moon, J.Y.; Chang, Y.; Kim, T.J.; Lee, G.H. Poly (D,L-lactide-co-glycolide) coated superparamagnetic iron oxide nanoparticles: Synthesis, characterization and in vivo study as MRI contrast agent. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313, 91–94. [Google Scholar] [CrossRef]
- Mohapatra, J.; Mitra, A.; Tyagi, H.; Bahadur, D.; Aslam, M. Iron Oxide Nanorods as High-Performance Magnetic Resonance Imaging Contrast Agents. Nanoscale 2015, 7, 9174–9184. [Google Scholar] [CrossRef] [PubMed]
- Bakoglidis, K.D.; Simeonidis, K.; Sakellari, D.; Stefanou, G.; Angelakeris, M. Size-dependent mechanisms in AC magnetic hyperthermia response of iron-oxide nanoparticles. IEEE Trans. Magn. 2012, 48, 1320–1323. [Google Scholar] [CrossRef]
- Martinez-Boubeta, C.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Serantes, D.; Baldomir, D.; Saghi, Z.; Midgley, P.A.; Conde-Leboran, I.; Serantes, D.; et al. Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep. 2013, 3, 1652. [Google Scholar] [CrossRef] [PubMed]
- Chourpa, I.; Douziech-Eyrolles, L.; Ngaboni-Okassa, L.; Fouquenet, J.-F.; Cohen-Jonathan, S.; Souce, M.; Marchais, H.; Dudois, P. Molecular composition of iron oxide nanoparticles, precursors for magnetic drug targeting, as characterized by confocal Raman microspectroscopy. Analyst 2005, 130, 1395–1403. [Google Scholar] [CrossRef]
- Jain, T.K.; Morales, M.A.; Sahoo, S.K.; Leslie-Pelecky, D.L.; Labhasetwar, V. Iron Oxide Nanoparticles for Sustained Delivery of Anticancer Agents. Mol. Pharm. 2005, 2, 194–205. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Xu, Z.; Sun, S. Controlled synthesis and chemical conversions of FeO nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 6329–6332. [Google Scholar] [CrossRef]
- Simeonidis, K.; Mourdikoudis, S.; Moulla, M.; Tsiaoussis, I.; Martinez-Boubeta, C.; Angelakeris, M.; Dendrinou-Samara, C.; Kalogirou, O. Controlled synthesis and phase characterization of Fe-based nanoparticles obtained by thermal decomposition. J. Magn. Magn. Mater. 2007, 316, 1–4. [Google Scholar] [CrossRef]
- Maity, D.; Kale, S.N.; Kaul-Ghanekar, R.; Xue, J.M.; Ding, J. Studies of magnetite nanoparticles synthesized by thermal decomposition of iron (III) acetylacetonate in tri(ethylene glycol). J. Magn. Magn. Mater. 2009, 321, 3093–3098. [Google Scholar] [CrossRef]
- Wan, J.; Cai, W.; Meng, X.; Liu, E. Monodisperse water-soluble magnetite nanoparticles prepared by polyol process for high-performance magnetic resonance imaging. Chem. Commun. 2007, 4, 5004–5006. [Google Scholar] [CrossRef]
- Chaianansutcharit, S.; Mekasuwandumrong, O.; Praserthdam, P. Synthesis of Fe2O3 nanoparticles in different reaction media. Ceram. Int. 2007, 33, 697–699. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, T.; Li, Y.; Zhao, Y.; Ye, Y.; Wu, W.; Tong, G. Effects of crystal size and sphere diameter on static magnetic and electromagnetic properties of monodisperse Fe3O4 microspheres. Mater. Chem. Phys. 2016, 173, 1–9. [Google Scholar] [CrossRef]
- Yuan, G.; Yuan, Y.; Xu, K.; Luo, Q. Biocompatible PEGylated Fe3O4 Nanoparticles as Photothermal Agents for Near-Infrared Light Modulated Cancer Therapy. Int. J. Mol. Sci. 2014, 15, 18776–18788. [Google Scholar] [CrossRef]
- Yan, A.; Liu, X.; Qiu, G.; Wu, H.; Yi, R.; Zhang, N.; Xu, J. Solvothermal synthesis and characterization of size-controlled Fe3O4 nanoparticles. J. Alloy. Compd. 2008, 458, 487–491. [Google Scholar] [CrossRef]
- Luo, J.; Wang, L.; Mott, D.; Njoki, P.N.; Kariuki, N.; Zhong, C.J.; He, T. Ternary alloy nanoparticles with controllable sizes and composition and electrocatalytic activity. J. Mater. Chem. 2006, 16, 1665–1673. [Google Scholar] [CrossRef]
- Shavel, A.; Rodríguez-González, B.; Spasova, M.; Farle, M.; Liz-Marzán, L.M. Synthesis and characterization of iron/iron oxide core/shell nanocubes. Adv. Funct. Mater. 2007, 17, 3870–3876. [Google Scholar] [CrossRef]
- Vijayakumar, R.; Koltypin, Y.; Felner, I.; Gedanken, A. Sonochemical synthesis and characterization of pure nanometer-sized Fe3O4 particles. Mater. Sci. Eng. A. 2000, 286, 101–105. [Google Scholar] [CrossRef]
- Jang, J.S.; Kim, H.G.; Reddy, V.R.; Bae, S.W.; Ji, S.M.; Lee, J.S. Photocatalytic water splitting over iron oxide nanoparticles intercalated in HTiNb(Ta)O5 layered compounds. J. Catal. 2005, 231, 213–222. [Google Scholar] [CrossRef]
- Park, S.; Ha, N.; Kim, J.; Kim, C. Size-controlled iron nanoparticles with lecithin for biomedical applications. J. Magn. Magn. Mater. 2006, 312, 386–389. [Google Scholar] [CrossRef]
- Miguel, O.B.; Gossuin, Y.; Morales, M.P.; Gillis, P.; Muller, R.N.; Veintemillas-Verdaguer, S. Comparative analysis of the 1H NMR relaxation enhancement produced by iron oxide and core-shell iron-iron oxide nanoparticles. Magn. Reson. Imaging 2007, 25, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Cabot, A.; Puntes, V.F.; Shevchenko, E.; Yin, Y.; Balcells, L.; Marcus, M.A.; Hughes, S.M.; Alivisatos, A.P. Vacancy Coalescence during Oxidation of Iron Nanoparticles. J. Am. Chem. Soc. 2007, 129, 10358–10360. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.; Lee, H.; Huang, Y.; Ko, I.; Kim, C. Control of Iron Nanoparticles Size and Shape by Thermal Decomposition Method. IEEE Trans. Magn. 2005, 41, 3388–3390. [Google Scholar] [CrossRef]
- Popovici, E.; Dumitrache, F.; Morjan, I.; Alexandrescu, R.; Ciupina, V.; Prodan, G.; Vekas, L.; Bica, D.; Marinica, O.; Vasile, E. Iron/iron oxides core-shell nanoparticles by laser pyrolysis: Structural characterization and enhanced particle dispersion. Appl. Surf. Sci. 2007, 254, 1048–1052. [Google Scholar] [CrossRef]
- Bonder, M.; Srinivasan, B.; Poirier, G.; Moriyama, T.; Kiick, K.L.; Hadjipanayis, G.C. In-vitro Heating With Polyethylene Glycol Coated Fe Nanoparticles. IEEE Trans. Magn. 2006, 97, 3602–3604. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Bonder, M.J.; Balakrishnan, S.; Wang, X.; Mao, H.; Hadjipanayis, G.C. Metallic iron nanoparticles for MRI contrast enhancement and local hyperthermia. Small 2008, 4, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Herzing, A.A.; Yan, W.; Li, X.; Koel, B.E.; Kiely, C.J.; Zhang, W.X. Determination of the oxide layer thickness in core-shell zerovalent iron nanoparticles. Langmuir 2008, 24, 4329–4334. [Google Scholar] [CrossRef]
- Zeng, Q.; Baker, I.; Loudis, J.A.; Liao, Y.; Hoopes, P.J.; Weaver, J.B. Fe/Fe oxide nanocomposite particles with large specific absorption rate for hyperthermia. Appl. Phys. Lett. 2007, 90, 233112–233114. [Google Scholar] [CrossRef]
- Somaskandan, K.; Veres, T.; Niewczas, M.; Simard, B. Surface protected and modified iron based core-shell nanoparticles for biological applications. New J. Chem. 2008, 32, 201–209. [Google Scholar] [CrossRef]
- Kotoulas, A.; Dendrinou-Samara, C.; Sarafidis, C.; Kehagias, H.; Arvanitidis, J.; Vourlias, G.; Angelakeris, M.; Kalogirou, O. Carbon-encapsulated cobalt nanoparticles: synthesis, properties, and magnetic particle hyperthermia efficiency. J. Nanoparticle Res. 2017, 19, 399. [Google Scholar] [CrossRef]
- Couto, G.G.; Klein, J.J.; Schreiner, W.H.; Mosca, D.H.; de Oliveira, A.J.A.; Zarbin, A.J.G. Nickel nanoparticles obtained by a modified polyol process: Synthesis, characterization, and magnetic properties. J. Colloids Interface Sci. 2007, 311, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Basti, H.; Tahar, L.B.; Smiri, L.S.; Herbst, F.; Vaulay, M.; Chau, F.; Ammar, S.; Benderbous, S. Catechol derivatives-coated Fe3O4 and γ-Fe2O3 nanoparticles as potential MRI contrast agents. J. Colloid Interface Sci. 2010, 341, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.H.; Cardoso, C.A.; Leite, E.R. Synthesis of colloidal magnetite nanocrystals using high molecular weight solvent. J. Mater. Chem. 2010, 20, 1167–1172. [Google Scholar] [CrossRef]
- Zhao, R.; Jia, K.; Wei, J.J.; Pu, J.X.; Liu, X.B. Hierarchically nanostructured Fe3O4 microspheres and their novel microwave electromagnetic properties. Mater. Lett. 2010, 64, 457–459. [Google Scholar] [CrossRef]
- Cao, S.-W.; Zhu, Y.; Ma, M.; Li, L.; Zhang, L. Hierarchically Nanostructured Magnetic Hollow Spheres of Fe3O4 and γ-Fe2O3: Preparation and Potential Application in Drug Delivery. J. Phys. Chem. C 2008, 112, 1851–1856. [Google Scholar] [CrossRef]
- Krishnan, K.M.; Pakhomov, A.B.; Bao, Y.; Blomqvist, P.; Chun, Y.; Gonzales, M.; Griffin, K.; Ji, X.; Roberts, B.K. Nanomagnetism and spin electronics: Materials, microstructure and novel properties. J. Mater. Sci. 2006, 41, 793–815. [Google Scholar] [CrossRef]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Stephen, Z.R.; Kievit, F.M.; Zhang, M. Magnetite nanoparticles for medical MR imaging. Mater. Today 2011, 14, 330–338. [Google Scholar] [CrossRef]
- Verges, M.A.; Costo, R.; Roca, A.G.; Marco, J.F.; Goya, G.F.; Serna, C.J.; Morales, M.P. Uniform and water stable magnetite nanoparticles with diameters around the monodomain–multidomain limit. J. Phys. D Appl. Phys. 2008, 41, 1343003–1343012. [Google Scholar]
- Hergt, R.; Dutz, S.; Muller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter. 2006, 18, 2919–2934. [Google Scholar] [CrossRef]
- Makridis, A.; Chatzitheodorou, I.; Topouridou, K.; Yavropoulou, M.P.; Angelakeris, M.; Dendrinou-Samara, C. A facile microwave synthetic route for ferrite nanoparticles with direct impact in magnetic particle hyperthermia. Mater. Sci. Eng. C 2016, 63, 663–670. [Google Scholar] [CrossRef]
- Hiergeist, R.; Andra, W.; Buske, N.; Hergt, R.; Hilger, I.; Richter, U.; Kaiser, W. Application of magnetite ferrofluids for hyperthermia. J. Magn. Magn. Mater. 1999, 201, 420–422. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Hergt, R.; Hiergeist, R.; Zeisberger, M.; Glockl, G.; Weitchies, W.; Ramires, L.P.; Hilger, I.; Kaiser, W.A. Enhancement of AC-losses of magnetic nanoparticles for heating applications. J. Magn. Magn. Mater. 2004, 280, 358–368. [Google Scholar] [CrossRef]
- Antonoglou, O.; Giannousi, K.; Arvanitidis, J.; Mourdikoudis, S.; Pantazaki, A.; Dendrinou-Samara, C. Elucidation of one step synthesis of PEGylated CuFe bimetallic nanoparticles. Antimicrobial activity of CuFe@PEG vs Cu@PEG. J. Inorg. Biochem. 2017, 177, 159–170. [Google Scholar] [CrossRef]
- Song, X.; Gao, L. Facile synthesis and hierarchical assembly of hollow nickel oxide architectures bearing enhanced photocatalytic properties. J. Phys. Chem. C 2008, 112, 15299–15305. [Google Scholar] [CrossRef]
- Qiao, R.; Zhang, X.L.; Qiu, R.; Kim, J.C.; Kang, Y.S. Preparation of Magnetic Hybrid Copolymer–Cobalt Hierarchical Hollow Spheres by Localized Ostwald Ripening. Chem. Mater. 2007, 19, 6485–6491. [Google Scholar] [CrossRef]
- Vamvakidis, K.; Katsikini, M.; Vourlias, G.; Angelakeris, M.; Paloura, E.C.; Dendrinou-Samara, C. Composition and hydrophicility control of Mn-doped ferrite (MnxFe3-xO4) nanoparticles induced by polyol differentiation. Dalton Trans. 2015, 44, 5396–5406. [Google Scholar] [CrossRef]
- Long, N.V.; Yang, Y.; Yuasa, M.; Thi, C.M.; Cao, Y.; Nann, T.; Nogami, M. Controlled synthesis and characterization of iron oxide nanostructures with potential applications for gas sensors and the environment. RSC Adv. 2014, 4, 6383–6390. [Google Scholar] [CrossRef]
- Shevchenko, E.V.; Talapin, D.V.; Schnablegger, H.; Kornowski, A.; Festin, O.; Svedlindh, P.; Haase, M.; Weller, H. Study of Nucleation and Growth in the Organometallic Synthesis of Magnetic Alloy Nanocrystals: The Role of Nucleation Rate in Size Control of CoPt3 Nanocrystals. J. Am. Chem. Soc. 2003, 125, 9090–9101. [Google Scholar] [CrossRef] [PubMed]
- De Dios, M.; Barroso, F.; Tojo, C.; Blanco, M.C.; López-Quintela, M.A. Effects of the reaction rate on the size control of nanoparticles synthesized in microemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2005, 270, 83–87. [Google Scholar] [CrossRef]
- Glavee, G.N.; Klabunde, K.J.; Sorensen, C.M.; Hadjipanayis, G.C. Chemistry of Borohydride Reduction of Iron (II) and Iron (III) Ions in Aqueous and Nonaqueous Media. Formation of Nanoscale Fe, FeB, and Fe2B Powders. Inorg. Chem. 1995, 34, 28–35. [Google Scholar] [CrossRef]
- Tao, A.R.; Habas, S.; Yang, P. Shape Control of Colloidal Metal Nanocrystals. Small 2008, 4, 310–325. [Google Scholar] [CrossRef]
- Chaikin, S.W.; Brown, W.G. Reduction of Aldehydes, Ketones and Acid Chlorides by Sodium Borohydride. J. Am. Chem. Soc. 1949, 71, 122–125. [Google Scholar] [CrossRef]
- Owusu, K.A.; Qu, L.; Li, J.; Wang, Z.; Zhao, K.; Yang, C.; Hercule, K.M.; Lin, C.; Shi, C.; Wei, Q.; et al. Low-crystalline iron oxide hydroxide nanoparticle anode for high-performance supercapacitors. Nat. Communiations 2017, 8, 14264. [Google Scholar] [CrossRef] [PubMed]
- Yathindranath, V.; Rebbouh, L.; Moore, D.F.; Miller, D.W.; Van Liero, J.; Hegmann, T. A versatile method for the reductive, one-pot synthesis of bare, hydrophilic and hydrophobic magnetite nanoparticles. Adv. Funct. Mater. 2011, 21, 1457–1464. [Google Scholar] [CrossRef]
- Shen, L.; Laibinis, P.E.; Hatton, T.A. Bilayer Surfactant Stabilized Magnetic Fluids: Synthesis and Interactions at Interfaces. Langmuir 1999, 15, 447–453. [Google Scholar] [CrossRef]
- Panda, R.N.; Gajbhiye, N.S.; Balaji, G. Magnetic properties of interacting single domain Fe3O4 particles. J. Alloy. Compd. 2001, 326, 50–53. [Google Scholar] [CrossRef]
- Skomski, R. Nanomagnetics. J. Phys. Condens. Matter. 2003, 15, 841–896. [Google Scholar] [CrossRef]
- Morales, M.P.; Veintemillas-Verdaguer, S.; Montero, M.I.; Serna, C.J.; Roig, A.; Casas, L.I.; Martinez, B.; Sandiumenge, F. Surface and Internal Spin Canting in γ-Fe2O3 Nanoparticles. Chem. Mater. 1999, 11, 3058–3064. [Google Scholar] [CrossRef]
- Lee, B.Y.; Lee, J.; Bae, C.J.; Park, J.-G.; Noh, H.-J.; Park, J.-H.; Hyeon, T. Large-Scale Synthesis of Uniform and Crystalline Magnetite Nanoparticles Using Reverse Micelles as Nanoreactors under Reflux Conditions. Adv. Funct. Mater. 2005, 15, 503–509. [Google Scholar] [CrossRef]
- Guardia, P.; Batlle-Brugal, B.; Roca, A.G.; Iglesias, O.; Morales, M.P.; Serna, C.J.; Labarta, A.; Battle, X. Surfactant effects in magnetite nanoparticles of controlled size. J. Magn. Magn. Mater. 2007, 316, 756–759. [Google Scholar] [CrossRef]
- Kittel, C.; Galt, J. Ferromagnetic Domain Theory. In Solid State Physics, 1st ed.; Seitz, F., Turnbull, D., Eds.; Academic Press Inc.: New York, NY, USA, 1956; Volume 3, pp. 437–564. [Google Scholar]
- Kittel, C. Theory of the Structure of Ferromagnetic Domains in Films and Small Particles. Phys. Rev. 1946, 70, 965–971. [Google Scholar] [CrossRef]
- Brown, W.F. The Fundamental Theorem of Fine-Ferromagnetic-Particle Theory. J. Appl. Phys. 1968, 39, 993–994. [Google Scholar] [CrossRef]
- Zhang, D.; Tong, Z.; Li, S.; Zhang, X.; Ying, A. Fabrication and characterization of hollow Fe3O4 nanospheres in a microemulsion. Mater. Lett. 2008, 62, 4053–4055. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, W.; Xiao, H.; Yang, Y.; Fu, S. Facile Synthesis of Metallic Co Hierarchical Nanostructured Microspheres by a Simple Solvothermal Process. J. Phys. Chem. C 2008, 112, 10073–10078. [Google Scholar] [CrossRef]
- Blanco-Mantecon, M.; O’Grady, K. Interaction and size effects in magnetic nanoparticles. J. Magn. Magn. Mater. 2006, 296, 124–133. [Google Scholar] [CrossRef]
- Nadeem, K.; Krenn, H.; Traussnig, T.; Wurschum, R.; Szabo, D.V.; Letofsky-Papst, I. Effect of dipolar and exchange interactions on magnetic blocking of maghemite nanoparticles. J. Magn. Magn. Mater. 2011, 323, 1998–2004. [Google Scholar] [CrossRef]
- Verdes, C.; Ruiz-Diaz, B.; Thompson, S.M.; Chantrell, R.W.; Stancu, A. Computational model of the magnetic and transport properties of interacting fine particles. Phys. Rev. B 2002, 65, 174417–174427. [Google Scholar] [CrossRef]
- Duong, B.; Khurshid, H.; Gangopadhyay, P.; Devkota, J.; Stojak, K.; Srikanth, H.; Tetard, L.; Norwood, R.A.; Peyghambarian, N.; Phan, M.H.; et al. Enhanced Magnetism in Highly Ordered Magnetite Nanoparticle-Filled Nanohole Arrays. Small 2014, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dormann, J.L.; Fiorani, D.; Tronc, E. On the models for interparticle interactions in nanoparticle assemblies: Comparison with experimental results. J. Magn. Magn. Mater. 1999, 202, 251–267. [Google Scholar] [CrossRef]
- Caruntu, D.; Caruntu, G.; O’Connor, C.J. Magnetic properties of variable-sized Fe3O4 nanoparticles synthesized from non-aqueous homogeneous solutions of polyols. J. Phys. D Appl. Phys. 2007, 40, 5801–5809. [Google Scholar] [CrossRef]
- Guimaraes, A.P. Principles of Nanomagnetism, 1st ed.; Springer: Heidelberg, Germany, 2009; pp. 57–104. [Google Scholar]
- Yamaura, M.; Camilo, R.L.; Sampaio, L.C.; Macedo, M.A.; Nakamura, M.; Toma, H.E. Preparation and characterization of (3-aminopropyl) triethoxysilane-coated magnetite nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217. [Google Scholar] [CrossRef]
- Dormann, J.L.; Fiorani, D.; Tronc, E. Magnetic relaxation in fine-particle systems. Adv. Chem. Phys. 1997, 98, 283–494. [Google Scholar]
- Makridis, A.; Curto, S.; van Rhoon, G.C.; Samaras, T.; Angelakeris, M. A standardization protocol for accurate evaluation of specific loss power in magnetic hyperthermia. J. Phys. D Appl. Phys. 2019, 52, 255001. [Google Scholar] [CrossRef]
- Simeonidis, K.; Martinez-Boubeta, C.; Balcells, L.; Monty, C.; Stavropoulos, G.; Mitrakas, M.; Matsakidou, A.; Vourlias, G.; Angelakeris, M. Fe-based nanoparticles as tunable magnetic particle hyperthermia agents. J. Appl. Phys. 2013, 114, 103904–103913. [Google Scholar] [CrossRef]
- Martinez-Βoubeta, C.; Simeonidis, K.; Serantes, D.; Conde-Leborán, I.; Kazakis, I.; Stefanou, G.; Peña, L.; Galceran, R.; Balcells, L.; Monty, C.; et al. Adjustable Hyperthermia Response of Self-Assembled Ferromagnetic Fe-MgO Core-Shell Nanoparticles by Tuning Dipole–Dipole Interactions. Adv. Funct. Mater. 2012, 22, 3737–3744. [Google Scholar] [CrossRef]
- Phong, P.T.; Nguyen, L.H.; Manh, D.H.; Lee, I.-J.; Phuc, N.X. Computer Simulations of Contributions of Neel and Brown Relaxation to Specific Loss Power of Magnetic Fluids in Hyperthermia. J. Electron. Mater. 2017, 46, 2393–2405. [Google Scholar] [CrossRef]
- Khandhar, A.P.; Ferguson, R.M.; Simon, J.A.; Krishnan, K.M. Tailored magnetic nanoparticles for optimizing magnetic fluid hyperthermia. J. Biomed. Mater. Res. 2011, 100, 728–737. [Google Scholar] [CrossRef] [Green Version]
- Périgo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and advances in magnetic hyperthermia. Appl. Phys. Rev. 2015, 2, 041302. [Google Scholar] [CrossRef] [Green Version]
- Angelakeris, Μ. Magnetic nanoparticles: A multifunctional vehicle for modern theranostics. Biochim. Biophys. Acta BBA Gen. Subj. 2017, 1861, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Myrovali, E.; Maniotis, N.; Makridis, A.; Terzopoulou, A.; Ntomprougkidis, V.; Simeonidis, K.; Sakellari, D.; Kalogirou, O.; Samaras, T.; Salikhov, R.; et al. Arrangement at the nanoscale: Effect on magnetic particle hyperthermia. Sci. Rep. 2016, 6, 37934. [Google Scholar] [CrossRef] [PubMed]
- Carrey, J.; Mehdaoui, B.; Respaud, M. Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: Application to magnetic hyperthermia optimization. J. Appl. Phys. 2011, 109, 083921–083939. [Google Scholar] [CrossRef]




| Sample | Initial Components in Flask | Hot Injection (100 °C) | Final Temperature (°C) |
|---|---|---|---|
| TD1 | 5 mmol Fe(acac)3 300 mmol TrEG (40 mL) 5 mmol NaBH4 | - | 260 |
| TD2 | 2.5 mmol Fe(acac)3 20 mmol PEG-1000 (20 g) | 2.5 mmol NaBH4 | 260 |
| TD3 | 2.5 mmol PEG-8000 (20 g) | 2.5 mmol Fe(acac)3 2.5 mmol NaBH4 | 260 |
| TD4 | 2.5 mmol Fe(acac)3 150 mmol TrEG (20 mL) 2.5 mmol N2H4·H2O | - | 260 |
| Sample | Reactants | Temperature (°C) |
|---|---|---|
| SV5 | 1.5 mmol Fe(acac)3 75 mmol TrEG (10 mL) | 200 |
| SV6 | 1.25 mmol Fe(acac)3 10 mmol PEG-1000 (10 g) | 200 |
| SV7 | 1.25 mmol Fe(acac)3 1.25 mmol PEG-8000 (10 g) | 200 |
| TD Samples | Nanoparticles’ Mean Size (nm) | Crystallites’ Mean Size (nm) | SV Samples | Crystallites’ Mean Size (nm) |
|---|---|---|---|---|
| TD1 (TrEG) | 12.8 (±3.3) | 11.8 | SV5 (TrEG) | 8.6 |
| TD2 (PEG-1000) | 7.7 (±1.6) | 8.1 | SV6 (PEG-1000) | 13.7 |
| TD3 (PEG-8000) | 4.1 (±1.0) | 4 | SV7 (PEG-8000) | 18.7 |
| TD4 (TrEG/hydrazine) | 10.5 (±2.2) | 11.9 | - | - |
| Sample | Ms (Am2/kgFe3O4) | Mr (Am2/kgFe3O4) | Mr/Ms (%) | Hc (mT) | Mean Size (nm) by TEM |
|---|---|---|---|---|---|
| TD1 | 73.7 | 34.2 | 46.3 | 17.8 | 12.8 |
| TD2 | 40.2 | 11.3 | 28.2 | 12.2 | 7.70 |
| TD3 | 12.6 | 0.9 | 6.80 | 18.4 | 4.10 |
| TD4 | 66.0 | 6.20 | 9.40 | 14.0 | 10.5 |
| Sample | Ms (Am2/kg) | Mr (Am2/kg) | Mr/Ms (%) | Hc (mT) | Crystallite Size (nm) by Scherrer’s Formula |
|---|---|---|---|---|---|
| SV5 | 64.1 | 16.2 | 25.3 | 17.6 | 8.60 |
| SV6 | 53.3 | 16.1 | 30.2 | 17.8 | 13.7 |
| SV7 | 46.3 | 11.0 | 23.8 | 18.5 | 18.7 |
| Sample | Ms (Am2/kg) | Precursor mol/ Surfactant mol | Sample | Ms (Am2/kg) | Precursor mol/Surfactant mol |
|---|---|---|---|---|---|
| TD1 | 65.6 | 5: 300 | SV5 | 64.1 | 1.25: 75 |
| TD2 | 34 | 2.5: 20 | SV6 | 53.3 | 1.25: 10 |
| TD3 | 10.3 | 2.5: 2.5 | SV7 | 46.3 | 1.25:1.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotoulas, A.; Dendrinou-Samara, C.; Angelakeris, M.; Kalogirou, O. The Effect of Polyol Composition on the Structural and Magnetic Properties of Magnetite Nanoparticles for Magnetic Particle Hyperthermia. Materials 2019, 12, 2663. https://doi.org/10.3390/ma12172663
Kotoulas A, Dendrinou-Samara C, Angelakeris M, Kalogirou O. The Effect of Polyol Composition on the Structural and Magnetic Properties of Magnetite Nanoparticles for Magnetic Particle Hyperthermia. Materials. 2019; 12(17):2663. https://doi.org/10.3390/ma12172663
Chicago/Turabian StyleKotoulas, Anastasios, Catherine Dendrinou-Samara, Mavroeidis Angelakeris, and Orestis Kalogirou. 2019. "The Effect of Polyol Composition on the Structural and Magnetic Properties of Magnetite Nanoparticles for Magnetic Particle Hyperthermia" Materials 12, no. 17: 2663. https://doi.org/10.3390/ma12172663






