Adhesion Behaviour of Primary Human Osteoblasts and Fibroblasts on Polyether Ether Ketone Compared with Titanium under In Vitro Lipopolysaccharide Incubation
Abstract
:1. Introduction
- When using PEEK, cell adhesion takes a place that is similar to that of titanium.
- The cellular inflammation behaviour towards PEEK is lower than that with titanium under LPS incubation.
2. Materials and Methods
2.1. Cell Seeding
2.2. Scanning Electron Microscopy (SEM)
2.3. Real-Time Polymerase Chain Reaction (PCR)
2.4. Immunocytochemical Marking
- SEM analysis: 2.5% glutaraldehyde (Carl Roth, Karlsruhe, Germany; in PBS) for 30 min;
- RT-PCR: no fixation; lysis of the cells by trizol (QIAzol, Qiagen, Hilden, Germany);
- Immunocytochemical marking:
- ○
- for LBP/TLR4 = 4% Paraformaldehyd solution (4% PFA in PBS) for 30 min;
- ○
- for phalloidin/vinculin = 3.7% PFA (+10% Methanol; Merk Millipore, Schwalbach, Germany) + 0.2% Triton X100-solution for 30 min.
3. Results
3.1. Scanning Electron Microscope Images
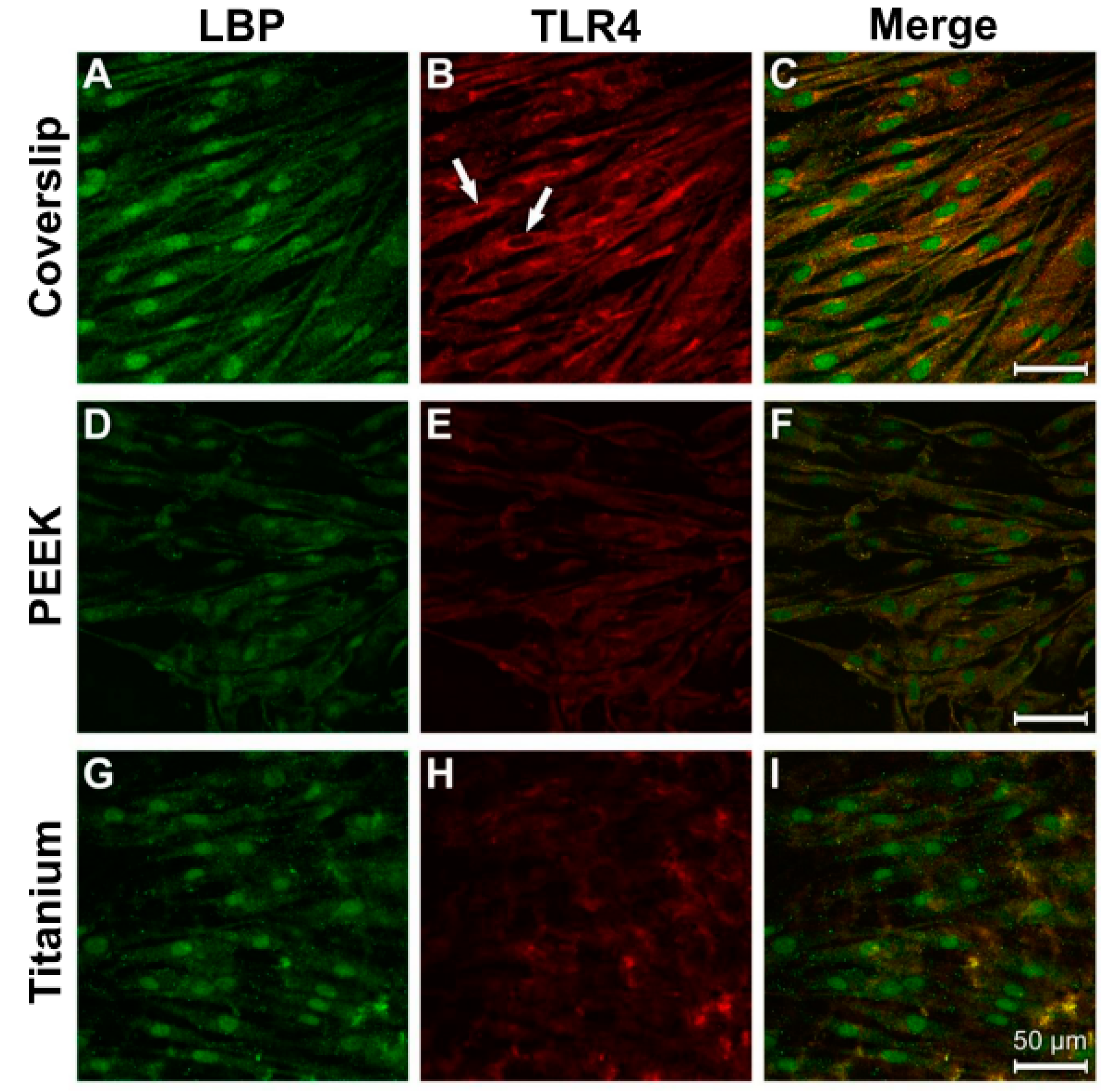
3.2. Real-Time PCR Testing for Expression of LBP and TLR4
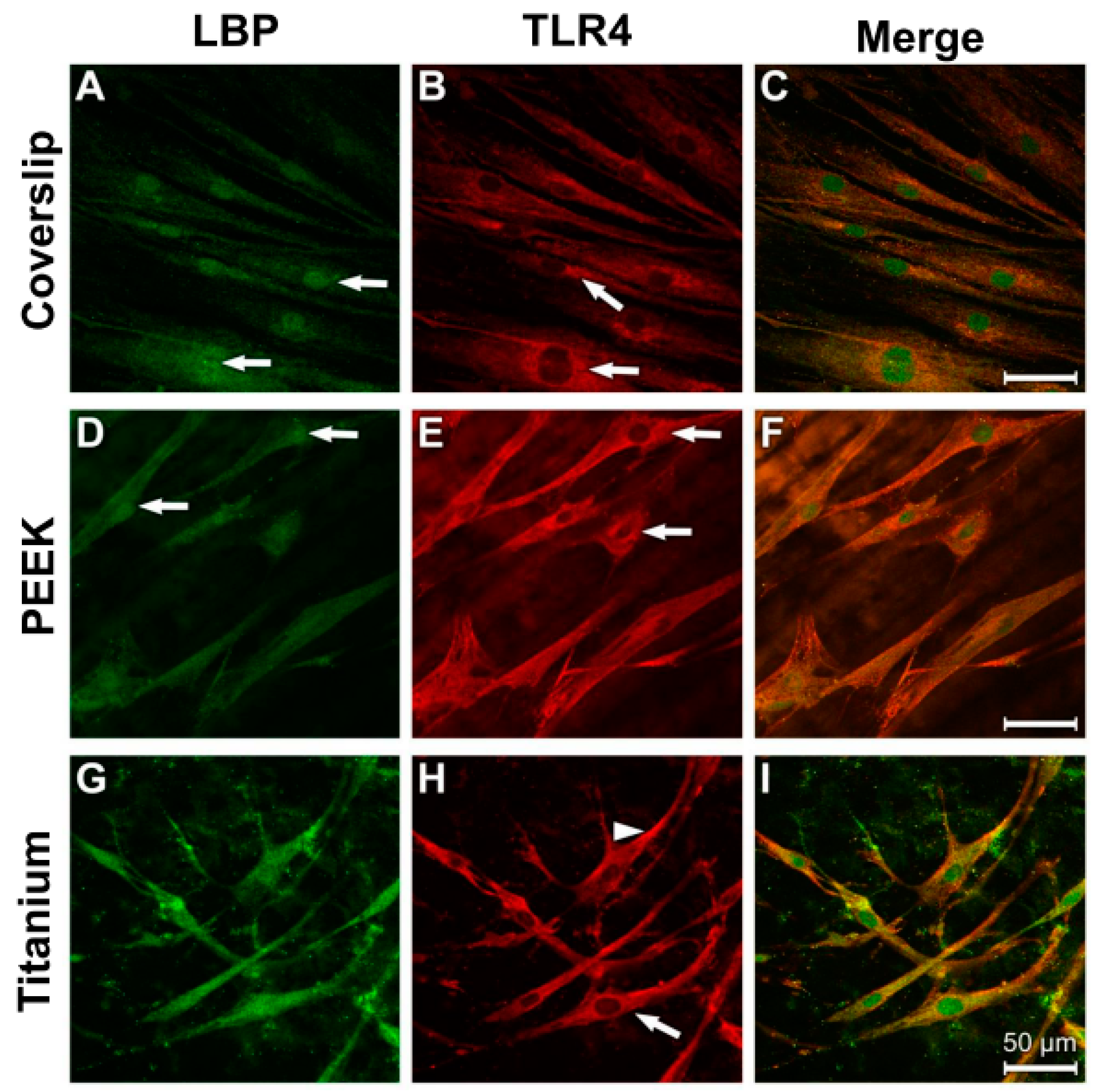
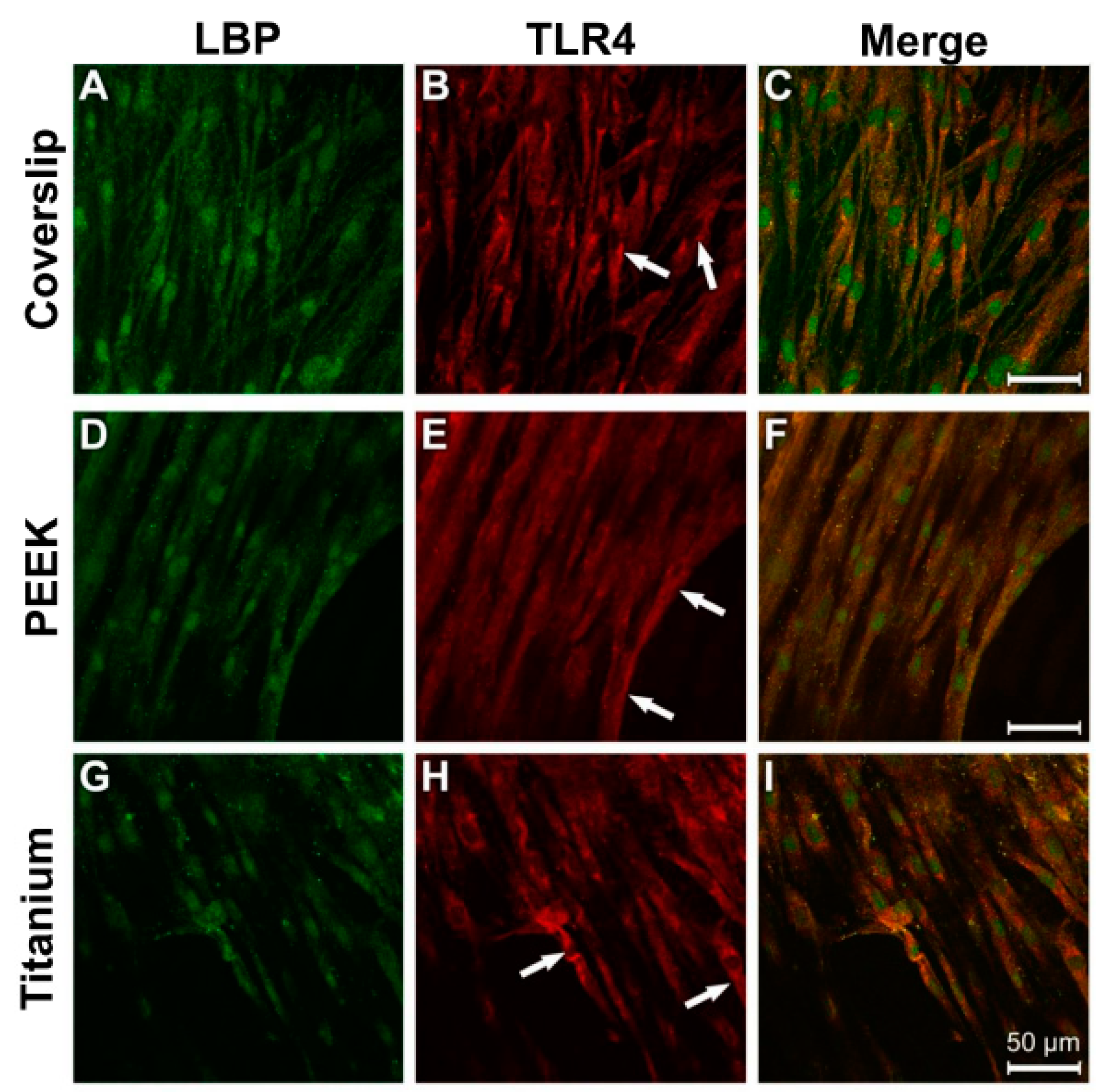
3.3. Evidence of Protein Expression of LBP and TLR4
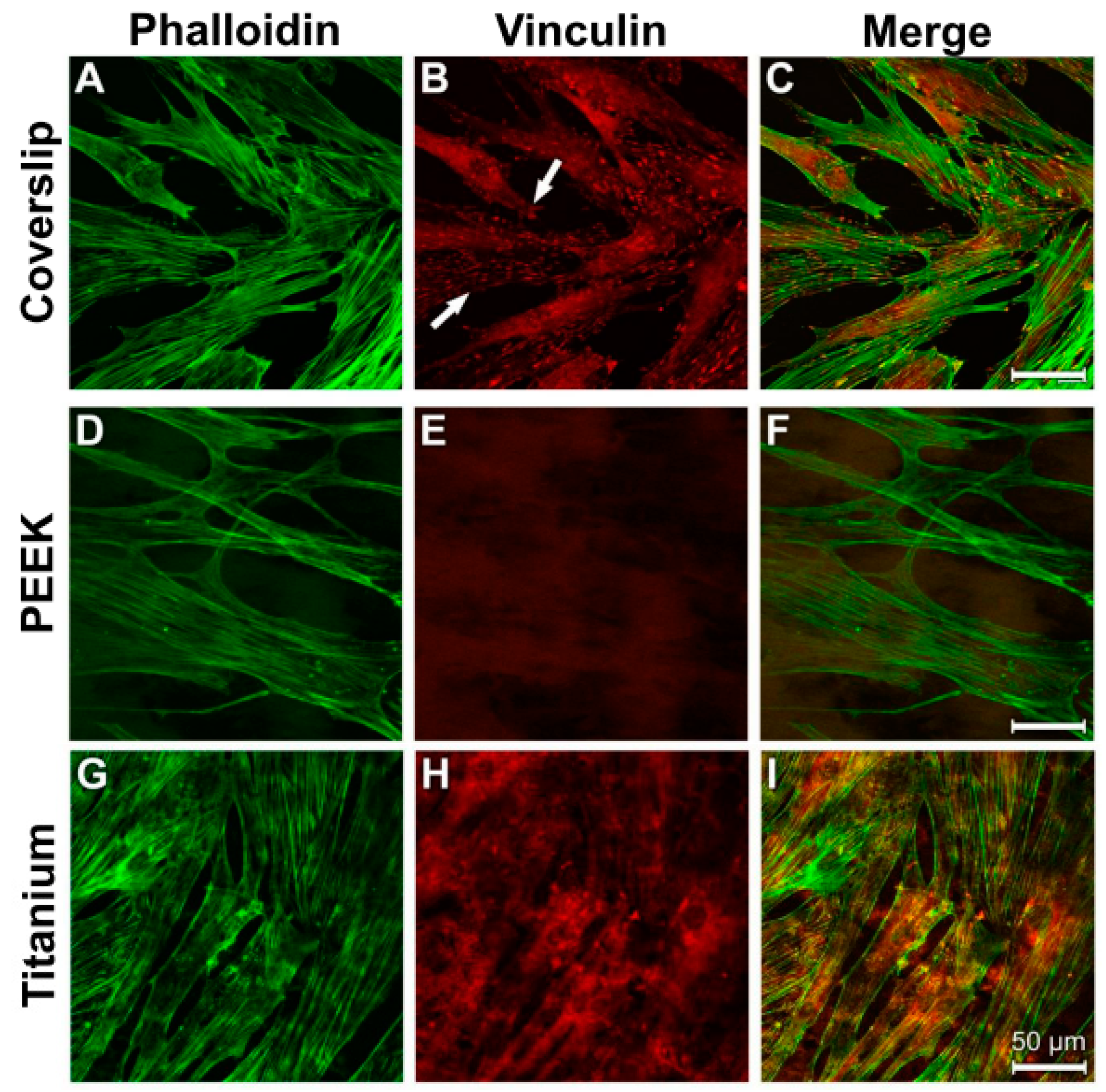
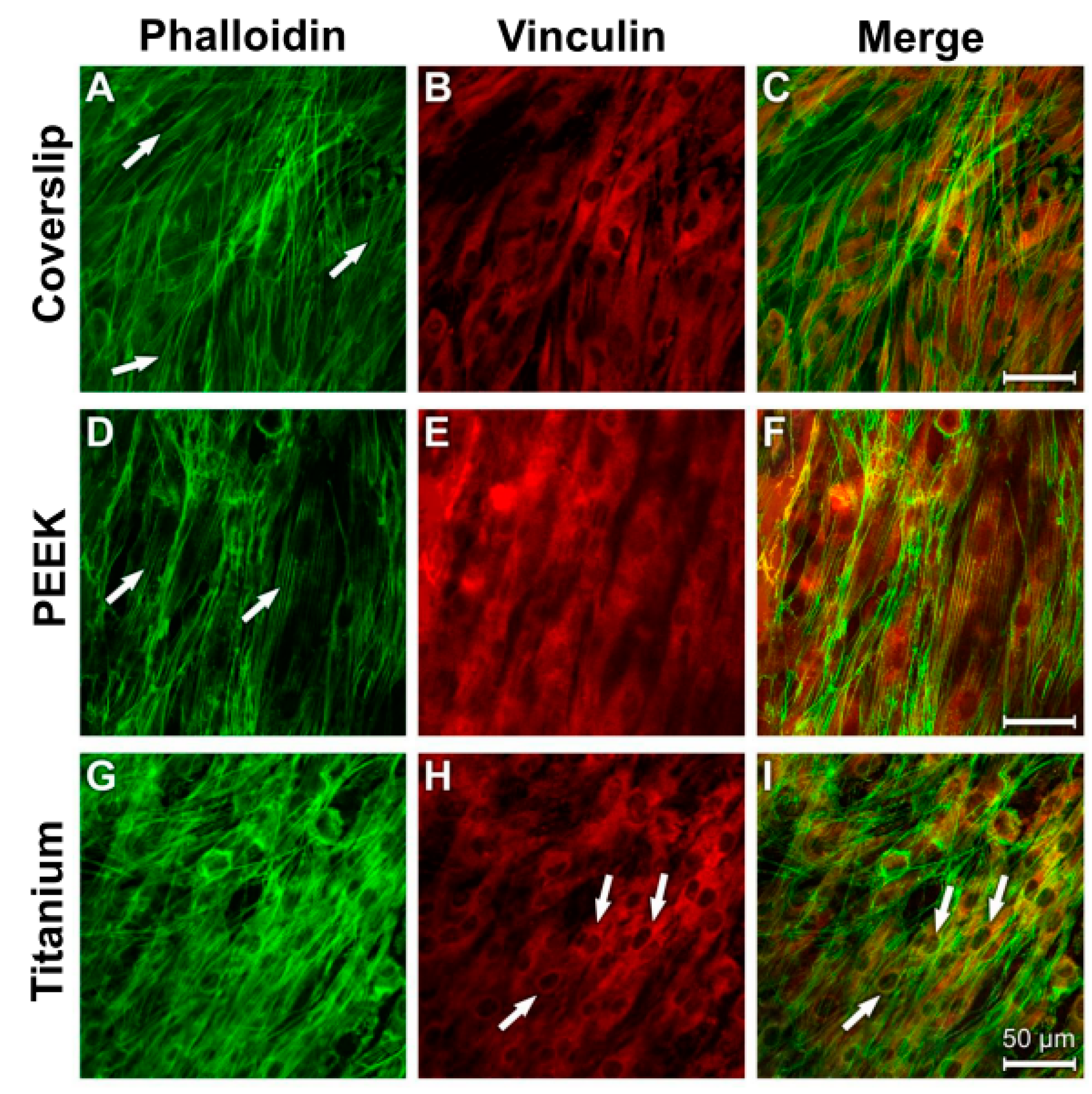
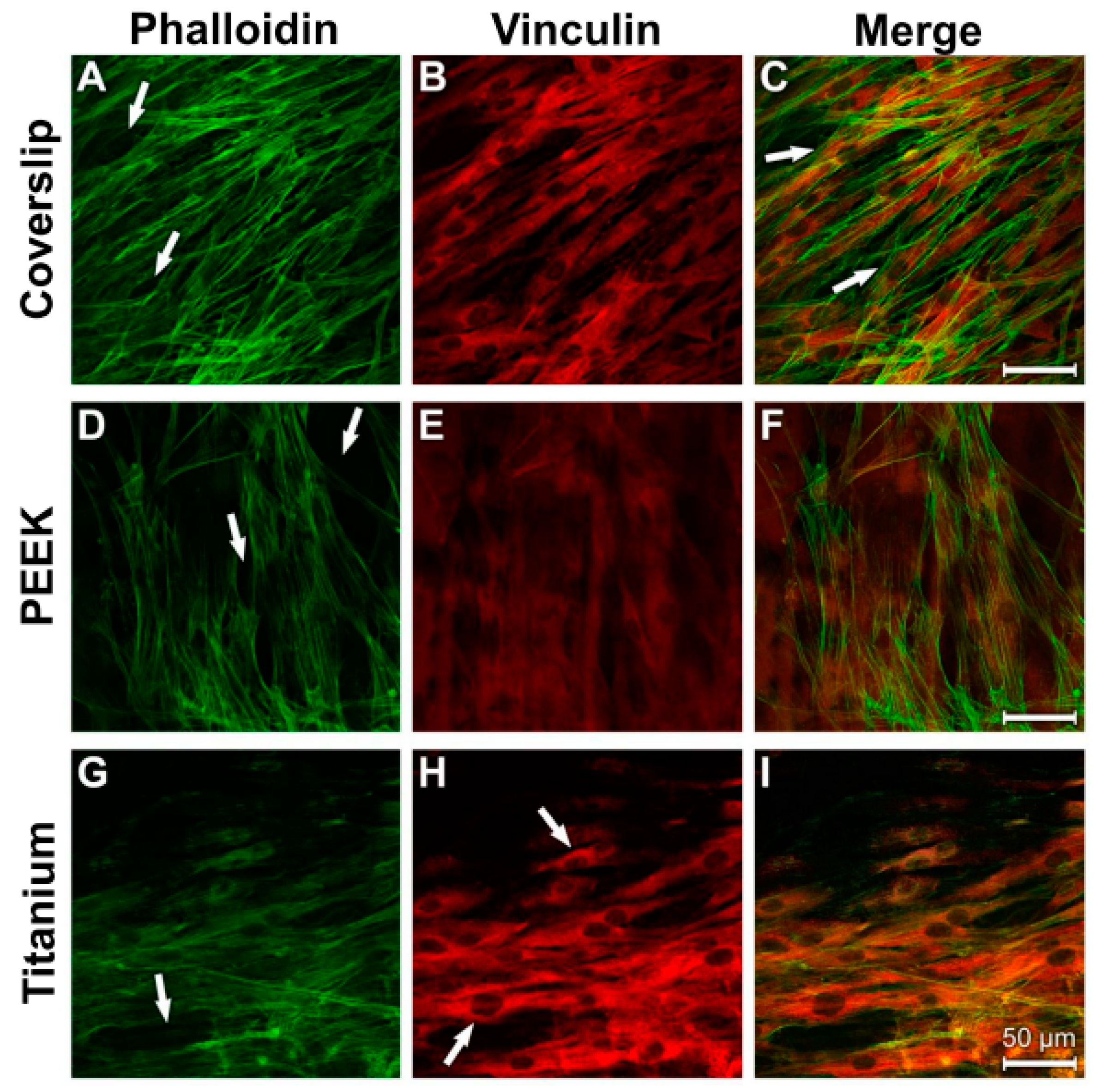
3.4. Analysis of Actin Cytoskeleton and Cell Adhesion Contacts (Vinculin) in Osteoblasts and Fibroblasts
4. Discussion
5. Conclusions
- PEEK enables the adhesion of human osteoblasts and fibroblasts under inflammatory environmental conditions that are similar to those of titanium.
- PEEK supports inflammatory processes to a lesser extent when compared with titanium.
Author Contributions
Funding
Conflicts of Interest
References
- Markhoff, J.; Krogull, M.; Schulze, C.; Rotsch, C.; Hunger, S.; Bader, R. Biocompatibility and Inflammatory Potential of Titanium Alloys Cultivated with Human Osteoblasts, Fibroblasts and Macrophages. Materials 2017, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.F.; Wang, L.; Zhang, Y.; Li, X.; Ma, Z.S.; Zou, J.W.; Lei, W.; Zhang, Z.Y. Effect of reactive oxygen species overproduction on osteogenesis of porous titanium implant in the present of diabetes mellitus. Biomaterials 2013, 34, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Gasik, M.; Braem, A.; Chaudhari, A.; Duyck, J.; Vleugels, J. Titanium implants with modified surfaces: Meta-analysis of in vivo osteointegration. Mater. Sci. Eng. C 2015, 49, 152–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romeo, E.; Lops, D.; Margutti, E.; Ghisolfi, M.; Chiapasco, M.; Vogel, G. Long-term survival and success of oral implants in the treatment of full and partial arches: A 7-year prospective study with the ITI dental implant system. Int. J. Oral Maxillofac. Implant. 2004, 19, 247–259. [Google Scholar]
- Wennerberg, A.; Albrektsson, T.; Chrcanovic, B. Long-term clinical outcome of implants with different surface modifications. Eur. J. Oral Implantol. 2018, 11, S123–S136. [Google Scholar]
- Javed, F.; Al-Hezaimi, K.; Almas, K.; Romanos, G.E. Is titanium sensitivity associated with allergic reactions in patients with dental implants? A systematic review. Clin. Implant. Dent. Relat. Res. 2013, 15, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Matusiewicz, H. Potential release of in vivo trace metals from metallic medical implants in the human body: From ions to nanoparticles—A systematic analytical review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef]
- Happe, A.; Sielker, S.; Hanisch, M.; Jung, S. The Biologic Effect of Particulate Titanium Contaminants of Dental Implants on Human Osteoblasts and Gingival Fibroblasts. Int. J. Oral Maxillofac. Implant. 2019, 34, 673–680. [Google Scholar] [CrossRef]
- Schwitalla, A.; Abou-Emara, M.; Spintig, T.; Lackmann, J.; Müller, W. Finite element analysis of the biomechanical effects of PEEK dental implants on the peri-implant bone. J. Biomech. 2015, 48, 1–7. [Google Scholar] [CrossRef]
- Canay, S.; Akça, K. Biomechanical Aspects of Bone-Level Diameter Shifting at Implant-Abutment Interface. Implant. Dent. 2009, 18, 239–248. [Google Scholar] [CrossRef]
- Da Cruz, M.; Marques, J.; Peñarrieta-Juanito, G.; Costa, M.; Souza, J.; Magini, R.; Miranda, G.; Silva, F.; Da Mata, A.D.S.P.; Caramês, J.M. Hard and Soft Tissue Cell Behavior on Polyetheretherketone, Zirconia, and Titanium Implant Materials. Int. J. Oral Maxillofac. Implant. 2019, 34, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Özkurt, Z.; Kazazoğlu, E. Zirconia Dental Implants: A Literature Review. J. Oral Implant. 2011, 37, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Heimer, S.; Schmidlin, P.R.; Stawarczyk, B. Discoloration of PMMA, composite, and PEEK. Clin. Oral Investig. 2017, 21, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Barkarmo, S.; Andersson, M.; Currie, F.; Kjellin, P.; Jimbo, R.; Johansson, C.; Stenport, V. Enhanced bone healing around nanohydroxyapatite-coated polyetheretherketone implants: An experimental study in rabbit bone. J. Biomater. Appl. 2014, 29, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Devine, D.M.; Hahn, J.; Richards, R.G.; Gruner, H.; Wieling, R.; Pearce, S.G. Coating of carbon fiber-reinforced polyetheretherketone implants with titanium to improve bone apposition. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 591–598. [Google Scholar] [CrossRef]
- Luo, H.; Xiong, G.; Yang, Z.; Raman, S.R.; Li, Q.; Ma, C.; Li, D.; Wang, Z.; Wan, Y. Preparation of three-dimensional braided carbon fiber-reinforced PEEK composites for potential load-bearing bone fixations. Part I. Mechanical properties and cytocompatibility. J. Mech. Behav. Biomed. Mater. 2014, 29, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, P.; Papathanasiou, I.; Polyzois, G. The Use of a Modified Poly-Ether-Ether-Ketone (PEEK) as an Alternative Framework Material for Removable Dental Prostheses. A Clinical Report. J. Prosthodont. 2016, 25, 580–584. [Google Scholar] [CrossRef]
- Almasi, D.; Iqbal, N.; Sadeghi, M.; Sudin, I.; Kadir, M.R.A.; Kamarul, T. Preparation Methods for Improving PEEK’s Bioactivity for Orthopedic and Dental Application: A Review. Int. J. Biomater. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Hanasono, M.M.; Goel, N.; Demonte, F. Calvarial Reconstruction with Polyetheretherketone Implants. Ann. Plast. Surg. 2009, 62, 653–655. [Google Scholar] [CrossRef]
- Ma, R.; Tang, T. Current Strategies to Improve the Bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.; Taguchi, Y.; Tominaga, K.; Umeda, M.; Tanaka, A. Porphyromonas gingivalis LPS inhibits osteoblastic differentiation and promotes pro-inflammatory cytokine production in human periodontal ligament stem cells. Arch. Oral Biol. 2014, 59, 167–175. [Google Scholar] [CrossRef]
- Bonsignore, L.A.; Anderson, J.R.; Lee, Z.; Goldberg, V.M.; Greenfield, E.M. Adherent lipopolysaccharide inhibits the osseointegration of orthopedic implants by impairing osteoblast differentiation. Bone 2013, 52, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Seydel, U.; Koch, M.H.; Brandenburg, K. Structural Polymorphisms of Rough Mutant Lipopolysaccharides Rd to Ra from Salmonella minnesota. J. Struct. Biol. 1993, 110, 232–243. [Google Scholar] [CrossRef]
- Vreugdenhil, A.C.; Snoek, A.M.; van ‘t Veer, C.; Greve, J.W.; Buurman, W.A. LPS-binding protein circulates in association with apoB-containing lipoproteins and enhances endotoxin-LDL/VLDL interaction. J. Clin. Investig. 2001, 107, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Schröder, N.W.J.; Heine, H.; Alexander, C.; Manukyan, M.; Eckert, J.; Hamann, L.; Göbel, U.B.; Schumann, R.R. Lipopolysaccharide Binding Protein Binds to Triacylated and Diacylated Lipopeptides and Mediates Innate Immune Responses. J. Immunol. 2004, 173, 2683–2691. [Google Scholar] [CrossRef] [Green Version]
- Schumann, R.; Leong, S.R.; Flaggs, G.W.; Gray, P.W.; Wright, S.D.; Mathison, J.C.; Tobias, P.S.; Ulevitch, R.J. Structure and function of lipopolysaccharide binding protein. Science 1990, 249, 1429–1431. [Google Scholar] [CrossRef]
- Palyu, E.; Lakatos, P.L.; Kiss, L.S.; Palatka, K.; Altorjay, I.; Antal-Szalmas, P.; Udvardy, M.; Molnar, T.; Farkas, K.; Veres, G.; et al. Serum lipopolysaccharide-binding protein and soluble CD14 are markers of disease activity in patients with Crohnʼs disease. Inflamm. Bowel Dis. 2011, 17, 767–777. [Google Scholar]
- Into, T.; Inomata, M.; Shibata, K.; Murakami, Y. Effect of the antimicrobial peptide LL-37 on Toll-like receptors 2-, 3- and 4-triggered expression of IL-6, IL-8 and CXCL10 in human gingival fibroblasts. Cell. Immunol. 2010, 264, 104–109. [Google Scholar] [CrossRef]
- Laugerette, F.; Furet, J.P.; Debard, C.; Daira, P.; Loizon, E.; Geloen, A.; Soulage, C.O.; Simonet, C.; Lefils-Lacourtablaise, J.; Bernoud-Hubac, N.; et al. Oil composition of high-fat diet affects metabolic inflammation differently in connection with endotoxin receptors in mice. Am. J. Physiol. Metab. 2012, 302, E374–E386. [Google Scholar] [CrossRef] [Green Version]
- Rochford, E.T.J.; Subbiahdoss, G.; Moriarty, T.F.; Poulsson, A.H.C.; Van Der Mei, H.C.; Busscher, H.J.; Richards, R.G. Anin vitroinvestigation of bacteria-osteoblast competition on oxygen plasma-modified PEEK. J. Biomed. Mater. Res. Part A 2014, 102, 4427–4434. [Google Scholar] [CrossRef]
- Cao, X.M.; Luo, X.G.; Liang, J.H.; Zhang, C.; Meng, X.P.; Guo, D.W. Critical selection of internal control genes for quantitative real-time RT-PCR studies in lipopolysaccharide-stimulated human THP-1 and K562 cells. Biochem. Biophys. Res. Commun. 2012, 427, 366–372. [Google Scholar] [CrossRef]
- Latz, E.; Visintin, A.; Lien, E.; Fitzgerald, K.A.; Monks, B.G.; Kurt-Jones, E.A.; Espevik, T.; Golenbock, D.T. Lipopolysaccharide Rapidly Traffics to and from the Golgi Apparatus with the Toll-like Receptor 4-MD-2-CD14 Complex in a Process That Is Distinct from the Initiation of Signal Transduction. J. Biol. Chem. 2002, 277, 47834–47843. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Bai, N.; Chang, A.; Zhang, Z.; Yin, J.; Shen, W.; Tian, Y.; Xiang, R.; Liu, C. ATF4 is directly recruited by TLR4 signaling and positively regulates TLR4-trigged cytokine production in human monocytes. Cell. Mol. Immunol. 2013, 10, 84–94. [Google Scholar] [CrossRef]
- Wolk, K.; Witte, E.; Hoffmann, U.; Doecke, W.D.; Endesfelder, S.; Asadullah, K.; Sterry, W.; Volk, H.D.; Wittig, B.M.; Sabat, R. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: A potential systemic role of IL-22 in Crohn’s disease. J. Immunol. 2007, 178, 5973–5981. [Google Scholar] [CrossRef]
- Yuen, C.Y.; Wong, S.L.; Lau, C.W.; Tsang, S.Y.; Xu, A.; Zhu, Z.; Ng, C.F.; Yao, X.; Kong, S.K.; Lee, H.K.; et al. From Skeleton to Cytoskeleton: Osteocalcin Transforms Vascular Fibroblasts to Myofibroblasts Via Angiotensin II and Toll-Like Receptor 4. Circ. Res. 2012, 111, e55–e66. [Google Scholar] [CrossRef]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The Future of Biologic Coatings for Orthopaedic Implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef]
- Bryers, J.D.; Giachelli, C.M.; Ratner, B.D. Engineering Biomaterials to Integrate and Heal: The Biocompatibility Paradigm Shifts. Biotechnol. Bioeng. 2012, 109, 1898–1911. [Google Scholar] [CrossRef]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef]
- Zhang, Z.; Shively, J.E. Generation of Novel Bone Forming Cells (Monoosteophils) from the Cathelicidin-Derived Peptide LL-37 Treated Monocytes. PLoS ONE 2010, 5, e13985. [Google Scholar] [CrossRef]
- Underwood, A.P.; Bennett, A.F. A comparison of the biological activities of the cell-adhesive proteins vitronectin and fibronectin. J. Cell Sci. 1989, 93, 641–649. [Google Scholar]
- Berrier, A.L.; Yamada, K.M. Cell–matrix adhesion. J. Cell. Physiol. 2007, 213, 565–573. [Google Scholar] [CrossRef]
- Minuth, W.W.; Denk, L.; Miess, C.; Glashauser, A. Peculiarities of the extracellular matrix in the interstitium of the renal stem/progenitor cell niche. Histochem. Cell Biol. 2011, 136, 321–334. [Google Scholar] [CrossRef]
- Rapuano, B.E.; Lee, J.J.E.; Macdonald, D.E. Titanium alloy surface oxide modulates the conformation of adsorbed fibronectin to enhance its binding to α5β1 integrins in osteoblasts. Eur. J. Oral Sci. 2012, 120, 185–194. [Google Scholar] [CrossRef]
- Guan, M.; Yao, W.; Liu, R.; Lam, K.S.; Nolta, J.; Jia, J.; Panganiban, B.; Meng, L.; Zhou, P.; Shahnazari, M.; et al. Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat. Med. 2012, 18, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Beutler, B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 2004, 430, 257–263. [Google Scholar] [CrossRef]
- Broughton, G.; Janis, J.E.; Attinger, C.E. The basic science of wound healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef]
- Fournier, B.P.; Larjava, H.; Häkkinen, L. Gingiva as a Source of Stem Cells with Therapeutic Potential. Stem Cells Dev. 2013, 22, 3157–3177. [Google Scholar] [CrossRef]
- Lee, C.T.; Huang, Y.W.; Zhu, L.; Weltman, R. Prevalences of peri-implantitis and peri-implant mucositis: Systematic review and meta-analysis. J. Dent. 2017, 62, 1–12. [Google Scholar] [CrossRef]
- Tilakaratne, A.; Soory, M. Osteoblastic responses to LPS, glucose-oxidised LDL and minocycline: Therapeutic targets for periodontal and cardiometabolic diseases. Recent Pat. Endocr. Metab. Immune Drug Discov. 2012, 6, 73–84. [Google Scholar] [CrossRef]
- Johansson, P.; Jimbo, R.; Kjellin, P.; Currie, F.; Chrcanovic, B.R.; Wennerberg, A. Biomechanical evaluation and surface characterization of a nano-modified surface on PEEK implants: A study in the rabbit tibia. Int. J. Nanomed. 2014, 9, 3903–3911. [Google Scholar] [CrossRef]
- Poulsson, A.H.; Eglin, D.; Zeiter, S.; Camenisch, K.; Sprecher, C.; Agarwal, Y.; Nehrbass, D.; Wilson, J.; Richards, R.G.; Zeiter, S. Osseointegration of machined, injection moulded and oxygen plasma modified PEEK implants in a sheep model. Biomaterials 2014, 35, 3717–3728. [Google Scholar] [CrossRef]
- Andrukhov, O.; Huber, R.; Shi, B.; Berner, S.; Rausch-Fan, X.; Moritz, A.; Spencer, N.D.; Schedle, A. Proliferation, behavior, and differentiation of osteoblasts on surfaces of different microroughness. Dent. Mater. 2016, 32, 1374–1384. [Google Scholar] [CrossRef]
- Lehnert, D.; Wehrle-Haller, B.; David, C.; Weiland, U.; Ballestrem, C.; Imhof, B.A.; Bastmeyer, M. Cell behaviour on micropatterned substrata: Limits of extracellular matrix geometry for spreading and adhesion. J. Cell Sci. 2004, 117, 41–52. [Google Scholar] [CrossRef]
- Tsou, H.K.; Hsieh, P.Y.; Chi, M.H.; Chung, C.J.; He, J.L. Improved osteoblast compatibility of medical-grade polyetheretherketone using arc ionplated rutile/anatase titanium dioxide films for spinal implants. J. Biomed. Mater. Res. Part A 2012, 100, 2787–2792. [Google Scholar] [CrossRef]
- Sagomonyants, K.B.; Jarman-Smith, M.L.; Devine, J.N.; Aronow, M.S.; Gronowicz, G.A. The in vitro response of human osteoblasts to polyetheretherketone (PEEK) substrates compared to commercially pure titanium. Biomaterials 2008, 29, 1563–1572. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Gittens, R.A.; Schneider, J.M.; Hyzy, S.L.; Haithcock, D.A.; Ullrich, P.F.; Schwartz, Z.; Boyan, B.D. Osteoblasts exhibit a more differentiated phenotype and increased bone morphogenetic protein production on titanium alloy substrates than on poly-ether-ether-ketone. Spine J. 2012, 12, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Marín-Pareja, N.; Salvagni, E.; Guillem-Marti, J.; Aparicio, C.; Ginebra, M.P. Collagen-functionalised titanium surfaces for biological sealing of dental implants: Effect of immobilisation process on fibroblasts response. Colloids Surf. B Biointerfaces 2014, 122, 601–610. [Google Scholar] [CrossRef]
- Miao, X.; Wang, D.; Xu, L.; Wang, J.; Zeng, D.; Lin, S.; Huang, C.; Liu, X.; Jiang, X. The response of human osteoblasts, epithelial cells, fibroblasts, macrophages and oral bacteria to nanostructured titanium surfaces: A systematic study. Int. J. Nanomed. 2017, 12, 1415–1430. [Google Scholar] [CrossRef]
- Chan, C.W.; Hussain, I.; Waugh, D.; Lawrence, J.; Man, H.; Waugh, D. Effect of laser treatment on the attachment and viability of mesenchymal stem cell responses on shape memory NiTi alloy. Mater. Sci. Eng. C 2014, 42, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Bandow, K.; Maeda, A.; Kakimoto, K.; Kusuyama, J.; Shamoto, M.; Ohnishi, T.; Matsuguchi, T. Molecular mechanisms of the inhibitory effect of lipopolysaccharide (LPS) on osteoblast differentiation. Biochem. Biophys. Res. Commun. 2010, 402, 755–761. [Google Scholar] [CrossRef]
- Lepper, P.M.; Kleber, M.E.; Grammer, T.B.; Hoffmann, K.; Dietz, S.; Winkelmann, B.R.; Boehm, B.O.; März, W. Lipopolysaccharide-binding protein (LBP) is associated with total and cardiovascular mortality in individuals with or without stable coronary artery disease—Results from the Ludwigshafen Risk and Cardiovascular Health Study (LURIC). Atherosclerosis 2011, 219, 291–297. [Google Scholar] [CrossRef]
- Herath, T.D.K.; Darveau, R.P.; Seneviratne, C.J.; Wang, C.Y.; Wang, Y.; Jin, L. Tetra-and Penta-Acylated Lipid A Structures of Porphyromonas gingivalis LPS Differentially Activate TLR4-Mediated NF-κB Signal Transduction Cascade and Immuno-Inflammatory Response in Human Gingival Fibroblasts. PLoS ONE 2013, 8, e58496. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Wang, Y.; Hu, Q. Effects of CD14 and TLR4 on LPS-mediated normal human skin fibroblast proliferation. Int. J. Clin. Exp. Med. 2015, 8, 2267–2272. [Google Scholar]
- Moon, Y.H.; Yoon, M.K.; Moon, J.S.; Kang, J.H.; Kim, S.H.; Yang, H.S.; Kim, M.S. Focal adhesion linker proteins expression of fibroblast related to adhesion in response to different transmucosal abutment surfaces. J. Adv. Prosthodont. 2013, 5, 341–350. [Google Scholar] [CrossRef]
- Guharoy, M.; Szabó, B.; Martos, S.C.; Kosol, S.; Tompa, P. Intrinsic Structural Disorder in Cytoskeletal Proteins. Cytoskeleton (Hoboken) 2013, 70, 550–571. [Google Scholar] [CrossRef] [Green Version]
- Richter, E.; Hitzler, H.; Zimmermann, H.; Hagedorn, R.; Fuhr, G. Trace formation during locomotion of L929 mouse fibroblasts continuously recorded by interference reflection microscopy (IRM). Cell Motil. Cytoskelet. 2000, 47, 38–47. [Google Scholar] [CrossRef]
- Rebl, H.; Finke, B.; Lange, R.; Weltmann, K.D.; Nebe, J.B. Impact of plasma chemistry versus titanium surface topography on osteoblast orientation. Acta Biomater. 2012, 8, 3840–3851. [Google Scholar] [CrossRef]
- Vandrovcová, M.; Bačáková, L. Adhesion, growth and differentiation of osteoblasts on surface-modified materials developed for bone implants. Physiol. Res. 2011, 60, 403–417. [Google Scholar]
- Takahashi, N.; Kobayashi, M.; Takaki, T.; Takano, K.; Miyata, M.; Okamatsu, Y.; Hasegawa, K.; Nishihara, T.; Yamamoto, M. Actinobacillus actinomycetemcomitans lipopolysaccharide stimulates collagen phagocytosis by human gingival fibroblasts. Oral Microbiol. Immunol. 2008, 23, 259–264. [Google Scholar] [CrossRef]
- El Awadly, T.A.; Radi, I.; El Khadem, A.; Osman, R. Can PEEK be an implant material? Evaluation of surface topography and wettability of filled versus unfilled PEEK with different surface roughness. J. Oral Implantol. 2017, 43, 456–461. [Google Scholar] [CrossRef]
- Schwitalla, A.D.; Zimmermann, T.; Spintig, T.; Abou-Emara, M.; Lackmann, J.; Müller, W.D.; Houshmand, A. Maximum insertion torque of a novel implant-abutment-interface design for PEEK dental implants. J. Mech. Behav. Biomed. Mater. 2018, 77, 85–89. [Google Scholar] [CrossRef]
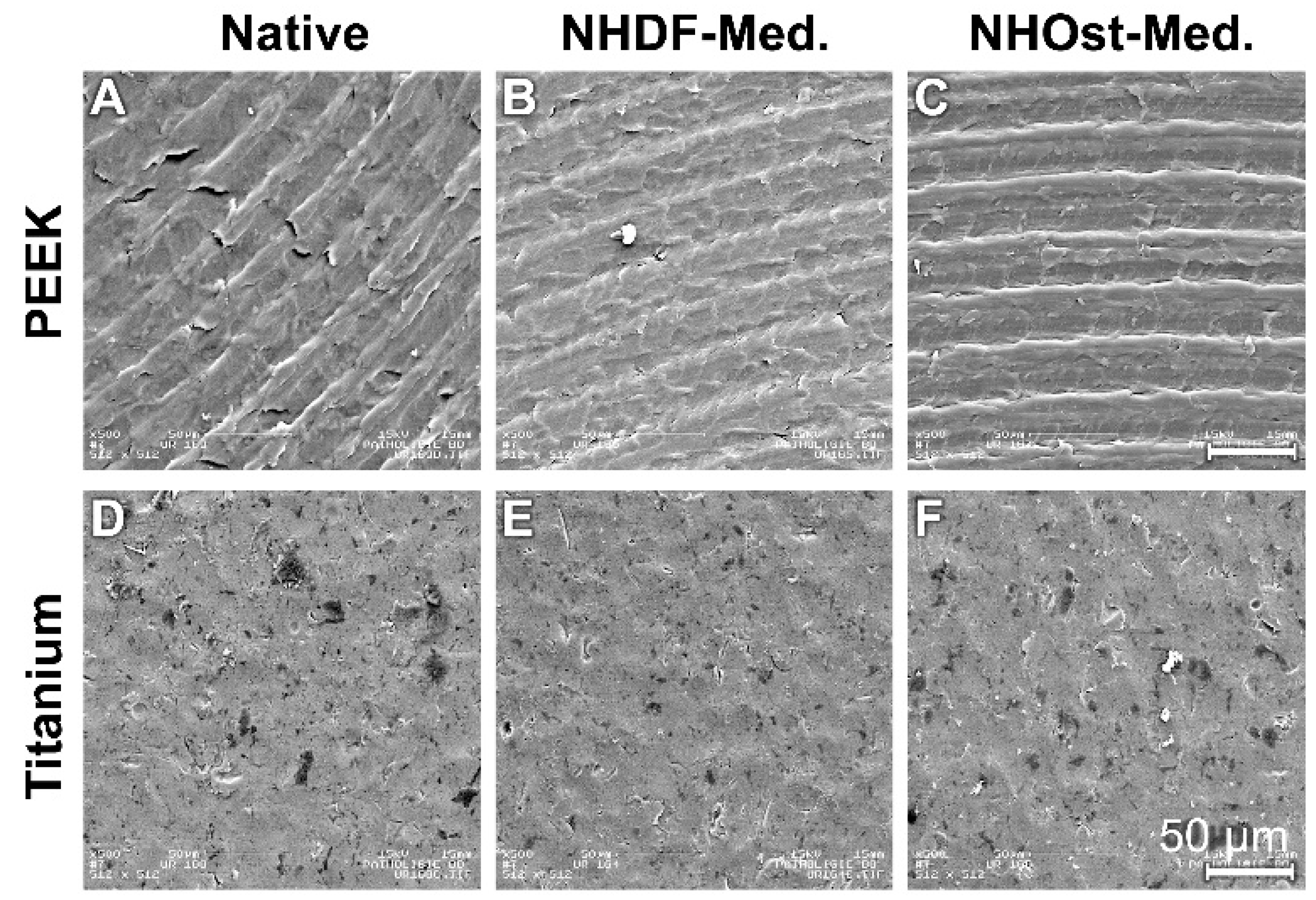

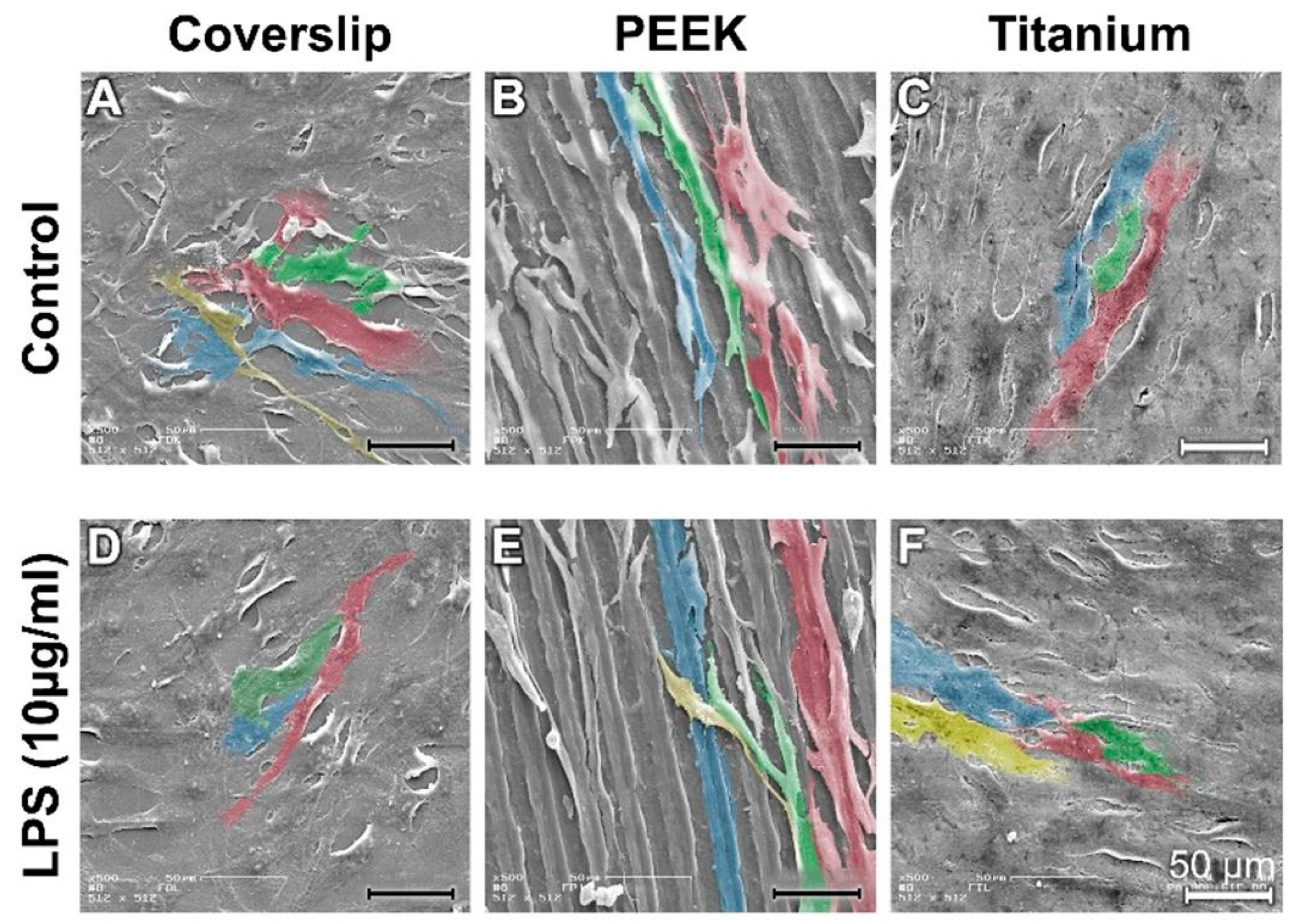
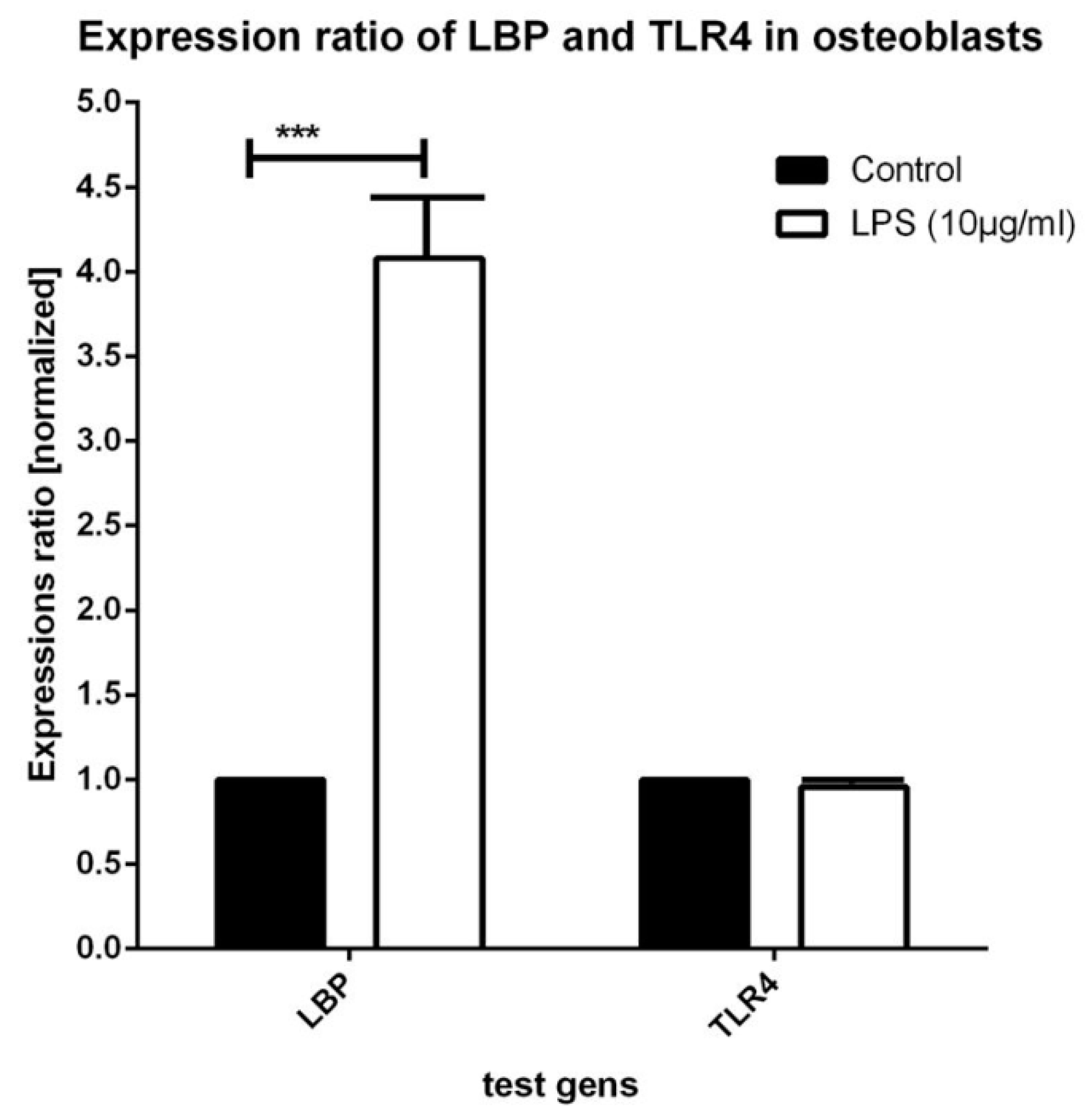
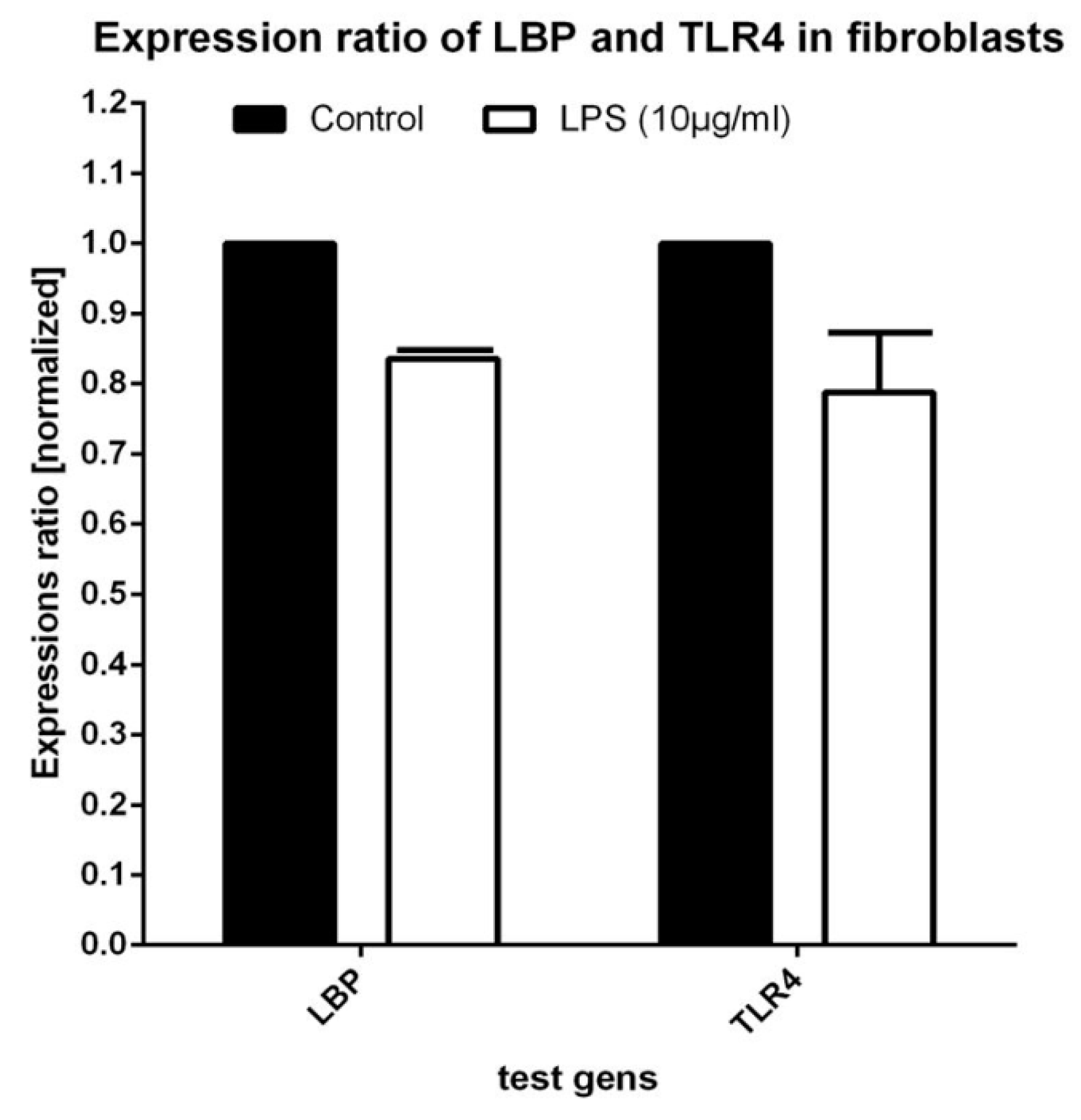


| Name | Company | Dilution Ratio |
|---|---|---|
| Alexa goat 488 anti-mouse | Invitrogen (Karlsruhe, Germany) | 1/1000 |
| Alexa goat 488 anti-rabbit | Invitrogen (Karlsruhe, Germany) | 1/1000 |
| Alexa goat 568 anti-mouse | Invitrogen (Karlsruhe, Germany) | 1/1000 |
| rabbit-anti-human LBP (PA5-21642) | Thermo Scientific (Watham, MA, USA) | 1/75 |
| Phalloidin Atto-488 | Sigma-Aldrich (Taufkirchen, Germany) | 1/1000 |
| Maus-anti-human TLR4 (76B357.1, (ab22048)) | Abcam (Cambridge, UK) | 1/75 |
| Monoclonal Anti-Vinculin (clone hVIN-1) | Sigma-Aldrich (Taufkirchen, Germany) | 1/500 |
| Primer | Function | Gen (Length) | Primer Length [bp] | Catalogue-No. |
|---|---|---|---|---|
| Hs_LBP_1_SG | lipopolysaccharide-binding protein | NM_004139 (1894 bp) | 79 | QT00027293 |
| Hs_TLR4_1_SG | Toll-like receptor 4 | NM_003266 (5781 bp) | 102 | QT00035238 |
| Hs_ACTB_1_SG | β-Actin | NM_001101 (1852 bp) | 146 | QT00095431 |
| Hs_CYC1_1_SG | Cytochrome C1 | NM_001916 (1251 bp) | 123 | QT00209454 |
| Hs_EIF4A2_1_SG | Eukaryotic initiation faktor 4A2 | NM_001967 (1905 bp) | 87 | QT00079226 |
| Hs_GAPDH_2_SG | Glycerinaldehyd-3-phosphat-dehydrogenase | NM_002046 (1421 bp) | 119 | QT01192646 |
| Hs_HMBS_1_SG | Hydroxymethylbilane Synthase | NM_000190 (1526 bp) | 107 | QT00014462 |
| Hs_RRN18s_1_SG | Ribosomal 18s RNA | X03205 (1869 bp) | 149 | QT00199367 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benz, K.; Schöbel, A.; Dietz, M.; Maurer, P.; Jackowski, J. Adhesion Behaviour of Primary Human Osteoblasts and Fibroblasts on Polyether Ether Ketone Compared with Titanium under In Vitro Lipopolysaccharide Incubation. Materials 2019, 12, 2739. https://doi.org/10.3390/ma12172739
Benz K, Schöbel A, Dietz M, Maurer P, Jackowski J. Adhesion Behaviour of Primary Human Osteoblasts and Fibroblasts on Polyether Ether Ketone Compared with Titanium under In Vitro Lipopolysaccharide Incubation. Materials. 2019; 12(17):2739. https://doi.org/10.3390/ma12172739
Chicago/Turabian StyleBenz, Korbinian, Andreas Schöbel, Marisa Dietz, Peter Maurer, and Jochen Jackowski. 2019. "Adhesion Behaviour of Primary Human Osteoblasts and Fibroblasts on Polyether Ether Ketone Compared with Titanium under In Vitro Lipopolysaccharide Incubation" Materials 12, no. 17: 2739. https://doi.org/10.3390/ma12172739





