Wear Resistant Coatings with a High Friction Coefficient Produced by Plasma Electrolytic Oxidation of Al Alloys in Electrolytes with Basalt Mineral Powder Additions
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Basalt Characterisation
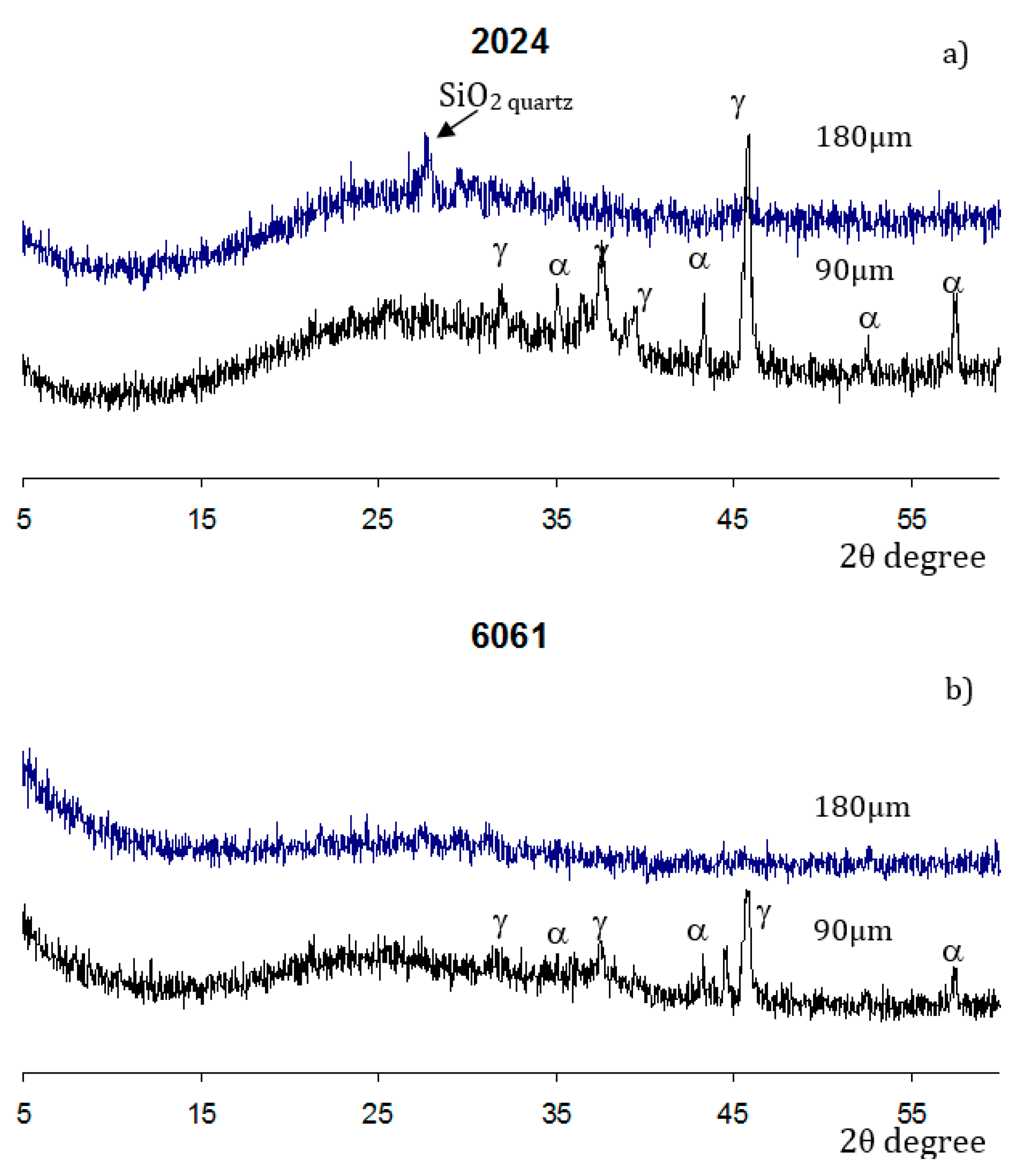
3.2. Coatings Structure and Composition
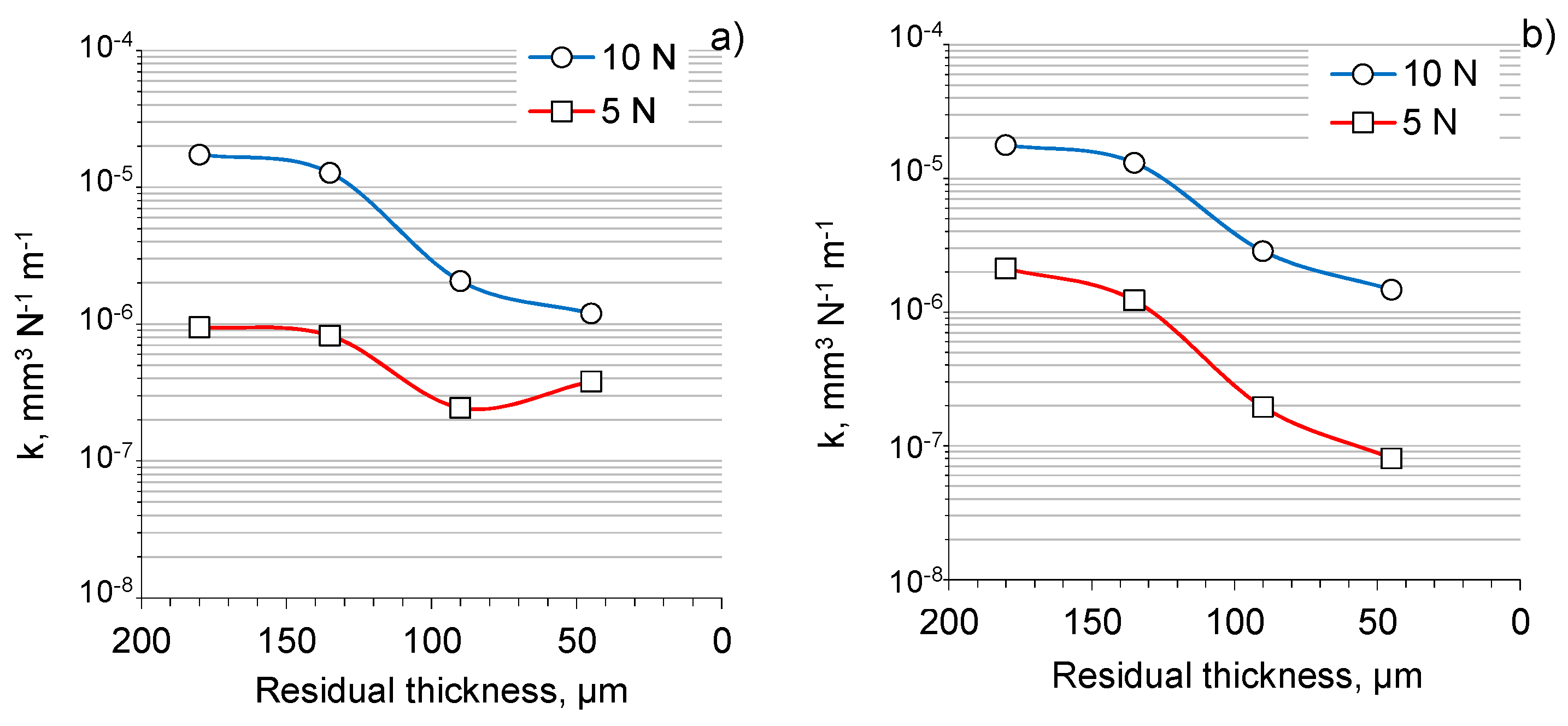
3.3. Tribological Properties
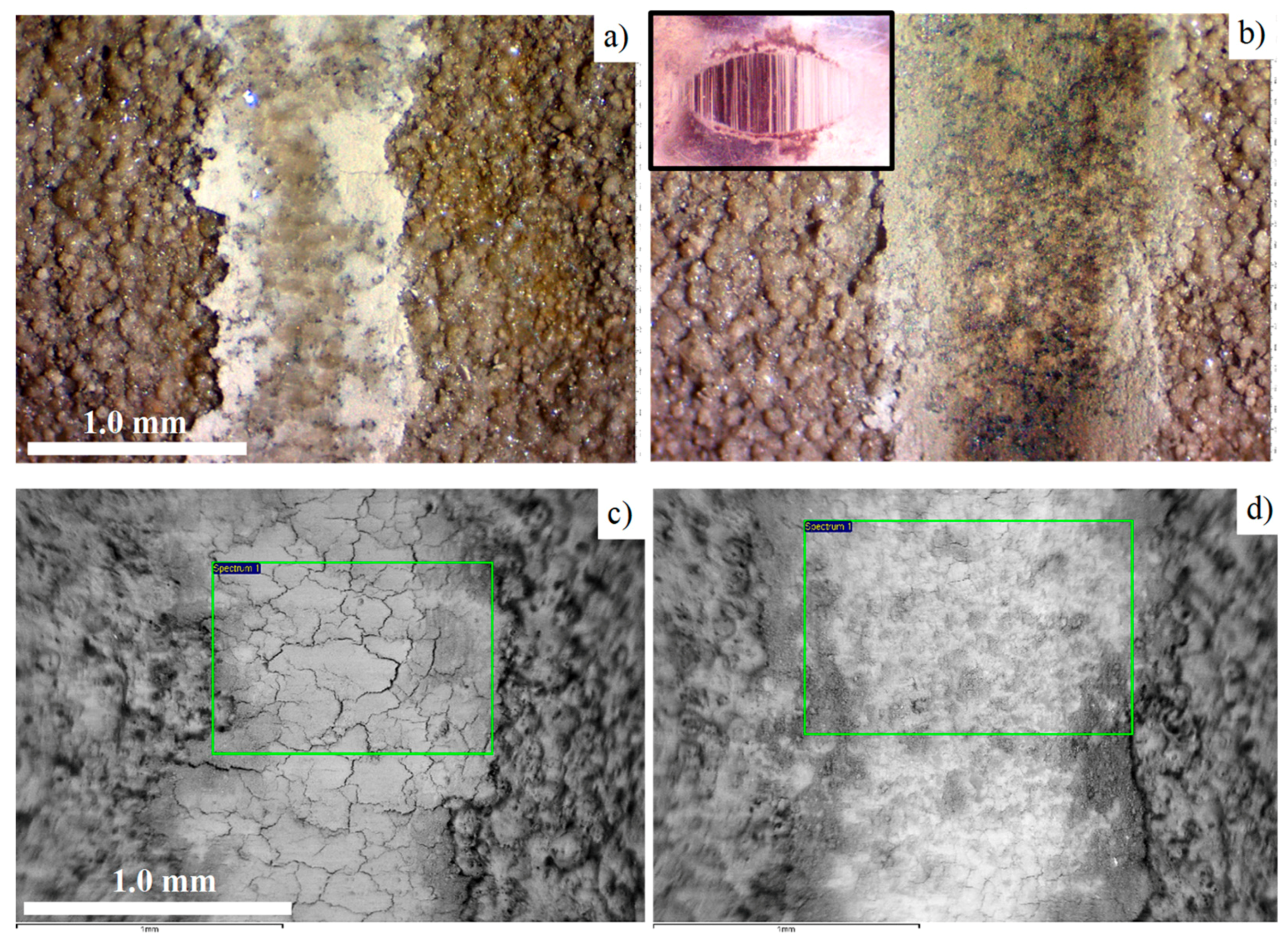
3.4. Analysis of the Modified Layer Wear Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kelly, J.C.; Sullivan, J.L.; Burnham, A.; Elgowainy, A. Impacts of Vehicle Weight Reduction via Material Substitution on Life-Cycle Greenhouse Gas Emissions. Environ. Sci. Technol. 2015, 49, 12535–12542. [Google Scholar] [CrossRef] [PubMed]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Walsh, F.C.; Low, C.T.J.; Wood, R.J.K.; Stevens, K.T.; Archer, J.; Poeton, A.R.; Ryder, A. Plasma electrolytic oxidation (PEO) for production of anodised coatings on lightweight metal (Al, Mg, Ti) alloys. Trans. IMF 2009, 87, 122–135. [Google Scholar] [CrossRef]
- Rao, V.D.N.; Cikanek, H.A.; Boyer, B.A.; Lesnevsky, L.N.; Tchernovsky, N.M.; Tjurin, N.V. Friction and Wear Characteristics of Micro-Arc Oxidation Coating for Light Weight, Wear Resistant, Powertrain Component Application. SAE Tech. Pap. 1997. [Google Scholar] [CrossRef]
- Krzymien, A.; Krzymien, P. Application of aluminum alloys for combustion engine bearings. J. KONES Powertrain Transp. 2007, 14, 233–238. [Google Scholar]
- Mistry, K.; Priest, M.; Shrestha, S. The potential of plasma electrolytic oxidized eutectic aluminium–silicon alloy as a cylinder wall surface for lightweight engine blocks. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2010, 224, 221–229. [Google Scholar] [CrossRef]
- Javidi, M.; Fadaee, H. Plasma electrolytic oxidation of 2024-T3 aluminum alloy and investigation on microstructure and wear behavior. Appl. Surf. Sci. 2013, 286, 212–219. [Google Scholar] [CrossRef]
- Malyshev, V.N.; Markov, G.A.; Fedorov, V.A.; Petrosyants, A.A.; Terleeva, O.P. Features of the structure and properties of coatings applied by the method of microarc oxidation. Chem. Pet. Eng. 1984, 20, 41–43. [Google Scholar] [CrossRef]
- Nie, X.; Wang, L.; Konca, E.; Alpas, A.T. Tribological behaviour of oxide/graphite composite coatings deposited using electrolytic plasma process. Surf. Coat. Technol. 2004, 188, 207–213. [Google Scholar] [CrossRef]
- Tseng, C.-C.; Lee, J.-L.; Kuo, T.-H.; Kuo, S.-N.; Tseng, K.-H. The influence of sodium tungstate concentration and anodizing conditions on microarc oxidation (MAO) coatings for aluminum alloy. Surf. Coat. Technol. 2012, 206, 3437–3443. [Google Scholar] [CrossRef]
- Malyshev, V.N. Modification of Friction Knots Work Surfaces on the Basis of Microarc Oxidation Method. Int. J. Sci. Res. Sci. Eng. Technol. 2016, 2, 464–480. [Google Scholar]
- Martini, C.; Ceschini, L.; Tarterini, F.; Paillard, J.M.; Curran, J.A. PEO layers obtained from mixed aluminate–phosphate baths on Ti–6Al–4V: Dry sliding behaviour and influence of a PTFE topcoat. Wear 2010, 269, 747–756. [Google Scholar] [CrossRef]
- Tian, J.; Luo, Z.; Qi, S.; Sun, X. Structure and antiwear behavior of micro-arc oxidized coatings on aluminum alloy. Surf. Coat. Technol. 2002, 154, 1–7. [Google Scholar] [CrossRef]
- Ceschini, L.; Martini, C.; Sambogna, G.; Tarterini, F. Dry Sliding Behaviour of Peo (Plasma Electrolytic Oxidation) Treated AA 2618/20% Al2O3p Composite. Mater. Sci. Forum 2011, 678, 61–74. [Google Scholar] [CrossRef]
- Ovundur, M.; Muhaffel, F.; Cimenoglu, H. Characterization and Tribological Properties of Hard Anodized and Micro Arc Oxidized 5754 Quality Aluminum Alloy. Tribol. Ind. 2015, 37, 55–59. [Google Scholar]
- Alnaqi, A.A.; Shrestha, S.; Barton, D.C.; Brooks, P.C. Optimisation of Alumina Coated Lightweight Brake Rotor. SAE Tech. Pap. 2014. [Google Scholar] [CrossRef] [Green Version]
- Alnaqi, A.A.; Kosarieh, S.; Barton, D.C.; Brooks, P.C.; Shrestha, S. Material characterisation of lightweight disc brake rotors. Proc. Inst. Mech. Eng. Part L: J. Mater. Des. Appl. 2018, 232, 555–565. [Google Scholar] [CrossRef]
- Xue, W.; Du, J.; Wu, X.; Lai, Y. Tribological Behavior of Microarc Oxidation Coatings on Aluminum Alloy. ISIJ Int. 2006, 46, 287–291. [Google Scholar] [CrossRef]
- Nie, X.; Leyland, A.; Song, H.; Yerokhin, A.L.; Dowey, S.J.; Matthews, A. Thickness effects on the mechanical properties of micro-arc discharge oxide coatings on aluminium alloys. Surf. Coat. Technol. 1999, 116, 1055–1060. [Google Scholar] [CrossRef]
- Godja, N.; Kiss, N.; Löcker, C.; Schindel, A.; Gavrilović, A.; Wosik, J.; Mann, R.; Wendrinsky, J.; Merstallinger, A.; Nauer, G.E. Preparation and characterization of spark-anodized Al-alloys: Physical, chemical and tribological properties. Tribol. Int. 2010, 43, 1253–1261. [Google Scholar] [CrossRef]
- Garshin, A.P.; Kulik, V.I.; Nilov, A.S. Braking friction materials based on fiber-reinforced composites with carbon and ceramic matrices. Refract. Ind. Ceram. 2008, 49, 391–396. [Google Scholar] [CrossRef]
- Guan, Q.F.; Li, G.Y.; Wang, H.Y.; An, J. Friction-wear characteristics of carbon fiber reinforced. J. Mater. Sci. 2004, 39, 641–643. [Google Scholar] [CrossRef]
- Bijwe, J. Composites as friction materials: Recent developments in non-asbestos fiber reinforced friction materials—A review. Polym. Compos. 1997, 18, 378–396. [Google Scholar] [CrossRef]
- Yi, G.; Yan, F. Mechanical and tribological properties of phenolic resin-based friction composites filled with several inorganic fillers. Wear 2007, 262, 121–129. [Google Scholar] [CrossRef]
- Terleeva, O.P.; Slonova, A.I.; Belevantsev, V.I.; Kireenko, I.B.; Ryzhikh, A.P. Correlations of electrolyte state and characteristics of microplasma coatings with quantity of transmitted electricity. Prot. Met. Phys. Chem. Surf. 2011, 47, 80–85. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; O Snizhko, L.; Gurevina, N.L.; Leyland, A.; Pilkington, A.; Matthews, A. Discharge characterization in plasma electrolytic oxidation of aluminium. J. Phys. D Appl. Phys. 2003, 36, 2110–2120. [Google Scholar] [CrossRef]
- Singha, K. A Short Review on Basalt Fiber. Int. J. Text. Sci. 2012, 1, 19–28. [Google Scholar]
- Gaetani, G.A.; Grove, T.L.; Bryan, W.B. The influence of water on the petrogenesis of subductionrelated igneous rocks. Nature 1993, 365, 332–334. [Google Scholar] [CrossRef]
- Malyshev, V.; Zorin, K. Features of microarc oxidation coatings formation technology in slurry electrolytes. Appl. Surf. Sci. 2007, 254, 1511–1516. [Google Scholar] [CrossRef]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions – A review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Borisov, A.M.; Krit, B.L.; Lyudin, V.B.; Morozova, N.V.; Suminov, I.V.; Apelfeld, A.V. Microarc oxidation in slurry electrolytes: A review. Surf. Eng. Appl. Electrochem. 2016, 52, 50–78. [Google Scholar] [CrossRef]
- Fatimah, S.; Kamil, M.; Kwon, J.; Kaseem, M.; Ko, Y. Dual incorporation of SiO2 and ZrO2 nanoparticles into the oxide layer on 6061 Al alloy via plasma electrolytic oxidation: Coating structure and corrosion properties. J. Alloy. Compd. 2017, 707, 358–364. [Google Scholar] [CrossRef]
- Song, J.-H.; Nam, K.-S.; Moon, J.-I.; Choi, Y.-J.; Lim, D.-Y. Influence of the duty cycle on structural and mechanical properties of oxide layers on Al-1050 by a plasma electrolytic oxidation process. Met. Mater. Int. 2014, 20, 451–458. [Google Scholar] [CrossRef]
- Wang, J.-H.; Du, M.-H.; Han, F.-Z.; Yang, J. Effects of the ratio of anodic and cathodic currents on the characteristics of micro-arc oxidation ceramic coatings on Al alloys. Appl. Surf. Sci. 2014, 292, 658–664. [Google Scholar] [CrossRef]
- Hakimizad, A.; Raeissi, K.; Golozar, M.A.; Lu, X.; Blawert, C.; Zheludkevich, M.L. The effect of pulse waveforms on surface morphology, composition and corrosion behavior of Al2O3 and Al2O3 /TiO2 nano-composite PEO coatings on 7075 aluminum alloy. Surf. Coat. Technol. 2017, 324, 208–221. [Google Scholar] [CrossRef]
- Sonova, A.I.; Terleeva, O.P. Morphology, structure, and phase composition of microplasma coatings formed on Al-Cu-Mg alloy. Prot. Met. 2008, 44, 65–75. [Google Scholar] [CrossRef]
- Curran, J.; Clyne, T. Thermo-physical properties of plasma electrolytic oxide coatings on aluminium. Surf. Coat. Technol. 2005, 199, 168–176. [Google Scholar] [CrossRef]






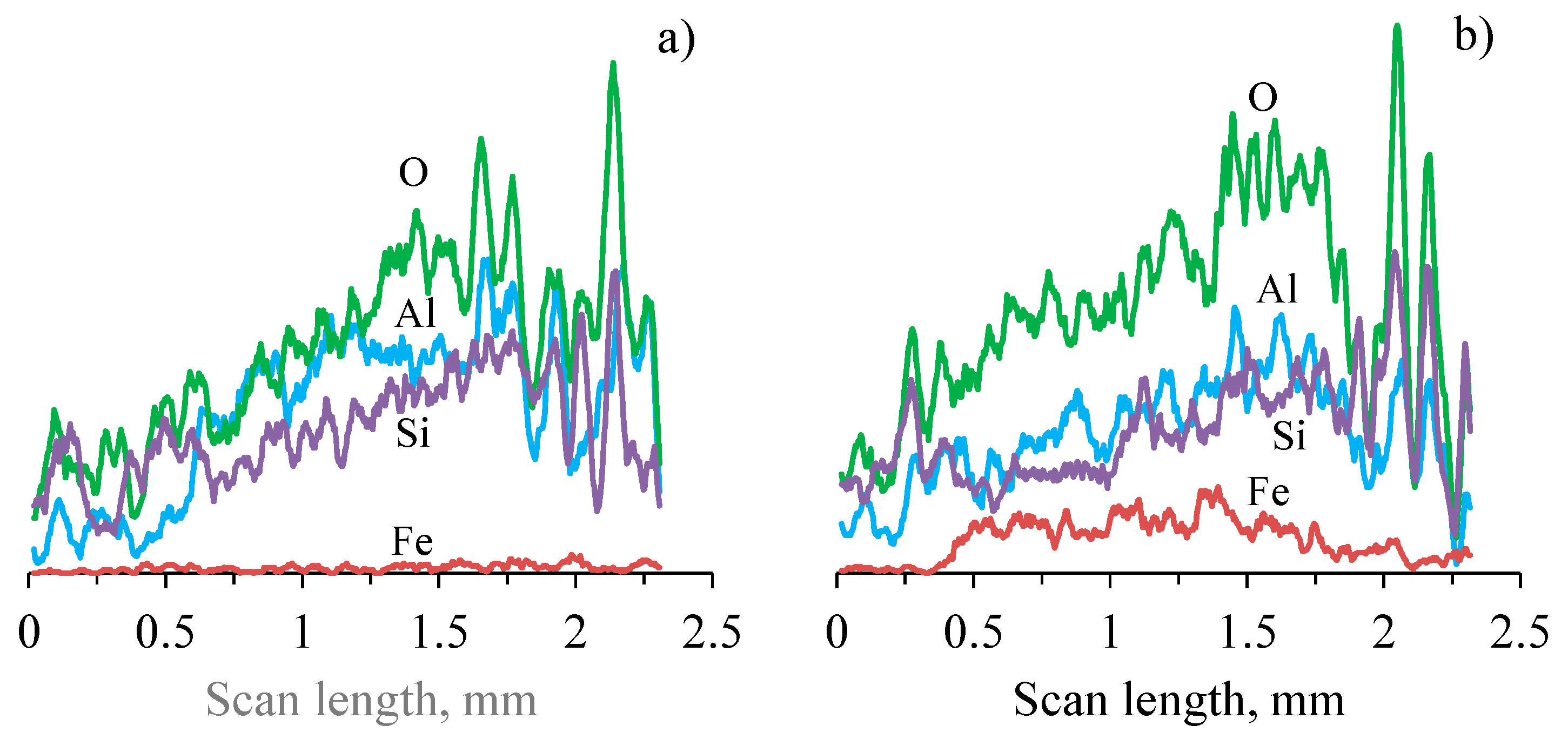
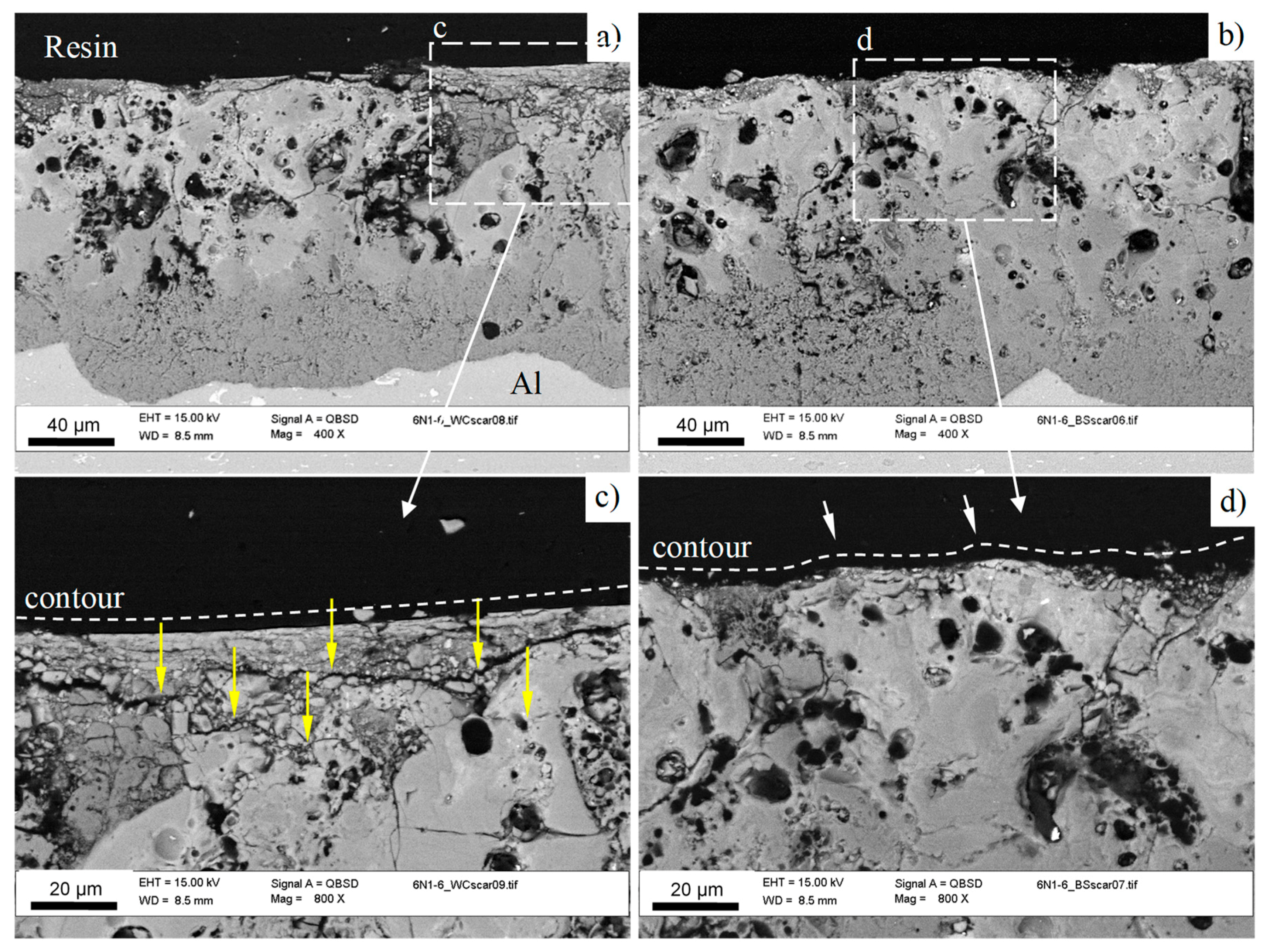
| Element | O | Na | Mg | Al | Si | S | K | Ca | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| at. % | 65.3 | 1.7 | 3.1 | 5.0 | 16.8 | 0.1 | 0.1 | 3.9 | 0.5 | 3.5 |
| Element | O | Na | Mg | Al | Si | P | K | Ca | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 2024, at. % | 65.9 | 6 | 1.1 | 7.6 | 8.8 | 5.2 | 1.7 | 1.6 | 2.1 |
| 6061, at. % | 67.2 | 4.1 | 0.9 | 5.5 | 13.1 | 3.3 | 1.9 | 1.7 | 2.3 |
| Element | O | Na | Mg | Al | Si | P | K | Ca | Ti | Cr | Fe | W |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BS, at. % | 69.0 | 4.2 | 0.7 | 8.2 | 6.7 | 4.3 | 1.3 | 1.0 | 0.1 | 0.1 | 4.3 | 0.0 |
| WC, at. % | 69.2 | 5.0 | 0.9 | 9.5 | 7.1 | 4.8 | 1.4 | 0.9 | 0.1 | 0.0 | 0.9 | 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terleeva, O.P.; Slonova, A.I.; Rogov, A.B.; Matthews, A.; Yerokhin, A. Wear Resistant Coatings with a High Friction Coefficient Produced by Plasma Electrolytic Oxidation of Al Alloys in Electrolytes with Basalt Mineral Powder Additions. Materials 2019, 12, 2738. https://doi.org/10.3390/ma12172738
Terleeva OP, Slonova AI, Rogov AB, Matthews A, Yerokhin A. Wear Resistant Coatings with a High Friction Coefficient Produced by Plasma Electrolytic Oxidation of Al Alloys in Electrolytes with Basalt Mineral Powder Additions. Materials. 2019; 12(17):2738. https://doi.org/10.3390/ma12172738
Chicago/Turabian StyleTerleeva, Olga P., Aleksandra I. Slonova, Aleksey B. Rogov, Allan Matthews, and Aleksey Yerokhin. 2019. "Wear Resistant Coatings with a High Friction Coefficient Produced by Plasma Electrolytic Oxidation of Al Alloys in Electrolytes with Basalt Mineral Powder Additions" Materials 12, no. 17: 2738. https://doi.org/10.3390/ma12172738





