Comparative Evaluation of Anticorrosive Properties of Mahaleb Seed Extract on Carbon Steel in Two Acidic Solutions
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials and Instrumentation
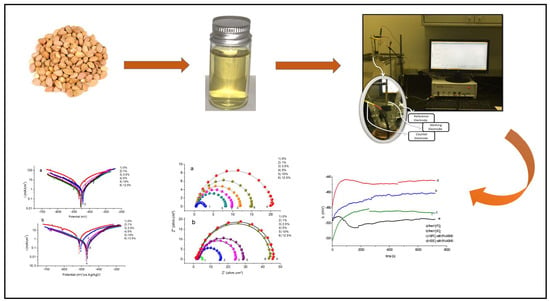
2.2. The Electrochemical Measurements
3. Results and Discussion
3.1. Effect of Inhibitor Concentration
3.1.1. Electrochemical Impedance Spectroscopy
3.1.2. Tafel Polarization
3.2. Potential-Time Measurements
3.3. The Thermodynamic Adsorption Parameter
3.4. Effect of Temperature Change
3.5. FTIR Spectra Analysis
3.6. SEM Image Investigation
3.7. The Role of ASMS in Corrosion Inhibition
4. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Aliofkhazraei, M. Developments in Corrosion Protection; Aliofkhazraei, M., Ed.; InTech: London, UK, 2014. [Google Scholar]
- Fekry, A.M.; Ameer, M.A. Corrosion Inhibition of Mild Steel in Acidic Media Using Newly Synthesized Heterocyclic Organic Molecules. Int. J. Hydrogen Energy 2010, 35, 7641–7651. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A. Schiff’s Base of Pyridyl Substituted Triazoles as New and Effective Corrosion Inhibitors for Mild Steel in Hydrochloric Acid Solution. Corros. Sci. 2014, 79, 5–15. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A.; Raja, P.B.; Fadaeinasab, M.; Qureshi, A.K.; Rahim, A.A.; Osman, H.; Litaudon, M.; Awang, K.; et al. Synergistic Inhibitor Effect of Cetylpyridinium Chloride and Other Halides on the Corrosion of Mild Steel in 0.5 M H2SO4. Corros. Sci. 2013, 66, 343–349. [Google Scholar]
- Abboud, Y.; Hammouti, B.; Abourriche, A.; Bennamara, A.; Hannache, H. 5-Naphthylazo-8-Hydroxyquinoline (5NA8HQ) as a Novel Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Solution. Res. Chem. Intermed. 2012, 38, 1591–1607. [Google Scholar] [CrossRef]
- Singh, A.; Lin, Y.; Liu, W.; Kuanhai, D.; Pan, J.; Huang, B.; Ren, C.; Zeng, D. A Study on the Inhibition of N80 Steel in 3.5% NaCl Solution Saturated with CO2 by Fruit Extract of Gingko Biloba. J. Taiwan Inst. Chem. Eng. 2014, 45, 1918–1926. [Google Scholar] [CrossRef]
- Rosliza, R.; Wan Nik, W.B.; Senin, H.B. The Effect of Inhibitor on the Corrosion of Aluminum Alloys in Acidic Solutions. Mater. Chem. Phys. 2008, 107, 281–288. [Google Scholar] [CrossRef]
- Oguzie, E.E. Corrosion Inhibition of Mild Steel in Hydrochloric Acid Solution by Methylene Blue Dye. Mater. Lett. 2005, 59, 1076–1079. [Google Scholar] [CrossRef]
- Ganash, A.A. Electrochemical Properties and Mechanistic Study of the Green Synthesis of Silver Nanoparticles Using Bardaqush Extract Solution. Mater. Res. Express 2019, 6, 065024. [Google Scholar] [CrossRef]
- Aisha, H.A.; Hind, H.A. Anticorrosive effects of leek seeds aqueous extract (allium ampeloprasumVar. kurrat) on aluminum alloys 6061, 7075 and 2024 in seawater. Org. Med. Chem. 2017, 3, 555624. [Google Scholar]
- Noor, E.A.; Al-moubaraki, A.; Alghanmi, R. Anti-Corrosive Behavior of Senna Aqueous Extract to Aluminum in Alkaline Solutions. Der Pharma Chemica 2017, 9, 51–59. [Google Scholar]
- Ganash, A.A. Theoretical and Experimental Studies of Dried Marjoram Leaves Extract as Green Inhibitor for Corrosion Protection of Steel Substrate in Acidic Solution. Chem. Eng. Commun. 2018, 205, 350–362. [Google Scholar] [CrossRef]
- Noor, E.A. Potential of Aqueous Extract of Hibiscus Sabdariffa Leaves for Inhibiting the Corrosion of Aluminum in Alkaline Solutions. J. Appl. Electrochem. 2009, 39, 1465–1475. [Google Scholar] [CrossRef]
- Ganash, A.A.; Baali, A.A.; Alajlani, L.A.; Alhrbi, R.F.; Sukkar, E.K. Evaluation of Shazab Extract as Eco-Friendly Green Corrosion Inhibitor for 420 Stainless Steel in Hydrochloric Acid. Mater. Res. Express 2019, 6, 096413. [Google Scholar] [CrossRef]
- Khadraoui, A.; Khelifa, A. Ethanolic Extract of Ruta Chalepensis as an Eco-Friendly Inhibitor of Acid Corrosion of Steel. Res. Chem. Intermed. 2013, 39, 3937–3948. [Google Scholar] [CrossRef]
- Bammou, L.; Belkhaouda, M.; Salghi, R.; Benali, O.; Zarrouk, A.; Zarrok, H.; Hammouti, B. Corrosion Inhibition of Steel in Sulfuric Acidic Solution by the Chenopodium Ambrosioides Extracts. J. Assoc. Arab Univ. Basic Appl. Sci. 2014, 16, 83–90. [Google Scholar] [CrossRef]
- Raja, P.B.; Sethuraman, M.G. Natural Products as Corrosion Inhibitor for Metals in Corrosive Media—A Review. Mater. Lett. 2008, 62, 113–116. [Google Scholar] [CrossRef]
- Elayyachy, M.; El Idrissi, A.; Hammouti, B. New Thio-Compounds as Corrosion Inhibitor for Steel in 1 M HCl. Corros. Sci. 2006, 48, 2470–2479. [Google Scholar] [CrossRef]
- Martinez, S.; Metikoš-Huković, M. A Nonlinear Kinetic Model Introduced for the Corrosion Inhibitive Properties of Some Organic Inhibitors. J. Appl. Electrochem. 2003, 33, 1137–1142. [Google Scholar] [CrossRef]
- Sherif, E.M.; Almajid, A.A.; Bairamov, A.K.; Al-zahrani, E. Corrosion of Monel-400 in Aerated Stagnant Arabian Gulf Seawater after Different Exposure Intervals. Int. J. Electrochem. Sci. 2011, 6, 5430–5444. [Google Scholar]
- Mondal, S.K.; Prasad, K.R.; Munichandraiah, N. Analysis of Electrochemical Impedance of Polyaniline Films Prepared by Galvanostatic, Potentiostatic and Potentiodynamic Methods. Synth. Met. 2005, 148, 275–286. [Google Scholar] [CrossRef]
- Umoren, S.A. Biomaterials for Corrosion Protection: Evaluation of Mustard Seed Extract as Eco-Friendly Corrosion Inhibitor for X60 Steel in Acid Media. J. Adhes. Sci. Technol. 2016, 30, 1858–1879. [Google Scholar] [CrossRef]
- Odewunmi, N.A.; Umoren, S.A.; Gasem, Z.M. Watermelon Waste Products as Green Corrosion Inhibitors for Mild Steel in HCl Solution. J. Environ. Chem. Eng. 2015, 3, 286–296. [Google Scholar] [CrossRef]
- Duran, B.; Duran, M. Experimental and Theoretical Studies on Protective Properties of Poly (Pyrrole-Co-N-Methyl Pyrrole) Coatings on Copper in Chloride Media. Corros. Sci. 2013, 69, 252–261. [Google Scholar]
- Khaled, K.F. Experimental and Atomistic Simulation Studies of Corrosion Inhibition of Copper by a New Benzotriazole Derivative in Acid Medium. Electrochim. Acta 2009, 54, 4345–4352. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Ehteshamzadeh, M.; Shahrabi, T. Protection of Mild Steel Corrosion with Schiff Bases in 0.5 M H2SO4 Solution. Electrochim. Acta 2007, 52, 3680–3685. [Google Scholar] [CrossRef]
- Umoren, S.A.; Gasem, Z.M.; Obot, I.B. Natural Products for Material Protection: Inhibition of Mild Steel Corrosion by Date Palm Seed Extracts in Acidic Media. Ind. Eng. Chem. Res. 2013, 52, 14855–14865. [Google Scholar] [CrossRef]
- Baskar, R.; Kesavan, D.; Gopiraman, M.; Subramanian, K. Corrosion Inhibition of Mild Steel in 1.0 M Hydrochloric Acid Medium by New Photo-Cross-Linkable Polymers. Prog. Org. Coat. 2014, 77, 836–844. [Google Scholar] [CrossRef]
- Thirumoolan, D.; Katkar, V.A.; Gunasekaran, G.; Kanai, T.; Anver Basha, K. Hyperbranched Poly(Cyanurateamine): A New Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Medium. Prog. Org. Coat. 2014, 77, 1253–1263. [Google Scholar] [CrossRef]
- Branzoi, V.; Golgovici, F.; Branzoi, F. Aluminium Corrosion in Hydrochloric Acid Solutions and the Effect of Some Organic Inhibitors. Mater. Chem. Phys. 2003, 78, 122–131. [Google Scholar] [CrossRef]
- Chetouani, A.; Aouniti, A.; Hammouti, B.; Benchat, N.; Benhadda, T.; Kertit, S. Corrosion Inhibitors for Iron in Hydrochloride Acid Solution by Newly Synthesised Pyridazine Derivatives. Corros. Sci. 2003, 45, 1675–1684. [Google Scholar] [CrossRef]
- Corrosion Inhibition of Iron in 1 M HCl by Some Gemini Surfactants in the Series of Alkanediyl-α,ω-Bis-(Dimethyl Tetradecyl Ammonium Bromide). Prog. Org. Coat. 2001, 43, 267–273. [CrossRef]
- Li, X.; Deng, S.; Xie, X. Experimental and Theoretical Study on Corrosion Inhibition of Oxime Compounds for Aluminium in HCl Solution. Corros. Sci. 2014, 81, 162–175. [Google Scholar] [CrossRef]
- Ehteram, A.N. Temperature Effects on the Corrosion Inhibition of Mild Steel in Acidic Solutions by Aqueous Extract of Fenugreek Leaves. Int. J. Electrochem. Sci. 2007, 2, 996–1017. [Google Scholar]
- Singh, A.; Singh, V.K.; Quraishi, M.A. Inhibition of Mild Steel Corrosion in HCl Solution Using Pipali (Piper Longum) Fruit Extract. Arab. J. Sci. Eng. 2013, 38, 85–97. [Google Scholar] [CrossRef]
- Al-Turkustani, A.M.; Arab, S.T.; Al-Qarni, L.S.S. Medicago Sative Plant as Safe Inhibitor on the Corrosion of Steel in 2.0M H2SO4 Solution. J. Saudi Chem. Soc. 2011, 15, 73–82. [Google Scholar] [CrossRef]
- El-Etre, A.Y. Inhibition of Aluminum Corrosion Using Opuntia Extract. Corros. Sci. 2003, 45, 2485–2495. [Google Scholar] [CrossRef]
- Larabi, L.; Harek, Y.; Benali, O.; Ghalem, S. Hydrazide Derivatives as Corrosion Inhibitors for Mild Steel in 1 M HCl. Prog. Org. Coat. 2005, 54, 256–262. [Google Scholar] [CrossRef]
- Abdallah, M. Rhodanine Azosulpha Drugs as Corrosion Inhibitors for Corrosion of 304 Stainless Steel in Hydrochloric Acid Solution. Corros. Sci. 2002, 44, 717–728. [Google Scholar] [CrossRef]
- Obot, I.B.; Ebenso, E.E.; Kabanda, M.M. Metronidazole as Environmentally Safe Corrosion Inhibitor for Mild Steel in 0.5 M HCl: Experimental and Theoretical Investigation. J. Environ. Chem. Eng. 2013, 1, 431–439. [Google Scholar] [CrossRef]
- Ita, B..; Offiong, O. The Study of the Inhibitory Properties of Benzoin, Benzil, Benzoin-(4-Phenylthiosemicarbazone) and Benzil-(4-Phenylthiosemicarbazone) on the Corrosion of Mild Steel in Hydrochloric Acid. Mater. Chem. Phys. 2001, 70, 330–335. [Google Scholar] [CrossRef]
- Wahdan, M.H.; Hermas, A.A.; Morad, M.S. Corrosion Inhibition of Carbon-Steels by Propargyltriphenylphosphonium Bromide in H2SO4 Solution. Mater. Chem. Phys. 2002, 76, 111–118. [Google Scholar] [CrossRef]
- Li, X.; Mu, G. Tween-40 as Corrosion Inhibitor for Cold Rolled Steel in Sulphuric Acid: Weight Loss Study, Electrochemical Characterization, and AFM. Appl. Surf. Sci. 2005, 252, 1254–1265. [Google Scholar] [CrossRef]
- Mu, G.N.; Li, X.; Li, F. Synergistic Inhibition between O-Phenanthroline and Chloride Ion on Cold Rolled Steel Corrosion in Phosphoric Acid. Mater. Chem. Phys. 2004, 86, 59–68. [Google Scholar] [CrossRef]
- Laidler, K.J. Reaction Kinetics: Homogeneous Gas Reactions; Pergamon: Oxford, UK, 2013; Volume 1. [Google Scholar]
- Yadav, D.K.; Maiti, B.; Quraishi, M.A. Electrochemical and Quantum Chemical Studies of 3,4-Dihydropyrimidin-2(1H)-Ones as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid Solution. Corros. Sci. 2010, 52, 3586–3598. [Google Scholar] [CrossRef]
- Tang, L.; Li, X.; Si, Y.; Mu, G.; Liu, G. The Synergistic Inhibition between 8-Hydroxyquinoline and Chloride Ion for the Corrosion of Cold Rolled Steel in 0.5 M Sulfuric Acid. Mater. Chem. Phys. 2006, 95, 29–38. [Google Scholar] [CrossRef]
- Gomma, G.K.; Wahdan, M.H. Schiff Bases as Corrosion Inhibitors for Aluminium in Hydrochloric Acid Solution. Mater. Chem. Phys. 1995, 39, 209–213. [Google Scholar] [CrossRef]
- Al-Moubaraki, A.H.; Al-Howiti, A.A.; Al-Dailami, M.M.; Al-Ghamdi, E.A. Role of Aqueous Extract of Celery (Apium Graveolens L.) Seeds against the Corrosion of Aluminium/Sodium Hydroxide Systems. J. Environ. Chem. Eng. 2017, 5, 4194–4205. [Google Scholar] [CrossRef]
- Kertit, S.; Aride, J.; Ben-Bachir, A.; Sghiri, A.; Elkholy, A.; Etman, M. Chemical and Electrochemical Inhibition Studies of Corrosion and Hydrogen Surface Embrittlement. I. Fe0.78B0.13Si0.09 Amorphous Alloy in Molar HCl. J. Appl. Electrochem. 1989, 19, 83–89. [Google Scholar] [CrossRef]
- Hermas, A.-E.A.; Abdel Salam, M.; Al-Juaid, S.S. In Situ Electrochemical Preparation of Multi-Walled Carbon Nanotubes/Polyaniline Composite on the Stainless Steel. Prog. Org. Coat. 2013, 76, 1810–1813. [Google Scholar] [CrossRef]










| Solution | CASMS (v/v %) | Rs (Ω cm2) | Rf (Ω cm2) | Cdl (μF cm−2) | Rct (Ω cm2) | Rp (Ω cm2) | σ |
|---|---|---|---|---|---|---|---|
| 2 M H2SO4 | 0.00 | 0.38 | 2.28 | 2767 | 0.08 | 2.37 | 0.00 |
| 1.00 | 0.50 | 6.77 | 2406 | 2.74 | 9.51 | 75.2 | |
| 3.50 | 0.49 | 9.97 | 1897 | 1.86 | 11.8 | 80.0 | |
| 5.00 | 0.41 | 11.1 | 1194 | 9.26 | 20.4 | 88.4 | |
| 10.0 | 0.58 | 7.91 | 6682 | 0.03 | 7.94 | 70.2 | |
| 12.5 | 0.40 | 0.19 | 2010 | 14.8 | 15.0 | 84.3 | |
| 2 M H3PO4 | 0.00 | 1.59 | 3.61 | 85.9 | 0.05 | 3.67 | 0.00 |
| 1.00 | 1.79 | 1.05 | 20.3 | 14.2 | 15.2 | 75.9 | |
| 3.50 | 1.78 | 2.01 | 20.0 | 14.3 | 16.3 | 77.5 | |
| 5.00 | 1.70 | 44.9 | 3.20 | 0.37 | 45.3 | 91.9 | |
| 10.0 | 2.02 | 1.09 | 168.0 | 27.1 | 28.2 | 86.9 | |
| 12.5 | 1.77 | 39.6 | 33.7 | 3.15 | 42.7 | 91.4 |
| Solution | CASMS(v/v %) | Ecorr(mV) | βa (mV dec−1) | βc (mV dec−1) | icorr (mA cm−2) | σ |
|---|---|---|---|---|---|---|
| 2 M H2SO4 | 0.00 | −469 | 139 | 144 | 7.11 | 0.00 |
| 1.00 | −460 | 104 | 148 | 2.66 | 62.5 | |
| 3.50 | −444 | 95.9 | 147 | 2.05 | 71.1 | |
| 5.00 | −458 | 89.6 | 131 | 0.98 | 86.2 | |
| 10.0 | −457 | 88.8 | 136 | 1.47 | 79.4 | |
| 12.5 | −454 | 96.5 | 168 | 2.17 | 69.5 | |
| 2 M H3PO4 | 0.00 | −513 | 149 | 157 | 4.11 | 0.00 |
| 1.00 | −504 | 115 | 150 | 1.58 | 61.6 | |
| 3.50 | −476 | 104 | 144 | 0.96 | 76.6 | |
| 5.00 | −469 | 83.1 | 136 | 0.43 | 89.6 | |
| 10.0 | −473 | 107 | 175 | 0.72 | 82.6 | |
| 12.5 | −466 | 86.2 | 155 | 0.67 | 83.8 |
| T °k | Parameter | 2 M H3PO4 | 2 M H2SO4 | ||
|---|---|---|---|---|---|
| Free | with 5% ASMS | Free | with 5% ASMS | ||
| Kads (L/mL) | 1.66 | 1.66 | 3.33 | ||
| ΔGads (kJ/mol) | −11.2 | −12.9 | |||
| ΔHads (kJ/mol) | −52.7 | −100 | |||
| Δ Sads (J/mol) | −139 | −293 | |||
| Ea (kJ/mol) | 24.2 | 80.6 | 42.7 | 79.7 | |
| ΔH* (kJ/mol) | 21.9 | 77.9 | 40.1 | 77.1 | |
| ΔS* (J/mol) | −160 | 11.3 | −92.8 | 14.4 | |
| 298 | ΔG* (kJ/mol) | 69.7 | 74.6 | 67.7 | 72.9 |
| 308 | 71.3 | 74.5 | 68.6 | 72.7 | |
| 318 | 72.9 | 74.4 | 69.5 | 72.6 | |
| 328 | 74.6 | 74.3 | 70.5 | 72.4 | |
| Compound Name | Compound Structure |
|---|---|
| Stigmasterol |  |
| Cholesterol |  |
| β-sitosterol |  |
| Heneicosane |  |
| Timnodonic |  |
| Linoleic acid |  |
| Oleic acid |  |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganash, A.A. Comparative Evaluation of Anticorrosive Properties of Mahaleb Seed Extract on Carbon Steel in Two Acidic Solutions. Materials 2019, 12, 3013. https://doi.org/10.3390/ma12183013
Ganash AA. Comparative Evaluation of Anticorrosive Properties of Mahaleb Seed Extract on Carbon Steel in Two Acidic Solutions. Materials. 2019; 12(18):3013. https://doi.org/10.3390/ma12183013
Chicago/Turabian StyleGanash, Aisha A. 2019. "Comparative Evaluation of Anticorrosive Properties of Mahaleb Seed Extract on Carbon Steel in Two Acidic Solutions" Materials 12, no. 18: 3013. https://doi.org/10.3390/ma12183013