Thermoluminescence Enhancement of LiMgPO4 Crystal Host by Tb3+ and Tm3+ Trivalent Rare-Earth Ions Co-doping
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Powder Form
2.1.2. Crystal Form
2.2. XRD Phases Analysis
2.3. Irradiations and TL Measurements
3. Results and Discussion
3.1. XRD Phases Analysis
3.2. TL Glow-Curve Shape Analysis
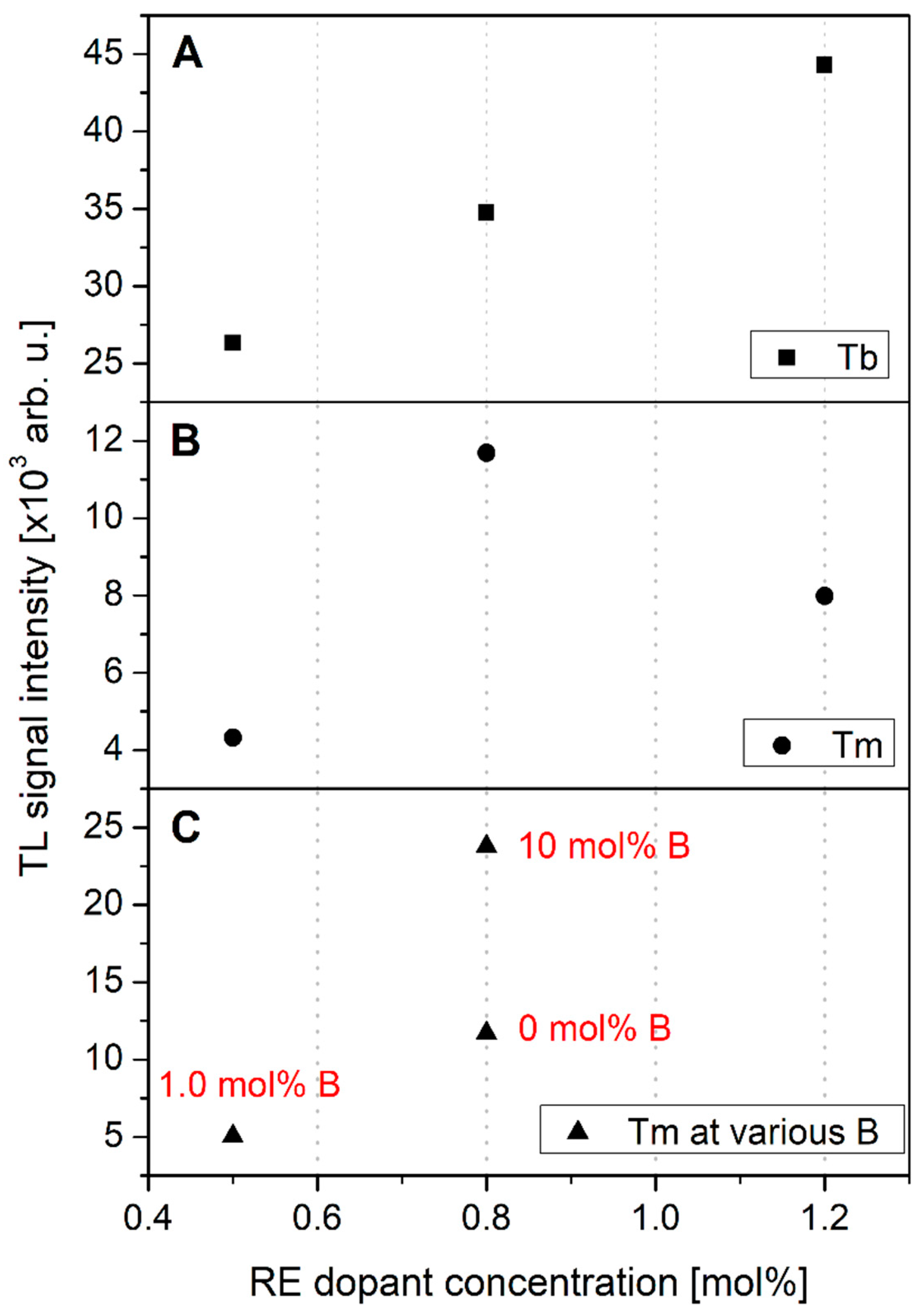
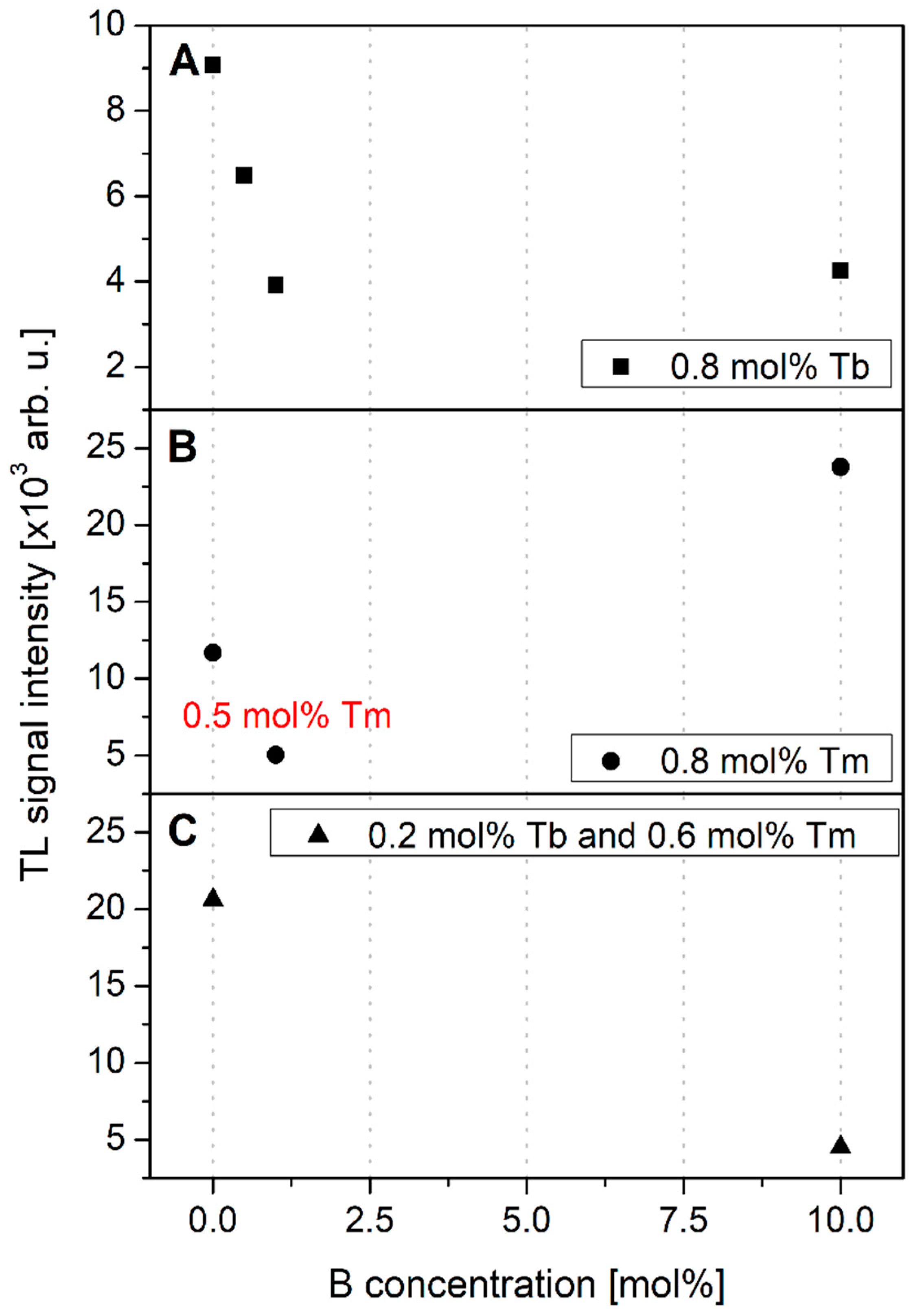
3.3. TL Response Dependence on Dopant Concentration
3.4. TL Emission Spectra Analysis
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Hanic, F.; Handlovic, M.; Burdova, K.; Majling, J. Crystal structure of lithium magnesium phosphate, LiMgPO4: Crystal chemistry of the olivine-type compounds. J. Cryst. Spectrosc. 1982, 12, 99–127. [Google Scholar] [CrossRef]
- Vuojola, J.; Soukka, T. Luminescent lanthanide reporters: New concepts for use in bioanalytical applications. Methods Appl. Fluoresc. 2014, 2, 012001. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tian, Y.; Zhao, Y.; Lin, J. Recent progress in luminescence tuning of Ce3+ and Eu2+ activated phosphors for pc-WLEDs. Chem. Soc. Rev. 2015, 44, 8688–8713. [Google Scholar] [CrossRef] [PubMed]
- Kulig, D.; Gieszczyk, W.; Bilski, P.; Marczewska, B.; Kłosowski, M. Thermoluminescence and optically stimulated Luminescence studies on LiMgPO4 crystallized by micro pulling down technique. Radiat. Meas. 2016, 85, 88–92. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Y.; Shi, L.; Seo, H.J. The luminescence characterization and structure of Eu2+ doped LiMgPO4. J. Phys. Condens. Matter 2010, 22, 235402. [Google Scholar] [CrossRef] [PubMed]
- Dhabekar, B.; Menon, S.N.; Alagu-Raja, E.; Bakshi, A.K.; Singh, A.K.; Chougaonkar, M.P.; Mayya, Y.S. LiMgPO4:Tb,B—A new sensitive OSL phosphor for dosimetry. Nucl. Instrum. Methods Phys. Res. B 2011, 269, 1844–1848. [Google Scholar] [CrossRef]
- Kumar, M.; Dhabekar, B.; Menon, S.N.; Chougaonkar, M.P.; Mayya, Y.S. LiMgPO4:Tb,B OSL phosphor—CW and LM OSL studies. Nucl. Instrum. Methods Phys. Res. B 2011, 269, 1849–1854. [Google Scholar] [CrossRef]
- Singh, A.K.; Menon, S.N.; Dhabekar, B.; Kadam, S.; Chougaonkar, M.P.; Mayya, Y.S. TL-OSL correlation studies of LiMgPO4:Tb,B dosimetric phosphor. Nucl. Instrum. Methods Phys. Res. B 2012, 274, 177–181. [Google Scholar] [CrossRef]
- Menon, S.N.; Dhabekar, B.; Alagu-Raja, E.; Chougaonkar, M.P. Preparation and TSL studies in Tb activatd LiMgPO4 phosphor. Radiat. Meas. 2012, 47, 236–240. [Google Scholar] [CrossRef]
- Gai, M.; Chen, Z.; Fan, Y.; Wang, J. Synthesis and luminescence in LiMgPO4:Tb,Sm,B phosphors with possible applications in real-time dosimetry. J. Rare Earths 2013, 31, 551–554. [Google Scholar] [CrossRef]
- Kumar, M.; Dhabekar, B.; Menon, S.N.; Bakshi, A.K.; Udhayakumar, J.; Chougaonkar, M.P.; Mayya, Y.S. Beta response of LiMgPO4:Tb,B based OSL discs for personnel monitoring applications. Radiat. Prot. Dosim. 2013, 155, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.N.; Kadam, S.; Dhabekar, B.; Singh, A.K.; Chougaonkar, M.P.; Babu, D.A.R.; Patra, A.K. Environmental monitoring using LiMgPO4:Tb,B based optically stimulated luminescence dosimeter. Radiat. Prot. Environ. 2013, 36, 146–149. [Google Scholar] [CrossRef]
- Baran, A.; Mahlik, S.; Grinberg, M.; Cai, P.; Kim, S.I.; Seo, H.J. Luminescence properties of different Eu sites in LiMgPO4:Eu2+,Eu3+. J. Phys. Condens. Matter 2014, 26, 385401. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Badiger, N.M. Energy absorption buildup factors, exposure buildup factors and Kerma for optically stimulated luminescence materials and their tissue equivalence for radiation dosimetry. Radiat. Phys. Chem. 2014, 104, 61–67. [Google Scholar] [CrossRef]
- Gai, M.Q.; Chen, Z.Y.; Fan, Y.W.; Yan, S.Y.; Xie, Y.X.; Wang, J.H.; Zhang, Y.G. Synthesis of LiMgPO4:Eu,Sm,B phosphors and investigation of their optically stimulated luminescence properties. Radiat. Meas. 2015, 78, 48–52. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, D.; Chang, C. Tunable emission and concentration quenching of Tb3+ in magnesium phosphate lithium. J. Alloys Compd. 2015, 627, 25–30. [Google Scholar] [CrossRef]
- Bajaj, N.S.; Palan, C.B.; Koparkar, K.A.; Kulkarni, M.S.; Omanwar, S.K. Preliminary results on effect of boron co-doping on CW-OSL and TL properties of LiMgPO4: Tb,B. J. Lumin. 2016, 175, 9–15. [Google Scholar] [CrossRef]
- Kulig, D.; Gieszczyk, W.; Bilski, P.; Marczewska, B.; Kłosowski, M. New OSL detectors based on LiMgPO4 crystals grown by micro pulling down method. Dosimetric properties vs. growth parameters. Radiat. Meas. 2016, 90, 303–307. [Google Scholar] [CrossRef]
- Marczewska, B.; Bilski, P.; Wróbel, D.; Kłosowski, M. Investigations of OSL properties of LiMgPO4: Tb,B based dosimeters. Radiat. Meas. 2016, 90, 265–268. [Google Scholar] [CrossRef]
- Barykina, Y.A.; Medvedeva, N.I.; Zubkov, V.G.; Kellerman, D.G. Luminescence of VO43− centers in LiMgPO4 and LiMgVO4: Effect of [PO4]3−/[VO4]3− substitution on the structure and optical properties. J. Alloys Compd. 2017, 709, 1–7. [Google Scholar] [CrossRef]
- Gieszczyk, W.; Kulig, D.; Bilski, P.; Marczewska, B.; Kłosowski, M. Analysis of TL and OSL kinetics in lithium magnesium phosphate crystals. Radiat. Meas. 2017, 106, 100–106. [Google Scholar] [CrossRef]
- Kulig, D.; Gieszczyk, W.; Marczewska, B.; Bilski, P.; Kłosowski, M.; Malthez, A.L.M.C. Comparative studies on OSL properties of LiMgPO4: Tb,B powders and crystals. Radiat. Meas. 2017, 106, 94–99. [Google Scholar] [CrossRef]
- Guo, J.; Tang, Q.; Zhang, C.; Luo, D.; Liu, X. Optically stimulated luminescence (OSL) of LiMgPO4: Tm,Tb phosphor. J. Rare Earths 2017, 35, 525–529. [Google Scholar] [CrossRef]
- Rawat, N.S.; Dhabekar, B.; Muthe, K.P.; Koul, D.K.; Datta, D. Detectioin of sub micro Gray dose levels using OSL phosphor LiMgPO4: Tb,B. Nucl. Instrum. Methods Phys. Res. B 2017, 397, 27–32. [Google Scholar] [CrossRef]
- Kellerman, D.G.; Medvedeva, N.I.; Kalinkin, M.O.; Syurdo, A.I.; Zubkov, V.G. Theoretical and experimental evidence of defects in LiMgPO4. J. Alloys Compd. 2018, 766, 626–636. [Google Scholar] [CrossRef]
- Menon, S.N.; Dhabecar, B.S.; Kadam, S.; Koul, D.K. Fading studies in LiMgPO4:Tb,B and synthesis of new LiMgPO4 based phosphor with better fading characteristics. Nucl. Instrum. Methods Phys. Res. B 2018, 436, 45–50. [Google Scholar] [CrossRef]
- Palan, C.B.; Bajaj, N.S.; Soni, A.; Omanwar, S.K. Synthesis and luminescence properties of Tb-doped LiMgPO phosphor. Bull. Mater. Sci. 2016, 39, 1157–1163. [Google Scholar] [CrossRef]
- Palan, C.B.; Bajaj, N.S.; Koparkar, K.A.; Soni, A.; Omanwar, S.K. TL/OSL properties of green emitting LiMgPO4:Tb3+,B (LMPTB) phosphor for radiation dosimetry. J. Inorg. Organomet. Polym. 2016, 26, 845–854. [Google Scholar] [CrossRef]
- Gieszczyk, W.; Bilski, P.; Kłosowski, M.; Nowak, T.; Malinowski, L. Thermoluminescent response of differently doped lithium magnesium phosphate (LiMgPO4, LMP) crystals to protons, neutrons and alpha particles. Radiat. Meas. 2018, 113, 14–19. [Google Scholar] [CrossRef]
- Fukuda, T.; Rudolph, P.; Uda, S. Fiber Crystal Growth from the Melt; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Fukuda, T.; Chani, V.I. Shaped Crystal Growth by Micro-Pulling-Down Technique; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Bos, A.J.J. Thermoluminescence as a research tool to investigate luminescence mechanisms. Materials 2017, 10, 1357. [Google Scholar] [CrossRef]
- Bos, A.J.J. Theory of thermoluminescence. Radiat. Meas. 2007, 41, S45–S56. [Google Scholar] [CrossRef]
- Bilski, P.; Gieszczyk, W.; Obryk, B.; Hodyr, K. Comparison of commercial thermoluminescent readers regarding high-dose high-temperature Measurements. Radiat. Meas. 2014, 65, 8–13. [Google Scholar] [CrossRef]
- Gieszczyk, W.; Bilski, P. A simplified numerical approach to non-radiation induced high-temperature signals in thermoluminescence. GlowVIEW—A useful tool for a multiple glow-curve analysis. Radiat. Meas. 2017, 107, 102–110. [Google Scholar] [CrossRef]
- Dorenbos, P. Systematic behavior in trivalent lanthanide charge transfer energies. J. Phys. Condens. Matter 2003, 15, 8417–8434. [Google Scholar] [CrossRef]
- Dorenbos, P. The Eu3+ charge transfer energy and the relation with the band gap of compounds. J. Lumin. 2005, 111, 89–104. [Google Scholar] [CrossRef]
- Dorenbos, P. Lanthanide charge transfer energies and related luminescence, charge carrier trapping, and redox phenomena. J. Alloys Compd. 2009, 488, 568–573. [Google Scholar] [CrossRef]
- Bos, A.J.J.; Dorenbos, P.; Bessiere, A.; Viana, B. Lanthanide energy levels in YPO4. Radiat. Meas. 2008, 43, 222–226. [Google Scholar] [CrossRef]
- Bos, A.J.J.; Poolton, N.R.J.; Wallinga, J.; Bessiere, A.; Dorenbos, P. Energy levels in YPO4:Ce3+,Sm3+ studied by thermally and optically stimulated luminescence. Radiat. Meas. 2010, 45, 343–346. [Google Scholar] [CrossRef]
- Bos, A.J.J.; Dorenbos, P.; Bessiere, A.; Lecointre, A.; Bedu, M.; Bettinelli, M.; Piccinelli, F. Study of TL glow curves of YPO4 double doped with lanthanide ions. Radiat. Meas. 2011, 46, 1410–1416. [Google Scholar] [CrossRef]
- Gieszczyk, W.; Bilski, P.; Kłosowski, M.; Mrozik, A.; Zorenko, T.; Witkiewicz, S.; Zorenko, Y. Luminescent properties of Tb and Eu activated AxB1−xAlO3 (A=Y, Lu, Gd; B=Lu; x=0, 0.5, 1) mixed oxides crystals prepared by micro-pulling-down method. Radiat. Meas. 2019, 126, 106140. [Google Scholar] [CrossRef]










| Host | Dopants Concentration [mol%] | Phase Purity [mass%] * | |||
|---|---|---|---|---|---|
| Tb | Tm | B | LiMgPO4 | Other Phases | |
| LiMgPO4 | - | - | - | 100 | - |
| 0.5 | - | - | 100 | - | |
| 0.8 | - | - | 98 | 2 (TbPO4) | |
| 1.2 | - | - | 100 | - | |
| - | 0.5 | - | 100 | - | |
| - | 0.8 | - | 94 | 6 (TmPO4) | |
| - | 1.2 | - | 100 | - | |
| 0.8 | 0.5 | - | 100 | - | |
| 0.8 | 1.0 | - | 100 | - | |
| 0.8 | 10 | - | 100 | - | |
| - | 0.5 | 1.0 | 100 | - | |
| - | 0.8 | 10 | 100 | - | |
| 0.2 | 0.6 | - | 100 | - | |
| 0.2 | 0.6 | 10 | 100 | - | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gieszczyk, W.; Marczewska, B.; Kłosowski, M.; Mrozik, A.; Bilski, P.; Sas-Bieniarz, A.; Goj, P.; Stoch, P. Thermoluminescence Enhancement of LiMgPO4 Crystal Host by Tb3+ and Tm3+ Trivalent Rare-Earth Ions Co-doping. Materials 2019, 12, 2861. https://doi.org/10.3390/ma12182861
Gieszczyk W, Marczewska B, Kłosowski M, Mrozik A, Bilski P, Sas-Bieniarz A, Goj P, Stoch P. Thermoluminescence Enhancement of LiMgPO4 Crystal Host by Tb3+ and Tm3+ Trivalent Rare-Earth Ions Co-doping. Materials. 2019; 12(18):2861. https://doi.org/10.3390/ma12182861
Chicago/Turabian StyleGieszczyk, Wojciech, Barbara Marczewska, Mariusz Kłosowski, Anna Mrozik, Paweł Bilski, Anna Sas-Bieniarz, Paweł Goj, and Paweł Stoch. 2019. "Thermoluminescence Enhancement of LiMgPO4 Crystal Host by Tb3+ and Tm3+ Trivalent Rare-Earth Ions Co-doping" Materials 12, no. 18: 2861. https://doi.org/10.3390/ma12182861





