A Review on the Corrosion Behaviour of Nanocoatings on Metallic Substrates
Abstract
:1. Introduction
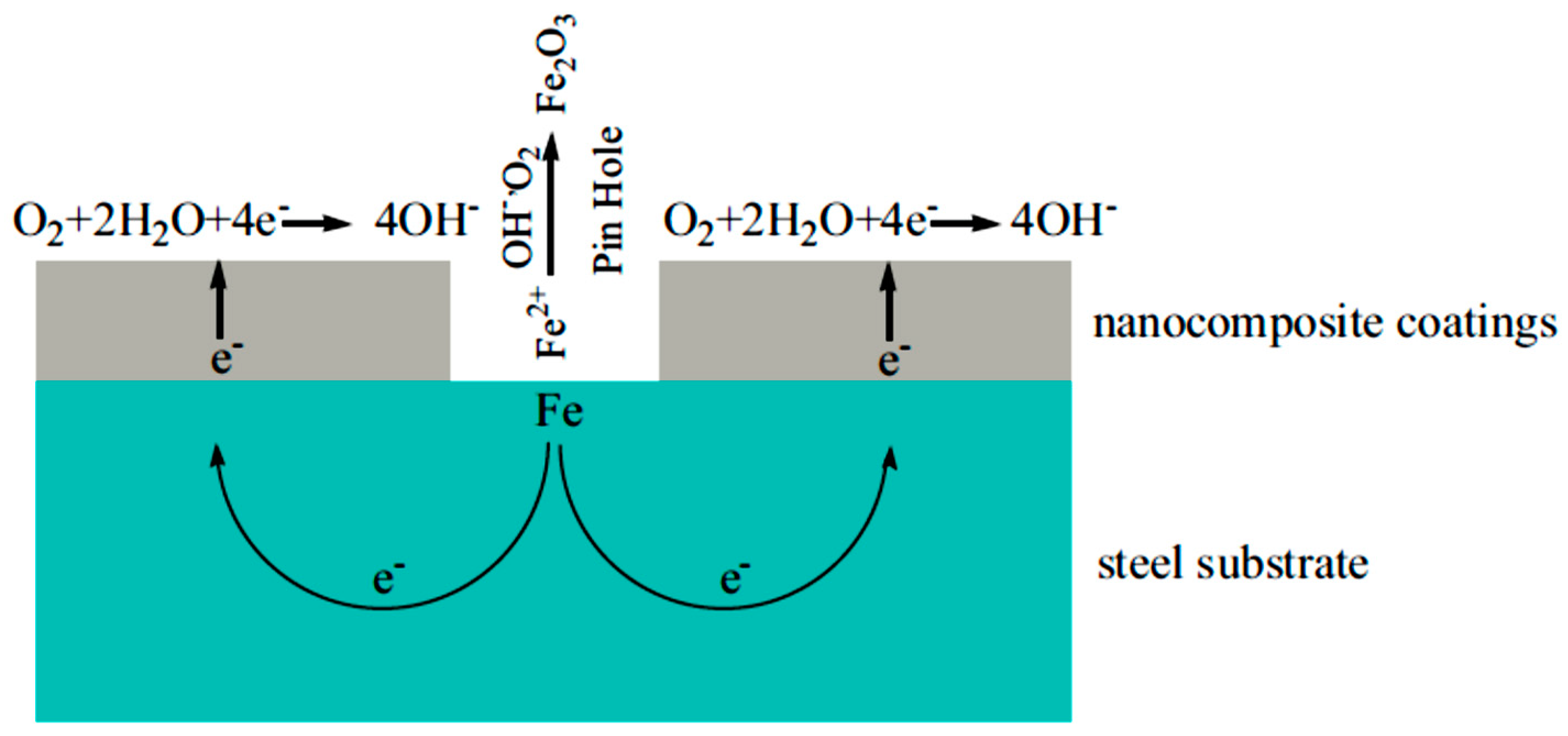
2. Nanocoating and Its Role in Corrosion Prevention
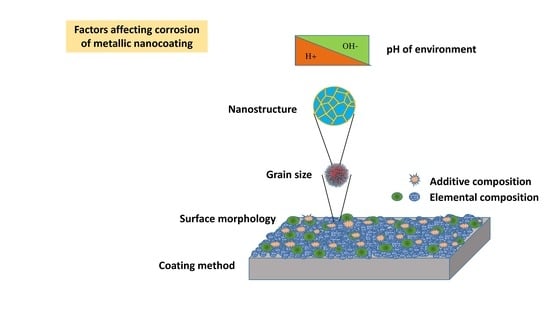
2.1. Metallic Nanocoating
2.1.1. Nanocoating Composition
2.1.2. Coating Structure Size
2.1.3. Nanocoatings’ Grain Size
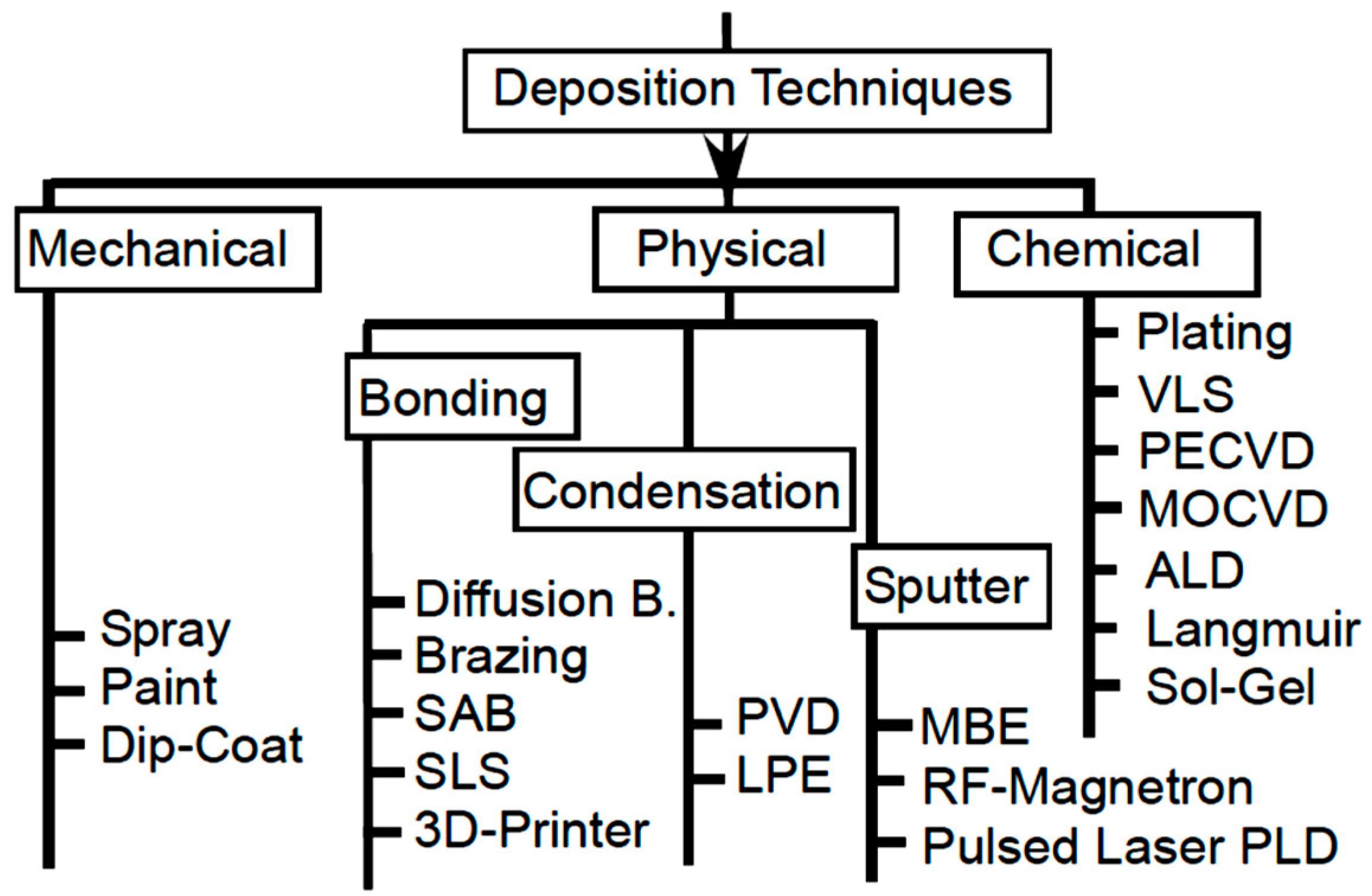
2.1.4. Coating Method
2.1.5. Additive Type and Concentration
2.1.6. pH of the Environment
2.1.7. Surface Morphology of the Nanocoating
2.2. Ceramic Nanocoating
2.2.1. Titanium Oxides Nanocoating
2.2.2. Alumina Nanocoating
2.2.3. Tantalum Oxide Nanocoating
2.2.4. Zirconia Nanocoating
| Nanomaterial Coating | Coating Thickness | Substrate | Electrolyte | Corrosion Resistance | Tested Conditions | Ecorr (V vs. SCE) | Icorr (µA/cm2) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Titanium Oxides | ||||||||
| TiO2 anatase NP (φ 15–18 nm) | 375 nm | 316L Stainless Steel | Ringer solution | Conformal thin layers of TiO2 formed an entire shield over the substrate | - | No values provided. Only graph | [71] | |
| TiO2 NP (φ 40 nm, pore size 5–8 nm) | 375 nm, 464 nm, 550 nm | 316L Stainless Steel | 0.5 mol/L NaCl solution | Best for 464-nm coating thickness. Increasing NaCl concentration or decreasing pH increased corrosion | 375-nm TiO2 thickness | −0.011 | 0.00897 | [73] |
| 464-nm thickness | 0.027 | 0.000105 | ||||||
| 550-nm thickness | −0.117 | 0.783 | ||||||
| Amorphous TiO2 NP films | 50 nm | 316L Stainless Steel | 3 wt.% NaCl solution | Conformal and dense thin layers of TiO2 formed an entire shield over the substrate | Bare stainless steel | −0.96 | 0.7 | [74] |
| TiO2 coated stainless steel | −0.63 | 0.063 | ||||||
| TiO2 thin films | 370 nm | 316L Stainless Steel | 0.5-M NaCl Solution | Best with the N-modified TiO2 surface | - | No values provided. Only graph | [77] | |
| Amorphous TiO2 NP layer over CrN coated SS | 90 nm | 316L Stainless Steel | 3 wt.% NaCl solution | Conformal thin layers of TiO2 formed an entire shield over the coated substrate | Bare stainless steel | −0.95 | 0.0026 | [75] |
| CrN single layer over stainless steel | −0.74 | 0.0019 | ||||||
| CrN/TiO2 over stainless steel | −0.49 | 0.00031 | ||||||
| Tantalum Oxides | ||||||||
| equiaxed ß Ta2O5 (avg. grain size ~20 nm) | 25 µm | Ti-6Al-4V | 3.5 wt.% NaCl solution | Enhanced for coated samples | Uncoated Ti-6Al-4V | −0.54 | 0.501 | [94] |
| Ta2O5 nanocoated Ti-6Al-4V | −0.26 | 0.117 | ||||||
| T2N (grain size 13 nm) | - | Ti-6Al-4V | 0.5 M H2SO4 solution | Increasing the acidity and temperature decreases the corrosion resistance | - | No potentiodynamic test done | [29] | |
| Thin films of tantalum oxide (Ta2O5) | 10, 50 nm | Carbon steel | 0.2-M NaCl solution | Icorr decreases with increasing grain size (best for 50 nm). Ta–O nanocoating better than Cr–O | Ta–O (10 nm) | −0.714 | 0.169 | [92] |
| Cr–O (10 nm) | −0.753 | 0.428 | ||||||
| Ta–O (50 nm) | −0.671 | 0.0348 | ||||||
| Cr–O (50 nm) | −0.693 | 0.208 | ||||||
| Thin films of tantalum oxide (Ta2O5) | 50 nm | Low alloy steel | 0.2-M NaCl solution | Corrosion rate of coated steel by FCAD is four times less than that of the ALD | ALD at pH 7 | −0.79 | 0.093 | [95] |
| FCAD at pH 7 | −0.67 | 0.039 | ||||||
| Aluminium Oxides | ||||||||
| Thin films of Al2O3 deposited | 50 nm | carbon steel | 0.2-M NaCl solution | Failed to protect the steel | - | No potentiodynamic test done | [87] | |
| Al2O3 (from 8–12 nm nanoparticles) | _ | 9Cr-1Mo steel | NaCl solution | Enhanced at both concentration compared to bare substrate, but the coating was susceptible to pitting under 200 ppm of Cl-conc. | - | No values provided. Only graph | [81] | |
| Al2O3 thin film deposited | 10 nm, 50 nm, and 100 nm | 100Cr6 carbon steel | Deaerated 0.2-M NaCl solution | Corrosion rate decreased by one, two, and four orders of magnitude for the coating thicknesses of 10 nm, 50 nm, and 100 nm, respectively | - | No values provided. Only graph | [85] | |
| Al2O3 thin film deposited | 50 nm | 100Cr6 carbon steel | Deaerated 0.2 M–NaCl solution | Enhanced for thermally ALD more than for plasma ALD one. | - | No values provided. Only graph | [88] | |
| Al2O3 thin film deposited | 10–50 nm | 100Cr6 carbon steel, Al2024-T3 alloy | Deaerated NaCl solution | Best corrosion for steel and Al alloy was at 150 °C and 50 °C, respectively. Better with PEALD than thermal ALD. | - | No potentiodynamic test done | [82] | |
| Al2O3 thin film deposited | 200 nm | X40CrMoV5-1 steel | 1-M HCl solution | Best with 300 °C deposition temperature | ALD at 150 °C | −0.43 | 670 | [83] |
| ALD at 225 °C | −0.447 | 190 | ||||||
| ALD at 300 °C | −0.456 | 50 | ||||||
| Al2O3 thin film deposited | 10, 20, 50 nm | Copper | Deaerated 0.5-M NaCl solution | 10 nm better than 50 nm | 10-nm thickness of Al2O3 | −0.356 | 0.15 | [89] |
| 20-nm thickness of Al2O3 | −0.336 | 1.52 | ||||||
| 50-nm thickness of Al2O3 | −0.308 | 2.71 | ||||||
| Al2O3 and Ta2O5 thin film deposited | 5–50 nm | 316L stainless steel | Deaerated 0.8-M NaCl solution | Better with higher thickness and higher deposition temperature. Al2O3 nanocoating better than Ta2O5. | - | No values provided. Only graph | [84] | |
| Zirconium Oxides | ||||||||
| Thin films of ZrO2 | 10, 35, 100 nm | Brass | Borate buffer (BB) and BB + 0.5-M NaCl solution | All showed a protective effect of the nanocoating. | - | No potentiodynamic test done | [105] | |
| Thin films of ZrO2 | 50–350 nm | Pre-oxidised 304L stainless steel | 0.1-M Na2PO4 solution | Best when oxidising the surface before coating | Uncoated substrate that is pre-oxidised with water and oxygen, and then with Fe2O3 | −0.2475 | 1.972 | [101] |
| Zirconia-coated substrate that is pre-oxidised with water and oxygen, and then with Fe2O3 | −0.1922 | 0.104 | ||||||
| Thin films of ZrO2 | 70–180 nm | Aluminum alloy AA6060 | Diluted Harrison solution (0.05 wt.% NaCl + 0.35 wt.% (NH4)2SO4) | Three dips of zirconia coating gave the same barrier properties as chromatised substrate | - | No values provided. Only graph | [27] | |
| Thin films of ZrO2 | 155 nm | 316L stainless steel | 1-M H2SO4 solution | Best with heat treatment temperature of 500 °C | Coated at 300 °C | −0.1814 | 3.11 | [109] |
| Coated SS at 500 °C | −0.152 | 0.65 | ||||||
| Coated SS at 600 °C | −0.1673 | 2.88 | ||||||
| Thin films of ZrO2 | - | AZ91D magnesium alloy | 3.5% NaCl solution | Best with treatment temperature of 360 °C | Zirconia-coated at 120 °C | −1.5651 | 1.98 | [108] |
| Zirconia-coated at 240 °C | −1.5468 | 1.43 | ||||||
| Zirconia-coated at 360 °C | −1.5155 | 0.526 | ||||||
| Thin films of ZrO2 | 0.4–0.6 µm | 316L stainless steel | Deaerated H2SO4 and in 3% NaCl solutions | Presented barrier properties in both acidic and neutral solutions | - | No values provided. Only graph | [103] | |
| Thin films of ZrO2 | 0.5–0.8 µm | 316L stainless steel | Hank solution | Better with samples treated at 400 °C more than 650 °C | - | No values provided. Only graph | [50] | |
| Thin films of ZrO2 | 500 nm | Alumina–silica refractory material | Molten borosilicate glass at 1400 °C for 162 h. | For zirconia-coated refractory, porosity and corrosion loss decreased by 18% and 16%, respectively. | - | No potentiodynamic test done | [107] | |
| Thin films of ZrO2 | 150 µm | Cp–Ti and Ti–13Nb–13Zr alloy | Hank solution | Almost same corrosion enhancement on the two substrate by coating with zirconia. | Al2O3-13 wt.% TiO2 coating on cp-Ti | 0.306 | 1.77 | [104] |
| ZrO2 coating on Ti–13Nb–13Zr | 0.516 | 3.79 | ||||||
| ZrO2 on cp–Ti substrate | 0.411 | 3.02 | ||||||
2.2.5. Other Ceramic Nanocoatings
2.3. Nanocomposite Coating
2.3.1. Polymeric Host Matrix of Nanocomposite Coatings
Conductive Polymer Matrix Nanocomposite Coating
Waterborne Polymer Nanocomposite Coating
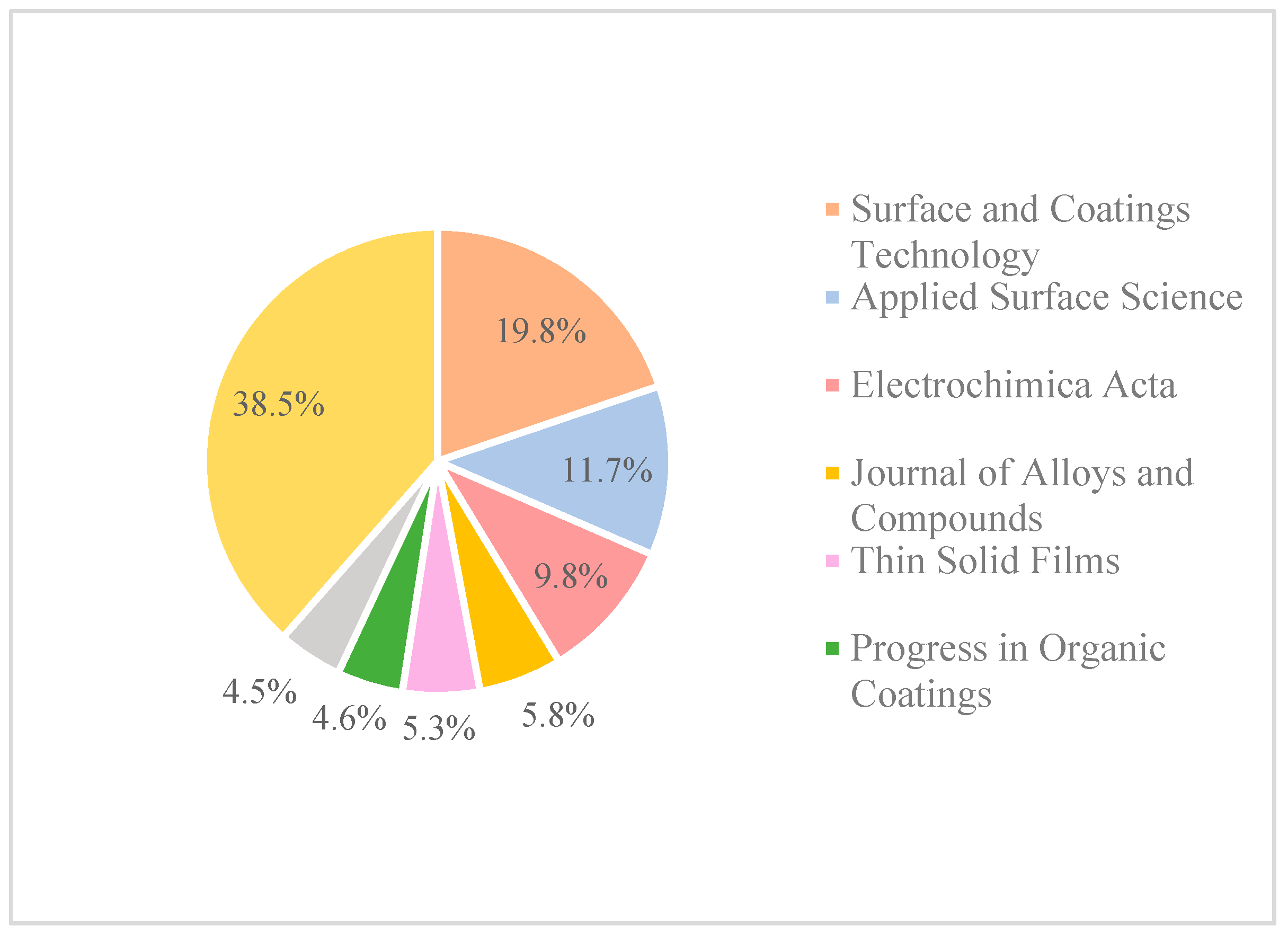
| Coating | Nanomaterial | Coating Thickness (µm) | Substrate | Electrolyte | Ecorr (V vs. SCE) | Icorr (μA/cm2) | Corrosion Resistance | Ref. |
|---|---|---|---|---|---|---|---|---|
| MWCNTs-epoxy | MWCNT diameter: 2–15 nm, length: 1–10 μm, layers: 5–20 | 500 | Steel | 5% NaCl solution | No potentiodynamic test done | Charge transfer resistance after the exposure to 5% NaCl is higher for the nanocoatings than for the neat coatings for both epoxy and vinyl chloride/vinyl acetate copolymer (VYHH) resins systems. | [127] | |
| MWCNTs-vinyl chloride/vinyl acetate copolymer | 200 | |||||||
| Al2O3-polymer (Xylan 1810/D1864) | Al2O3 particle size 50 nm | 80–100 | Low carbon steel | 3 wt.% NaCl solution | No values provided. Only graph | Small improvement in the corrosion resistance when 10 wt.% of Al2O3 filler were added to the polymer matrix compared to only the polymer coating, and significant improvement when compared to bare carbon steel. | [128] | |
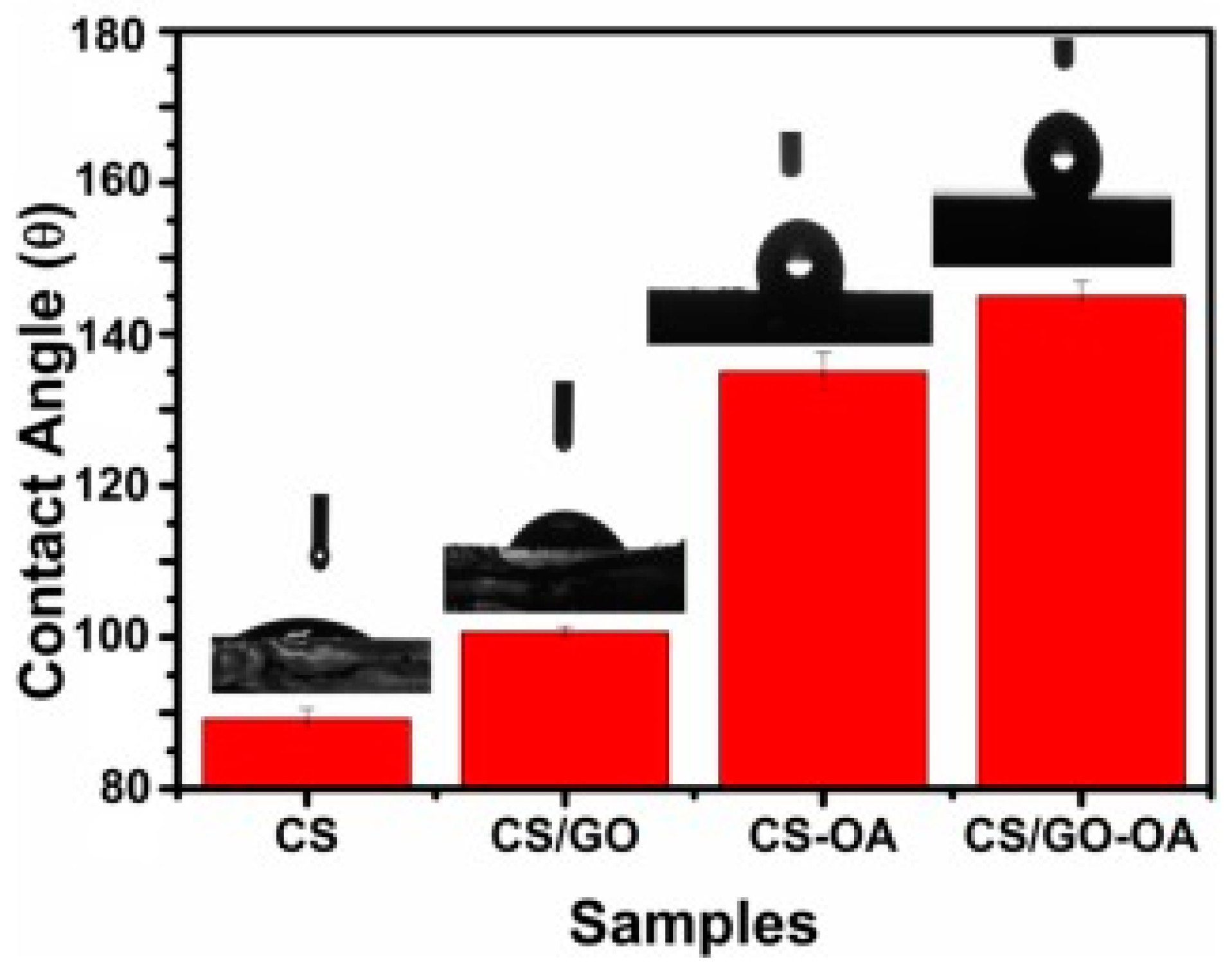
| No coating | GO platelet thickness: 1.3 nm, flake size: 3 µm | 3.5 | Carbon steel | 3.5 wt.% NaCl solutions | −0.790 | 84.4 | CS/GO-OA hydrophobic film has the lowest corrosion current and corrosion rate. Nanolayers maintained long-term anti-corrosive stability, which is correlated with hydrophobicity and permeability. Optimal filler concentration: 3 wt.% filler | [120] |
| CS | −0.707 | 18.72 | ||||||
| CS/GO | −0.722 | 15.4 | ||||||
| CS/GO-OA | −0.374 | 3.9 | ||||||
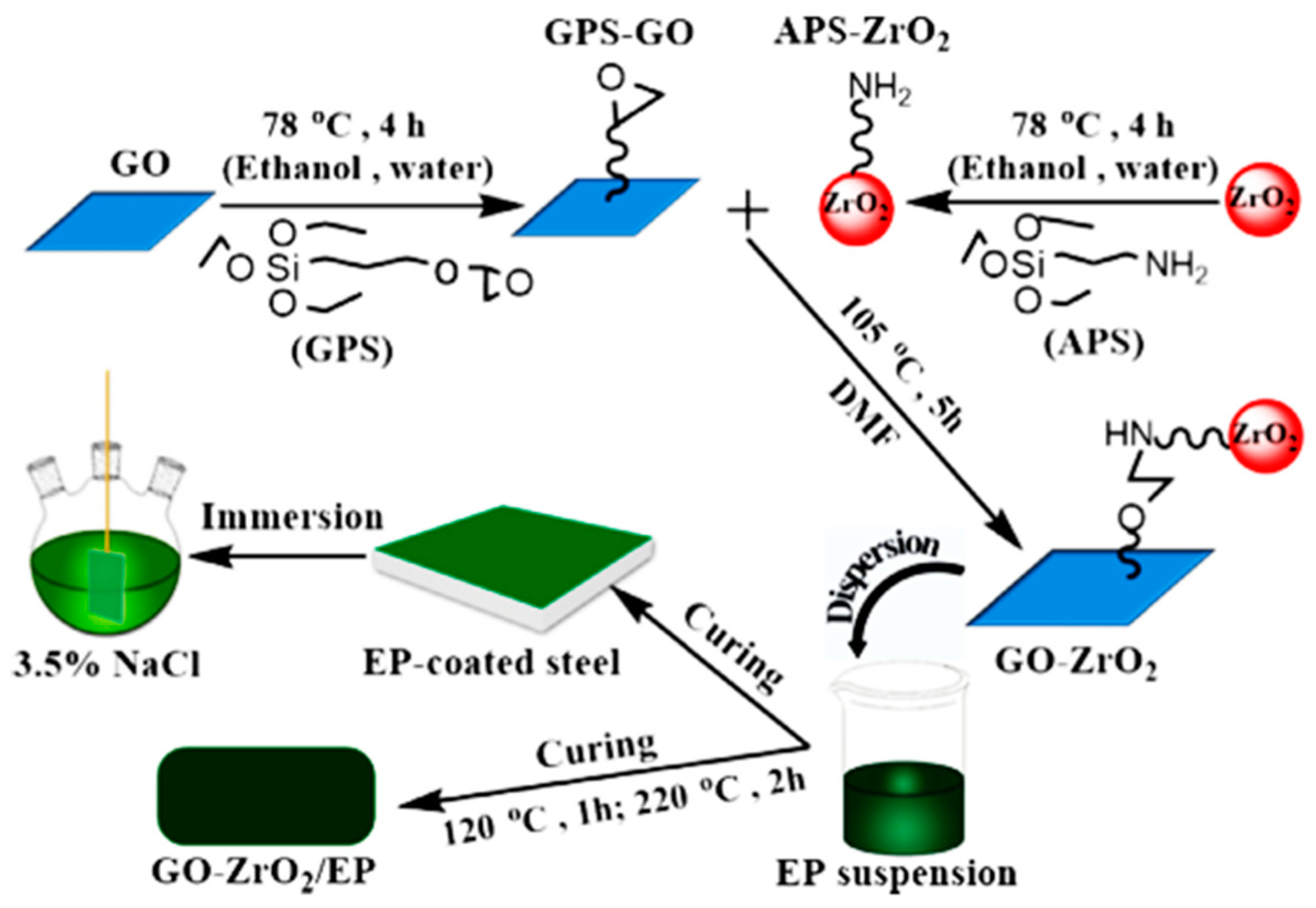
| GO–ZrO2 in EP matrix | 60-nm ZrO2 nanoparticles and GO | 65 | Steel | 3.5 wt.% NaCl solutions | –0.432 | 0.370 | Well-dispersed GO–ZrO2 embedded in an epoxy resin (EP) matrix provided a superior barrier effect due to their two-dimensional sheet and plugging tiny pores properties Optimal filler concentration 2 wt.% of GO–ZrO2 | [129] |
| SiO2/P(St-BA) in fluoropolymer (matrix) | 10–20-nm SiO2 nanoparticles | Mild steel | 3.5 wt.% NaCl solutions, pH 7 | 0.796 | 0.031 | A 4 wt.% SiO2 concentration has the best corrosion resistance by increasing the barrier properties | [130] | |
| Conductive Polymer Nanocomposite | ||||||||
| TiO2–polyaniline–polyvinyl butyral (PVB) | 75–105-nm TiO2 particles | 15–17 | Stainless steel | 3.5 wt.% NaCl solutions | No values provided. Only graph | A 100-times improvement in the corrosion resistance, especially for polyaniline prepared with 4.18 wt.% nano-TiO2 | [136] | |
| PVAc | 5–7-nm PANI particles 16-nm ZnO particle | Stainless steel | 3.5 wt.% NaCl solutions | No values provided. Only graph | After 15 days of immersion in the electrolyte, all showed a superior corrosion resistance for the hybrid coating PVAc-ZnO-Pani compared to the others | [137] | ||
| PVAc–ZnO | ||||||||
| PVAc–ZnO–Pani | ||||||||
| Graphene–polyaniline (PANI/G) | Graphene nanoflake thickness 0.569 ± 0.231 | 0.566 ± 0.322 | Mild steel | 0.1 M HCl, pH = 1 | −0.532 | 0.572 | Best corrosion resistance obtained at optimal concentration = 0.2% | [138] |
| CaCO3–polyaniline | 20–56-nm CaCO3 nanoparticles | 50 | Mild steel | 5 wt.% HCl solution | No potentiodynamic test done | Corrosion rate of alkyd coating is found to decrease with the increase of the polyaniline (PANI)-CaCO3 (PAC) nanocomposite loading in alkyd resin | [121] | |
| 5 wt.% NaOH solution | ||||||||
| 5 wt.% NaCl solution | No values provided. Only graph | |||||||
| no coating | _ | _ | Copper | 5000-ppm NaCl solution | −0.331 | 5.2 | [139] | |
| PANI | −0.078 | 1.8 | ||||||
| PANI/G | −0.282 | 0.1 | ||||||
| Waterborne Polymer | ||||||||
| Fe3O4– epoxy acrylate (EpAc)– butylated melamine formaldehyde (BMF) | 10–30-nm Fe3O4 nanoparticles | 108–142 | Mild steel | 3.5 wt.% HCl solution | −0.694 | 0.215 | Best corrosion resistance at 2.5 wt.% concentration of Fe3O4. Same behaviour when tested in NaCl, best resistance at 2.5 wt.% concentration | [142] |
| 3.5 wt.% NaOH solution | −0.222 | 50.8 | ||||||
| Tap water | −0.512 | 5.343 | ||||||
| Fe2O3 alkyd | 10–30-nm Fe2O3 nanoparticles | _ | Mild steel | Salt spray | No potentiodynamic test done | A coating system with higher concentration of nano-Fe2O3 particles (0.3 wt.%) showed best corrosion resistance, UV resistance, scratch resistance, and abrasion resistance | [145] | |
| ZnO alkyd-nano | 35–40-nm ZnO nanoparticles | 9–10 | Mild steel | Salt spray | No potentiodynamic test done | Addition of extremely small concentration of nano-ZnO can improve the corrosion resistance, scratch resistance, and abrasion resistance of the coating | [146] | |
2.3.2. Metallic Host Matrix Nanocomposite Coatings
Electroless Nickel Nanocomposite Coating
3. Conclusions
4. Challenges of Corrosion Studies of the Nanocoatings
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stansbury, E.E.; Buchanan, R.A. Fundamentals of Elecrochemical Corrosion; ASM International: Materials Park, OH, USA, 2000; ISBN 0-87170-676-8. [Google Scholar]
- Baena, L.M.; Gómez, M.; Calderón, J.A. Aggressiveness of a 20% bioethanol-80% gasoline mixture on autoparts: I behavior of metallic materials and evaluation of their electrochemical properties. Fuel 2012, 95, 320–328. [Google Scholar] [CrossRef]
- ASM International. The Effects and Economic Impact of Corrosion. In Corrosion: Understanding the Basics; ASM International: Materials Park, OH, USA, 2000; pp. 1–21. [Google Scholar]
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study; NACE International: Houston, TX, USA, 2016. [Google Scholar]
- Koc, G.H.; Brongers, M.P.H.; Thompson, N.G.; Virmani, Y.P.; Payer, J.H. Corrosion Costs and Preventive Strategies in the United States; NACE International: McLean, VA, USA, 2002. [Google Scholar]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Prentice-Hall Inc.: Upper Saddle River, NJ, USA, 1996; ISBN 0133599930. [Google Scholar]
- HSE’s Hazardous Installations Directorate. External Corrosion Management Inspection Project; Health and Safety Executive: Conroe, TX, USA, 2010.
- Lamaka, S.V.; Zheludkevich, M.L.; Yasakau, K.A.; Montemor, M.F.; Ferreira, M.G.S. High effective organic corrosion inhibitors for 2024 aluminium alloy. Electrochim. Acta 2007, 52, 7231–7247. [Google Scholar] [CrossRef]
- Rahmani, K.; Jadidian, R.; Haghtalab, S. Evaluation of inhibitors and biocides on the corrosion, scaling and biofouling control of carbon steel and copper-nickel alloys in a power plant cooling water system. Desalination 2015, 393, 174–185. [Google Scholar] [CrossRef]
- Cho, C.P.; Kwon, O.S.; Lee, Y.J. Effects of the sulfur content of liquefied petroleum gas on regulated and unregulated emissions from liquefied petroleum gas vehicle. Fuel 2014, 137, 328–334. [Google Scholar] [CrossRef]
- Du, D.; Chen, K.; Lu, H.; Zhang, L.; Shi, X.; Xu, X.; Andresen, P.L. Effects of chloride and oxygen on stress corrosion cracking of cold worked 316/316L austenitic stainless steel in high temperature water. Eval. Program Plan. 2016, 110, 134–142. [Google Scholar] [CrossRef]
- Calderón, J.A.; Jiménez, J.P.; Zuleta, A.A. Improvement of the erosion-corrosion resistance of magnesium by electroless Ni-P/Ni(OH)2-ceramic nanoparticle composite coatings. Surf. Coat. Technol. 2016, 304, 167–178. [Google Scholar] [CrossRef]
- Telegdi, J.; Szabó, T.; Románszki, L.; Pávai, M. The use of nano-/microlayers, self-healing and slow-release coatings to prevent corrosion and biofouling. In Handbook of Smart Coatings for Materials Protection; Woodhead Publishing: Cambridge, UK, 2014; pp. 135–182. ISBN 9780857096883. [Google Scholar] [Green Version]
- Wang, S.; Ma, Z.; Liao, Z.; Song, J.; Yang, K.; Liu, W. Study on improved tribological properties by alloying copper to CP-Ti and Ti–6Al–4V alloy. Mater. Sci. Eng. C 2015, 57, 123–132. [Google Scholar] [CrossRef]
- Singh, R. Coating for Corrosion Prevention. In Corrosion Control for Offshore Structures: Cathodic Protection and High Efficiency Coating; Gulf Professional Publishing: Waltham, MA, USA, 2014; pp. 115–129. [Google Scholar]
- Samimiã, A.; Zarinabadi, S. An Analysis of Polyethylene Coating Corrosion in Oil and Gas Pipelines. J. Am. Sci. 2011, 7, 1032–1036. [Google Scholar]
- Van Velson, N.; Flannery, M. Performance Life Testing of a Nanoscale Coating for Erosion and Corrosion Protection in Copper Microchannel Coolers. In Proceedings of the 15th IEEE Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (ITherm), Las Vegas, NV, USA, 31 May–3 June 2016; pp. 662–669. [Google Scholar]
- Saji, V.S. The impact of nanotechnology on reducing corrosion cost. In Corrosion Protection and Control Using Nanomaterials; Saji, V.S., Cook, R., Eds.; Woodhead Publishing Limited: Philadelphia, PA, USA, 2012; pp. 3–15. ISBN 9781845699499. [Google Scholar]
- Mingming, Y.; Yedong, H.; Ying, Z.; Quixia, Y. Al2O3-Y2O3 Nano- and Micro-composite coatings on Fe-9Cr-Mo. J. Rare Earth 2006, 24, 587–590. [Google Scholar]
- Dariva, C.G.; Galio, A.F. Corrosion Inhibitors—Principles, Mechanisms and Applications. In Developments in Corrosion Protection; IntechOpen Limited: London, UK, 2014; p. 16. ISBN 978-953-51-1223-5. [Google Scholar]
- Bashir, S.; Liu, J.L. Nanomaterials and Their Application. In Advanced Nanomaterials and Their Applications in Renewable Energy; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 1–50. ISBN 9780128017081. [Google Scholar]
- Schaefer, H.-E. Nanoscience; Springer: Berlin/Heidelberg, Germany; Stuttgart, Germany, 2010; Volume 1, ISBN 9788578110796. [Google Scholar]
- Yousaf, S.; Alhnan, M.A.; Abdallah, A.; Abdallah, B.; Khan, I.; Ahmed, W. Nanocoatings in medicine: Antiquity and modern times. In Emerging Nanotechnologies for Manufacturing; Ahmed, W., Jackson, M.J., Eds.; Elsevier Inc.: Oxford, UK, 2015; pp. 418–443. ISBN 9780323289900. [Google Scholar]
- van Lente, H.; van Til, J.I. Articulation of sustainability in the emerging field of nanocoatings. J. Clean. Prod. 2008, 16, 967–976. [Google Scholar] [CrossRef]
- Schuh, C.A.; Nieh, T.G.; Iwasaki, H. The effect of solid solution W additions on the mechanical properties of nanocrystalline Ni. Acta Mater. 2003, 51, 431–443. [Google Scholar] [CrossRef]
- Sriraman, K.R.; Strauss, H.W.; Brahimi, S.; Chromik, R.R.; Szpunar, J.A.; Osborne, J.H.; Yue, S. Tribological behavior of electrodeposited Zn, Zn–Ni, Cd and Cd–Ti coatings on low carbon steel substrates. Tribiol. Int. 2012, 56, 107–120. [Google Scholar] [CrossRef]
- Andreatta, F.; Aldighieri, P.; Paussa, L.; Di Maggio, R.; Rossi, S.; Fedrizzi, L. Electrochemical behaviour of ZrO2 sol–gel pre-treatments on AA6060 aluminium alloy. Electrochim. Acta 2007, 52, 7545–7555. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hu, Y.; Li, C. Comparative Study on Optical Properties and Scratch Resistance of Nanocomposite Coatings Incorporated with Flame Spray Pyrolyzed Silica Modified via in-situ Route and ex-situ Route. J. Mater. Sci. Technol. 2016, 32, 251–258. [Google Scholar] [CrossRef]
- Ma, J.; Xu, J.; Jiang, S.; Munroe, P.; Xie, Z. Effectsof pH value and temperature on the corrosion behavior of a Ta2N nanoceramic coating in simulated polymer electrolyte membrane fuel cell environment. Ceram. Int. 2016, 42, 16833–16851. [Google Scholar] [CrossRef]
- Hibbard, G.; Aust, K.T.; Palumbo, G.; Erb, U. Thermal Stability of Electrodeposited Nanocrystalline Cobalt. Scr. Mater. 2001, 44, 513–518. [Google Scholar] [CrossRef]
- McGee, J.D.; Thomas, S.S.; Bammel, B.D.; Bryden, T.R. Release on Demand Corrosion Inhibitor Composition. U.S. Patent No. 8241524, 14 August 2012. [Google Scholar]
- Youssef, K.M.S.; Koch, C.C.; Fedkiw, P.S. Improved corrosion behavior of nanocrystalline zinc produced by pulse-current electrodeposition. Corros. Sci. 2004, 46, 51–64. [Google Scholar] [CrossRef]
- Boostani, H.; Modirrousta, S. Review of Nanocoatings for Building Application. Procedia Eng. 2016, 145, 1541–1548. [Google Scholar] [CrossRef] [Green Version]
- Khanna, A.S. Nanotechnology in High Performance Paint Coatings. Asian J. Exp. Sci. 2008, 21, 25–32. [Google Scholar]
- Beyene, F.G. A review on nanocoating of metallic structures to improve hardness and maintaining toughness. i-Manager’s J. Mater. Sci. 2016, 4, 32–41. [Google Scholar]
- Mahapatro, A. Bio-functional nano-coatings on metallic biomaterials. Mater. Sci. Eng. C 2015, 55, 227–251. [Google Scholar] [CrossRef]
- Wunderlich, W. The Atomistic Structure of Metal/Ceramic Interfaces is the Key Issue for Developing Better Properties. Metals 2014, 4, 410–427. [Google Scholar] [CrossRef]
- Agarwala, V.; Agarwala, R.C.; Daniel, B.S.S. Development of nanograined metallic materials by bulk and coating techniques. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2006, 36, 3–16. [Google Scholar] [CrossRef]
- Hamdy, A.S. Corrosion Protection Performance via Nano-Coatings Technologies. Recent Pat. Mater. Sci. 2010, 3, 258–267. [Google Scholar] [CrossRef]
- Gao, W.; Li, Z. Nano-Structured Alloy and Composite Coatings for High Temperature Applications. Mater. Res. 2004, 7, 175–182. [Google Scholar] [CrossRef]
- GAMRY Instruments. Compliance Voltage—How Much is Enough? GAMRY Instruments: Warminster, PA, USA, 2014. [Google Scholar]
- Wang, L.; Zhang, J.; Gao, Y.; Xue, Q.; Hu, L.; Xu, T. Grain size effect in corrosion behavior of electrodeposited nanocrystalline Ni coatings in alkaline solution. Scr. Mater. 2006, 55, 657–660. [Google Scholar] [CrossRef]
- Wang, L.; Lin, Y.; Zeng, Z.; Liu, W.; Xue, Q.; Hu, L.; Zhang, J. Electrochemical corrosion behavior of nanocrystalline Co coatings explained by higher grain boundary density. Electrochim. Acta 2007, 52, 4342–4350. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Dey, G.K.; Dusane, R.O.; Grover, A.K. Improved pitting corrosion behaviour of electrodeposited nanocrystalline Ni-Cu alloys in 3.0 wt.% NaCl solution. J. Alloys Compd. 2006, 426, 235–243. [Google Scholar] [CrossRef]
- Lu, H.; Li, Y.; Wang, F. Enhancement of the electrochemical behavior for Cu–70Zr alloy by grain refinement. Surf. Coat. Technol. 2006, 201, 3393–3398. [Google Scholar] [CrossRef]
- Guan, X.; Wang, Y.; Xue, Q.; Wang, L. Toward high load bearing capacity and corrosion resistance Cr/Cr2N nano-multilayer coatings against seawater attack. Surf. Coat. Technol. 2015, 282, 78–85. [Google Scholar] [CrossRef]
- Pramod Kumar, U.; Kennady, C.J.; Zhou, Q. Effect of salicylaldehyde on microstructure and corrosion resistance of electrodeposited nanocrystalline Ni–W alloy coatings. Surf. Coat. Technol. 2015, 283, 148–155. [Google Scholar] [CrossRef]
- Mosavat, S.H.; Shariat, M.H.; Bahrololoom, M.E. Study of corrosion performance of electrodeposited nanocrystalline Zn-Ni alloy coatings. Corros. Sci. 2012, 59, 81–87. [Google Scholar] [CrossRef]
- Selvi, V.E.; Seenivasan, H.; Rajam, K.S. Electrochemical corrosion behavior of pulse and DC electrodeposited Co-P coatings. Surf. Coat. Technol. 2012, 206, 2199–2206. [Google Scholar] [CrossRef]
- Espitia-Cabrera, I.; Orozco-Hernández, H.; Torres-Sánchez, R.; Contreras-García, M.E.; Bartolo-Pe’rez, P.; Martínez, L. Synthesis of nanostructured zirconia electrodeposited films on AISI 316L stainless steel and its behaviour in corrosion resistance assessment. Mater. Lett. 2003, 58, 191–195. [Google Scholar] [CrossRef]
- Li, Q.; Yang, X.; Zhang, L.; Wang, J.; Chen, B. Corrosion resistance and mechanical properties of pulse electrodeposited Ni-TiO2 composite coating for sintered NdFeB magnet. J. Alloys Compd. 2009, 482, 339–344. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Q.; Zhang, J.; Yang, P.; Song, H.; An, M. Electrodeposition of nanocrystalline Zn-Ni coatings with single gamma phase from an alkaline bath. Surf. Coat. Technol. 2015, 270, 47–56. [Google Scholar] [CrossRef]
- Afshari, V.; Dehghanian, C. Effects of grain size on the electrochemical corrosion behaviour of electrodeposited nanocrystalline Fe coatings in alkaline solution. Corros. Sci. 2009, 51, 1844–1849. [Google Scholar] [CrossRef]
- Aledresse, A.; Alfantazi, A. A study on the corrosion behavior of nanostructured electrodeposited cobalt. J. Mater. Sci. 2004, 39, 1523–1526. [Google Scholar] [CrossRef]
- Mirak, M.; Alizadeh, M.; Ghaffari, M. Characterization, mechanical properties and corrosion resistance of biocompatible Zn-HA/TiO2 nanocomposite coatings. J. Mech. Behav. Biomed. Mater. 2016, 62, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.D.N.; Ghosh, R. Electroless nickel—phosphorus coatings to protect steel reinforcement bars from chloride induced corrosion. Surf. Coat. Technol. 2006, 201, 90–101. [Google Scholar] [CrossRef]
- Longfei, Z.O.U.; Shoufu, L.U.O.; Pengxing, L.I. A study on the anodic polarization behaviours of electroless nickel coatings in acidic, alkaline and neutral solutions. Surf. Coat. Technol. 1988, 36, 455–462. [Google Scholar] [CrossRef]
- Jung, H.; Alfantazi, A. An electrochemical impedance spectroscopy and polarization study of nanocrystalline Co and Co-P alloy in 0.1 M H2SO4 solution. Electrochim. Acta 2006, 51, 1806–1814. [Google Scholar] [CrossRef]
- Meng, G.; Li, Y.; Shao, Y.; Zhang, T.; Wang, Y.; Wang, F.; Cheng, X.; Dong, C.; Li, X. Effect of Microstructures on Corrosion Behavior of Nickel Coatings: (I) Abnormal Grain Size Effect on Corrosion Behavior. J. Mater. Sci. Technol. 2015, 31, 1186–1192. [Google Scholar] [CrossRef]
- Chianpairot, A.; Lothongkum, G.; Schuh, C.A.; Boonyongmaneerat, Y. Corrosion of nanocrystalline Ni–W alloys in alkaline and acidic 3.5 wt.% NaCl solutions. Corros. Sci. 2011, 53, 1066–1071. [Google Scholar] [CrossRef]
- Lowenheim, F.A. Modern Electroplating; John Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Saber, K.; Koch, C.C.; Fedkiw, P.S. Pulse current electrodeposition of nanocrystalline zinc. Mater. Sci. Eng. A 2003, 341, 174–181. [Google Scholar] [CrossRef]
- Ramanauskas, R.; Gudavičiute, L.; Juškenas, R.; Ščit, O. Structural and corrosion characterization of pulse plated nanocrystalline zinc coatings. Electrochim. Acta 2007, 53, 1801–1810. [Google Scholar] [CrossRef]
- Basavanna, S.; Arthoba Naik, Y. Study of the effect of new brightener on Zn-Ni alloy electrodeposition from acid sulphate bath. J. Appl. Electrochem. 2011, 41, 535–541. [Google Scholar] [CrossRef]
- Hassani, S.; Raeissi, K.; Azzi, M.; Li, D.; Golozar, M.A.; Szpunar, J.A. Improving the corrosion and tribocorrosion resistance of Ni-Co nanocrystalline coatings in NaOH solution. Corros. Sci. 2009, 51, 2371–2379. [Google Scholar] [CrossRef]
- Watchman, J.D.; Haber, R.A. Ceramic Films and Coatings—An overview. In Ceramic Films and Coatings; Noyes Publications: New York, NY, USA, 1993; p. 1. [Google Scholar]
- Callister, W.D.; Rethwisch, D.G. Fundamentals of Materials Science and Engineering an Integerated Approach, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Giolando, D.M. Transparent self-cleaning coating applicable to solar energy consisting of nano-crystals of titanium dioxide in fluorine doped tin dioxide. Sol. Energy 2016, 124, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Lorencik, S.; Yu, Q.L.; Brouwers, H.J.H. Photocatalytic coating for indoor air purification: Synergetic effect of photocatalyst dosage and silica modification. Chem. Eng. J. 2016, 306, 942–952. [Google Scholar] [CrossRef]
- Shen, G.X.; Chen, Y.C.; Lin, L.; Lin, C.J.; Scantlebury, D. Study on a hydrophobic nano-TiO2 coating and its properties for corrosion protection of metals. Electrochim. Acta 2005, 50, 5083–5089. [Google Scholar] [CrossRef]
- Kontos, A.I.; Kontos, A.G.; Tsoukleris, D.S.; Vlachos, G.D.; Falaras, P. Superhydrophilicity and photocatalytic property of nanocrystalline titania sol–gel films. Thin Solid Films 2007, 515, 7370–7375. [Google Scholar] [CrossRef]
- Shen, G.X.; Chen, Y.C.; Lin, C.J. Corrosion protection of 316L stainless steel by a TiO2 nanoparticle coating prepared by sol–gel method. Thin Solid Films 2005, 489, 130–136. [Google Scholar] [CrossRef]
- Shan, C.X.; Hou, X.; Choy, K. Corrosion resistance of TiO2 films grown on stainless steel by atomic layer deposition. Surf. Coat. Technol. 2008, 202, 2399–2402. [Google Scholar] [CrossRef]
- Shan, C.X.; Hou, X.; Choy, K.; Choquet, P. Improvement in corrosion resistance of CrN coated stainless steel by conformal TiO2 deposition. Surf. Coat. Technol. 2008, 202, 2147–2151. [Google Scholar] [CrossRef]
- Deyab, M.A.; Keera, S.T. Effect of nano-TiO2 particles size on the corrosion resistance of alkyd coating. Mater. Chem. Phys. 2014, 146, 406–411. [Google Scholar] [CrossRef]
- Yun, H.; Li, J.; Chen, H.; Lin, C. A study on the N-, S- and Cl-modified nano-TiO2 coatings for corrosion protection of stainless steel. Electrochim. Acta 2007, 52, 6679–6685. [Google Scholar] [CrossRef]
- Calle, E.; Ortega, P.; Von Gastrow, G.; Martín, I.; Savin, H. Long-term stability of Al2O3 passivated black silicon. Energy Procedia 2016, 92, 341–346. [Google Scholar] [CrossRef]
- Ali, K.; Choi, K.; Jo, J.; Woo, Y. High rate roll-to-roll atmospheric atomic layer deposition of Al2O3 thin films towards gas diffusion barriers on polymers. Mater. Lett. 2014, 136, 90–94. [Google Scholar] [CrossRef]
- Wuu, D.; Lin, C.; Chen, C.; Lee, H.; Huang, J. Properties of double-layer Al2O3/TiO2 antireflection coatings by liquid phase deposition. Thin Solid Films 2015, 584, 248–252. [Google Scholar] [CrossRef]
- Ruhi, G.; Modi, O.P.; Singh, I.B. Corrosion behaviour of nano structured sol-gel alumina coated 9Cr–1Mo ferritic steel in chloride bearing environments. Surf. Coat. Technol. 2009, 204, 359–365. [Google Scholar] [CrossRef]
- Potts, S.E.; Schmalz, L.; Fenker, M.; Díaz, B.; Swiatowska, J.; Maurice, V.; Seyeux, A.; Marcus, P.; Radnoczi, G.; Toth, L.; et al. Ultra-Thin Aluminium Oxide Films Deposited by Plasma-Enhanced Atomic Layer Deposition for Corrosion Protection. J. Electrochem. Soc. 2011, 158, 132–138. [Google Scholar] [CrossRef]
- Borylo, P.; Lukaszkowicz, K.; Szindler, M.; Kubacki, J.; Balin, K.; Basiaga, M.; Szewczenko, J. Structure and properties of Al2O3 thin films deposited by ALD process. Vacuum 2016, 131, 319–326. [Google Scholar] [CrossRef]
- Díaz, B.; Swiatowska, J.; Maurice, V.; Seyeux, A.; Normand, B.; Härkönen, E.; Ritala, M.; Marcus, P. Electrochemical and time-of-flight secondary ion mass spectrometry analysis of ultra-thin metal oxide (Al2O3 and Ta2O5) coatings deposited by atomic layer deposition on stainless steel. Electrochim. Acta 2011, 56, 10516–10523. [Google Scholar] [CrossRef]
- Díaz, B.; Härkönen, E.; Jolanta, Ś.; Maurice, V.; Seyeux, A.; Marcus, P.; Ritala, M. Low-temperature atomic layer deposition of Al2O3 thin coatings for corrosion protection of steel: Surface and electrochemical analysis. Corros. Sci. 2011, 53, 2168–2175. [Google Scholar] [CrossRef]
- Langereis, E.; Creatore, M.; Heil, S.B.S.; Van De Sanden, M.C.M.; Kessels, W.M.M. Plasma-assisted atomic layer deposition of Al2O3 moisture permeation. Appl. Phys. Lett. 2006, 89, 2–4. [Google Scholar] [CrossRef]
- Díaz, B.; Härkönen, E.; Maurice, V.; Swiatowska, J.; Seyeux, A.; Ritala, M.; Marcus, P. Failure mechanism of thin Al2O3 coatings grown by atomic layer deposition for corrosion protection of carbon steel. Electrochim. Acta 2011, 56, 9609–9618. [Google Scholar] [CrossRef]
- Härkönen, E.; Potts, S.E.; Kessels, W.M.M.; Díaz, B.; Seyeux, A.; Jolanta, Ś.; Maurice, V.; Marcus, P.; Radnóczi, G.; Tóth, L.; et al. Hydrogen—ARgon plasma pre-treatment for improving the anti-corrosion properties of thin Al2O3 films deposited using atomic layer deposition on steel. Thin Solid Films 2013, 534, 384–393. [Google Scholar] [CrossRef]
- Mirhashemihaghighia, S.; SwiatowskaMaurice, J.; Maurice, V.; Seyeux, A.; Klein, L.H.; Salmi, E.; Ritala, M.; Marcus, P. The role of surface preparation in corrosion protection of copper with nanometer-thick ALD alumina coatings. Appl. Surf. Sci. 2016, 387, 1054–1061. [Google Scholar] [CrossRef]
- Chaneliere, C.; Autran, J.L.; Devine, R.A.B.; Balland, B. Tantalum pentoxide (Ta2O5) thin films for advanced dielectric applications. Mater. Sci. Eng. 1998, 22, 269–322. [Google Scholar] [CrossRef]
- Rahmati, B.; Sarhan, A.A.D.; Zalnezhad, E.; Kamiab, Z.; Dabbagh, A.; Choudhury, D. Development of tantalum oxide (Ta-O) thin film coating on biomedical Ti-6Al-4V alloy to enhance mechanical properties and biocompatibility. Ceram. Int. 2016, 42, 466–480. [Google Scholar] [CrossRef]
- Díaz, B.; Jolanta, Ś.; Maurice, V.; Pisarek, M.; Seyeux, A.; Zanna, S.; Tervakangas, S.; Kolehmainen, J.; Marcus, P. Chromium and tantalum oxide nanocoatings prepared by filtered cathodic arc deposition for corrosion protection of carbon steel. Surf. Coat. Technol. 2012, 206, 3903–3910. [Google Scholar] [CrossRef]
- McKinley, K.A.; Sandler, N.P. Tantalum pentoxide for advanced DRAM applications. Thin Solid Films 1996, 291, 440–446. [Google Scholar] [CrossRef]
- Hu, W.; Xu, J.; Lu, X.; Hu, D.; Tao, H.; Munroe, P.; Xie, Z. Corrosion and wear behaviours of a reactive-sputter-deposited Ta2O5 nanoceramic coating. Appl. Surf. Sci. 2016, 368, 177–190. [Google Scholar] [CrossRef]
- Díaz, B.; Swiatowska, J.; Maurice, V.; Seyeux, A.; Härkönen, E.; Ritala, M.; Tervakangas, S.; Kolehmainen, J.; Marcus, P. Tantalum oxide nanocoatings prepared by atomic layer and filtered cathodic arc deposition for corrosion protection of steel: Comparative surface and electrochemical analysis. Electrochim. Acta 2013, 90, 232–245. [Google Scholar] [CrossRef]
- Moulzolf, S.C.; Lad, R.J.; Blau, P.J. Microstructural effects on the friction and wear of zirconia films in unlubricated sliding contact. Thin Solid Films 1999, 347, 220–225. [Google Scholar] [CrossRef]
- Naga, S.M.; Hassan, A.M.; Awaad, M. Physical and mechanical properties of Ta2O5 doped zirconia-toughened alumina (ZTA) composites. Ceram. Int. 2015, 41, 6248–6255. [Google Scholar] [CrossRef]
- Venkataraj, S.; Kappertz, O.; Liesch, C.; Detemple, R.; Jayavel, R. Thermal stability of sputtered zirconium oxide films. Vacuum 2004, 75, 7–16. [Google Scholar] [CrossRef]
- Emeline, A.V.; Kuzmin, G.N.; Basov, L.L.; Serpone, N. Photoactivity and photoselectivity of a dielectric metal-oxide photocatalyst (ZrO2) probed by the photoinduced reduction of oxygen and oxidation of hydrogen. J. Photochem. Photobiol. A Chem. 2005, 174, 214–221. [Google Scholar] [CrossRef]
- Cao, X.Q.; Vassen, R.; Stoever, D. Ceramic materials for thermal barrier coatings. J. Eur. Ceram. Soc. 2004, 24, 1–10. [Google Scholar] [CrossRef]
- Garg, N.; Bera, S.; Mangamma, G.; Das, C.R.; Kamaruddin, S.; Velmurugan, S. Electrochemical and adhesion properties of hydrothermally deposited nano-ZrO2 coatings on oxide layers of stainless steel. Surf. Coat. Technol. 2015, 281, 98–108. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Y.; Ye, C.; Zhang, P. Preparation and characterization of doped sol–gel zirconia films. Ceram. Int. 2002, 28, 349–354. [Google Scholar] [CrossRef]
- Atik, M.; de Neto, P.L.; Avaca, L.A.; Aegertera, M.A. Sol-Gel Thin Films for Corrosion Protection. Ceram. Int. 1995, 21, 403–406. [Google Scholar] [CrossRef]
- Richard, C.; Kowandy, C.; Landoulsi, J.; Geetha, M.; Ramasawmy, H. Corrosion and wear behavior of thermally sprayed nano ceramic coatings on commercially pure Titanium and Ti–13Nb–13Zr substrates. Int. J. Refract. Met. Hard Mater. 2010, 28, 115–123. [Google Scholar] [CrossRef]
- Holgado, J.P.; Yubero, F.; Espinos, J.P. Corrosion resistant ZrO2 thin films prepared at room temperature by ion beam induced chemical vapour deposition. Surf. Coat. Technol. 2002, 152, 449–453. [Google Scholar] [CrossRef]
- Viazzi, C.; Deboni, A.; Zoppas, J.; Bonino, J.; Ansart, F. Synthesis of Yttria Stabilized Zirconia by sol–gel route: Influence of experimental parameters and large scale production. Solid State Sci. 2006, 8, 1023–1028. [Google Scholar] [CrossRef] [Green Version]
- Kessman, A.J.; Ramji, K.; Morris, N.J.; Cairns, D.R. Zirconia sol–gel coatings on alumina–silica refractory material for improved corrosion resistance. Surf. Coat. Technol. 2009, 204, 477–483. [Google Scholar] [CrossRef]
- Li, Q.; Zhong, X.; Hu, J.; Kang, W. Preparation and corrosion resistance studies of zirconia coating on fluorinated AZ91D magnesium alloy. Progress Org. Coat. 2008, 63, 222–227. [Google Scholar] [CrossRef]
- Nouri, E.; Shahmiri, M.; Reza, H.; Talayian, F. Investigation of structural evolution and electrochemical behaviour of zirconia thin films on the 316L stainless steel substrate formed via sol–gel process. Surf. Coat. Technol. 2011, 205, 5109–5115. [Google Scholar] [CrossRef]
- López Ibáñez, R.; Martín, F.; Ramos-Barrado, J.R.; Leinen, D. Optimization of spray pyrolysis zirconia coatings on aluminized steel. Surf. Coat. Technol. 2006, 200, 6368–6372. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-Inhibiting Coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Sharma, M.; Singh, H. Preparation and coating of nano-ceramic on orthopaedic implant material using electrostatic spray deposition. JMADE 2015, 88, 278–286. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Nguyen, T.A.; Carriere, P.; Ngo Xuan, C. Nanocomposite Coatings: Preparation, Characterization, Properties, and Applications. Int. J. Corros. 2018, 2018, 4749501. [Google Scholar] [CrossRef]
- Li, X.; Guerieri, P.; Zhou, W.; Huang, C.; Zachariah, M.R. Direct deposit laminate nanocomposites with enhanced propellent properties. ACS Appl. Mater. Interfaces 2015, 7, 9103–9109. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shen, X.; Jiang, L.; Wang, K.; Chen, K. Solvothermal synthesis and characterization of sandwich-like graphene/ZnO nanocomposites. Appl. Surf. Sci. 2010, 256, 2826–2830. [Google Scholar] [CrossRef]
- Mittal, V. Polymer Nanocomposites: Synthesis, Microstructure, and Properties. In Optimization of Polymer Nanocomposite Properties; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 1–19. ISBN 9783527325214. [Google Scholar]
- Oliveira, M.; Machado, A. Preparation of polymer-based nanocomposites by different routes. Nanocomp. Synth. Charact. Appl. 2013, 1–22. [Google Scholar]
- Tyan, H.-L.; Liu, Y.-C.; Wei, K.-H. Thermally and Mechanically Enhanced Clay/Polyimide Nanocomposite via Reactive Organoclay. Chem. Mater. 1999, 11, 1942–1947. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Sadasivuni, K.K.; Ponnamma, D.; Al-Maadeed, M.A.A. Oleic acid-grafted chitosan/graphene oxide composite coating for corrosion protection of carbon steel. Carbohydr. Polym. 2016, 151, 871–878. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Sonawane, S.H. New approach for simultaneous enhancement of anticorrosive and mechanical properties of coatings: Application of water repellent nano CaCO3-PANI emulsion nanocomposite in alkyd resin. Chem. Eng. J. 2010, 156, 177–183. [Google Scholar] [CrossRef]
- Fernando, R.H. Nanocomposite and nanostructured coatings: Recent advancements. In Nanotechnology Applications in Coatings-ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2009; Volume 1008, pp. 2–21. [Google Scholar]
- Park, C.; Ounaies, Z.; Watson, K.A.; Crooks, R.E.; Smith, J.; Lowther, S.E.; Connell, J.W.; Siochi, E.J.; Harrison, J.S.; Clair, T.L.S. Dispersion of single wall carbon nanotubes by in situ polymerization under sonication. Chem. Phys. Lett. 2002, 364, 303–308. [Google Scholar] [CrossRef]
- Noh, M.W.; Lee, D.C. Synthesis and characterization of PS-clay nanocomposite by emulsion polymerization. Polym. Bull. 1999, 42, 619–626. [Google Scholar] [CrossRef]
- Shi, X.; Gan, Z. Preparation and characterization of poly(propylene carbonate)/montmorillonite nanocomposites by solution intercalation. Eur. Polym. J. 2007, 43, 4852–4858. [Google Scholar] [CrossRef]
- Shen, Z.; Simon, G.P.; Cheng, Y.-B. Comparison of solution intercalation and melt intercalation of polymer–clay nanocomposites. Polymer 2002, 43, 4251–4260. [Google Scholar] [CrossRef]
- Aglan, A.; Allie, A.; Ludwick, A.; Koons, L. Formulation and evaluation of nano-structured polymeric coatings for corrosion protection. Surf. Coat. Technol. 2007, 202, 370–378. [Google Scholar] [CrossRef]
- Wang, Y.; Lim, S.; Luo, J.L.; Xu, Z.H. Tribological and corrosion behaviors of Al2O3/polymer nanocomposite coatings. Wear 2006, 260, 976–983. [Google Scholar] [CrossRef]
- Di, H.; Yu, Z.; Ma, Y.; Zhang, C.; Li, F.; Lv, L.; Pan, Y.; Shi, H.; He, Y. Corrosion-resistant hybrid coatings based on graphene oxide–zirconia dioxide/epoxy system. J. Taiwan Inst. Chem. Eng. 2016, 67, 511–520. [Google Scholar] [CrossRef]
- Chen, L.; Song, R.G.; Li, X.W.; Guo, Y.Q.; Wang, C.; Jiang, Y. The improvement of corrosion resistance of fluoropolymer coatings by SiO2/poly(styrene-co-butyl acrylate) nanocomposite particles. Appl. Surf. Sci. 2015, 353, 254–262. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Yang, J.; Li, Y.; Qiu, H.; Yang, J. Reinforcement of graphene and its derivatives on the anticorrosive properties of waterborne polyurethane coatings. Compos. Sci. Technol. 2016, 129, 30–37. [Google Scholar] [CrossRef]
- Tang, X.Z.; Mu, C.; Zhu, W.; Yan, X.; Hu, X.; Yang, J. Flexible polyurethane composites prepared by incorporation of polyethylenimine-modified slightly reduced graphene oxide. Carbon 2016, 98, 432–440. [Google Scholar] [CrossRef]
- Fan, Y.; He, Y.; Luo, P.; Shi, T.; Chen, X. Pulse Current Electrodeposition and Properties of Ni-W-GO Composite Coatings. J. Electrochem. Soc. 2016, 163, D68–D73. [Google Scholar] [CrossRef]
- Rout, T.K.; Jha, G.; Singh, A.K.; Bandyopadhyay, N.; Mohanty, O.N. Development of conducting polyaniline coating: A novel approach to superior corrosion resistance. Surf. Coat. Technol. 2003, 167, 16–24. [Google Scholar] [CrossRef]
- Saji, V.S.; Thomas, J. Nanomaterials for corrosion control. Curr. Sci. 2007, 92, 51–55. [Google Scholar]
- Radhakrishnan, S.; Siju, C.R.; Mahanta, D.; Patil, S.; Madras, G. Conducting polyaniline-nano-TiO2 composites for smart corrosion resistant coatings. Electrochim. Acta 2009, 54, 1249–1254. [Google Scholar] [CrossRef]
- Patil, R.C.; Radhakrishnan, S. Conducting polymer based hybrid nano-composites for enhanced corrosion protective coatings. Progress Org. Coat. 2006, 57, 332–336. [Google Scholar] [CrossRef]
- Mahato, N.; Cho, M.H. Graphene integrated polyaniline nanostructured composite coating for protecting steels from corrosion: Synthesis, characterization, and protection mechanism of the coating material in acidic environment. Constr. Build. Mater. 2016, 115, 618–633. [Google Scholar] [CrossRef]
- Jafari, Y.; Ghoreishi, S.M.; Shabani-Nooshabadi, M. Polyaniline/Graphene nanocomposite coatings on copper: Electropolymerization, characterization, and evaluation of corrosion protection performance. Synth. Met. 2016, 217, 220–230. [Google Scholar] [CrossRef]
- Ammar, A.U.; Shahid, M.; Ahmed, M.K.; Khan, M.; Khalid, A.; Khan, Z.A. Electrochemical study of polymer and ceramic-based nanocomposite coatings for corrosion protection of cast iron pipeline. Materials 2018, 9, 332. [Google Scholar] [CrossRef]
- Oueiny, C.; Berlioz, S.; Perrin, F.X. Carbon nanotube-polyaniline composites. Progress Polym. Sci. 2014, 39, 707–748. [Google Scholar] [CrossRef]
- Rahman, O.U.; Kashif, M.; Ahmad, S. Nanoferrite dispersed waterborne epoxy-acrylate: Anticorrosive nanocomposite coatings. Progress Org. Coat. 2015, 80, 77–86. [Google Scholar] [CrossRef]
- Prieto, J. Painting the future. Eur. Coat. J. 2010, 4, 20–25. [Google Scholar]
- Wang, S.; Ang, H.M.; Tade, M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: State of the art. Environ. Int. 2007, 33, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Dhoke, S.K.; Mangal Sinha, T.J.; Khanna, A.S. Effect of nano-Fe2O3 particles particles on the corrosion behavior of alkyd based waterborne coatings. J. Coat. Technol. Res. 2009, 6, 353–368. [Google Scholar] [CrossRef]
- Dhoke, S.K.; Khanna, A.S.; Sinha, T.J.M. Effect of nano-ZnO particles on the corrosion behavior of alkyd-based waterborne coatings. Progress Org. Coat. 2009, 64, 371–382. [Google Scholar] [CrossRef]
- Dehgahi, S.; Amini, R.; Alizadeh, M. Corrosion, Passivation and Wear Behaviors of Electrodeposited Ni-Al2O3-SiC Nano-composite Coatings. Surf. Coat. Technol. 2016. [Google Scholar] [CrossRef]
- Vaezi, M.R.; Sadrnezhaad, S.K.; Nikzad, L. Electrodeposition of Ni-SiC nano-composite coatings and evaluation of wear and corrosion resistance and electroplating characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 176–182. [Google Scholar] [CrossRef]
- Benea, L.; Wenger, F.; Ponthiaux, P.; Celis, J.P. Tribocorrosion behaviour of Ni–SiC nano-structured composite coatings obtained by electrodeposition. Wear 2009, 266, 398–405. [Google Scholar] [CrossRef]
- Benea, L.; Luigi, P.; Borello, A.; Martelli, S. Wear corrosion properties of nano-structured SiC-nickel composite coatings obtained by electroplating. Wear 2002, 249, 995–1003. [Google Scholar] [CrossRef]
- Cai, C.; Zhu, X.B.; Zheng, G.Q.; Yuan, Y.N.; Huang, X.Q.; Cao, F.H.; Yang, J.F.; Zhang, B. Electrodeposition and characterization of nano-structured Ni-SiC composite films. Surf. Coat. Technol. 2011, 205, 3448–3454. [Google Scholar] [CrossRef]
- Yao, Y.; Yao, S.; Zhang, L.; Wang, H. Electrodeposition and mechanical and corrosion resistance properties of Ni-W/SiC nanocomposite coatings. Mater. Lett. 2007, 61, 67–70. [Google Scholar] [CrossRef]
- Shi, L.; Sun, C.; Gao, P.; Zhou, F.; Liu, W. Mechanical properties and wear and corrosion resistance of electrodeposited Ni-Co/SiC nanocomposite coating. Appl. Surf. Sci. 2006, 252, 3591–3599. [Google Scholar] [CrossRef]
- Feng, Q.; Li, T.; Teng, H.; Zhang, X.; Zhang, Y.; Liu, C.; Jin, J. Investigation on the corrosion and oxidation resistance of Ni-Al2O3 nano-composite coatings prepared by sediment co-deposition. Surf. Coat. Technol. 2008, 202, 4137–4144. [Google Scholar] [CrossRef]
- Ciubotariu, A.C.; Benea, L.; Lakatos-Varsanyi, M.; Dragan, V. Electrochemical impedance spectroscopy and corrosion behaviour of Al2O3-Ni nano composite coatings. Electrochim. Acta 2008, 53, 4557–4563. [Google Scholar] [CrossRef]
- Wuhrer, R.; Yeung, W.Y. A comparative study of magnetron co-sputtered nanocrystalline titanium aluminium and chromium aluminium nitride coatings. Scr. Mater. 2004, 50, 1461–1466. [Google Scholar] [CrossRef]
- Kim, G.S.; Lee, S.Y. Microstructure and mechanical properties of AlCrN films deposited by CFUBMS. Surf. Coat. Technol. 2006, 201, 4361–4366. [Google Scholar] [CrossRef]
- Kayani, A.; Buchanan, T.L.; Kopczyk, M.; Collins, C.; Lucas, J.; Lund, K.; Hutchison, R.; Gannon, P.E.; Deibert, M.C.; Smith, R.J.; et al. Oxidation resistance of magnetron-sputtered CrAlN coatings on 430 steel at 800 °C. Surf. Coat. Technol. 2006, 201, 4460–4466. [Google Scholar] [CrossRef]
- Yu, C.Y.; Wang, S.B.; Li, T.B.; Zhang, Z.X. Tribological behaviour of CrAlN coatings at 600 °C. Surf. Eng. 2013, 29, 318–321. [Google Scholar] [CrossRef]
- Swadźba, L.; Maciejny, A.; Formanek, B.; Liberski, P.; Podolski, P.; Mendala, B.; Gabriel, H.; Poznańska, A. Influence of coatings obtained by PVD on the properties of aircraft compressor blades. Surf. Coat. Technol. 1996, 78, 137–143. [Google Scholar] [CrossRef]
- Jehn, H.A.; Thiergarten, F.; Ebersbach, E.; Fabian, D. Characterization of PVD (Ti, Cr)Nxhard coatings. Surf. Coat. Technol. 1991, 50, 45–52. [Google Scholar] [CrossRef]
- Yang, Q.; McKellar, R. Nanolayered CrAlTiN and multilayered CrAlTiN-AlTiN coatings for solid particle erosion protection. Tribol. Int. 2015, 83, 12–20. [Google Scholar] [CrossRef]
- Cabrera, G.; Torres, F.; Caicedo, J.C.; Aperador, W.; Amaya, C.; Prieto, P. Improvement of electrochemical surface properties in steel substrates using a nanostructured CrN/AlN multilayer coating. J. Mater. Eng. Perform. 2012, 21, 128–136. [Google Scholar] [CrossRef]
- Geng, M.; He, G.; Sun, Z.; Chen, J.; Yang, Z.; Li, Y. Corrosion Damage Mechanism of TiN/ZrN Nanoscale Multilayer Anti-Erosion Coating. Coatings 2018, 8, 400. [Google Scholar] [CrossRef]
- Lin, C.H.; Duh, J.G. Electrochemical impedance spectroscopy (EIS) study on corrosion performance of CrAlSiN coated steels in 3.5 wt.% NaCl solution. Surf. Coat. Technol. 2009, 204, 784–787. [Google Scholar] [CrossRef]
- Mallory, G.O.; Hajdu, J.B. Electroless Plating: Fundamentals and Applications; American Electroplaters and Surface Finishers Society: Orlando, FL, USA, 1990; ISBN 0936569077. [Google Scholar]
- Agarwala, R.C.; Agarwala, V. Electroless alloy/composite coatings: A review. Sadhana 2003, 28, 475–493. [Google Scholar] [CrossRef]
- Sudagar, J.; Lian, J.; Sha, W. Electroless nickel, alloy, composite and nano coatings—A critical review. J. Alloys Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef]
- Hamdy, A.S.; Shoeib, M.A.; Hady, H.; Abdel Salam, O.F. Corrosion behavior of electroless Ni-P alloy coatings containing tungsten or nano-scattered alumina composite in 3.5% NaCl solution. Surf. Coat. Technol. 2007, 202, 162–171. [Google Scholar] [CrossRef]
- Hosseini, J. Bodaghi, a Corrosion Behavior of Electroless Ni-P-TiO2 Nanocomposite Coatings and Optimization of Process Parameters Using Taguchi Method. Port. Electrochim. Acta 2013, 31, 11–20. [Google Scholar] [CrossRef]
- Tamilarasan, T.R.; Rajendran, R.; Rajagopal, G.; Sudagar, J. Effect of surfactants on the coating properties and corrosion behaviour of Ni–P–nano-TiO2 coatings. Surf. Coat. Technol. 2015, 276, 320–326. [Google Scholar] [CrossRef]
- Ranganatha, S.; Venkatesha, T.V.; Vathsala, K. Development of electroless Ni-Zn-P/nano-TiO2 composite coatings and their properties. Appl. Surf. Sci. 2010, 256, 7377–7383. [Google Scholar] [CrossRef]
- Allahkaram, S.R.; Nazari, M.H.; Mamaghani, S.; Zarebidaki, A. Characterization and corrosion behavior of electroless Ni-P/nano-SiC coating inside the CO2 containing media in the presence of acetic acid. Mater. Des. 2011, 32, 750–755. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Allahkaram, S.R. Corrosion resistance enhancement of Ni-P electroless coatings by incorporation of nano-SiO2 particles. Mater. Des. 2011, 32, 133–138. [Google Scholar] [CrossRef]
- Bigdeli, F.; Allahkaram, S.R. An investigation on corrosion resistance of as-applied and heat treated Ni-P/nanoSiC coatings. Mater. Des. 2009, 30, 4450–4453. [Google Scholar] [CrossRef]













| Nanomaterial Coating | Coating Thickness | Substrate | Electrolyte | Corrosion Resistance | Tested Conditions | Ecorr (V vs. SCE) | Icorr (µA/cm2) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Multi-layers of nano Cr/Cr2N | Individual Cr layer was 21 nm | 316L Stainless steel | Artificial seawater solution | Best with the highest thickness ratio of Cr:Cr2N of 1.3 (lowest porosity) | Plain 316-L stainless steel | −0.59 | 1.23 | [46] |
| 1.3 thickness Cr/Cr2N | −0.38 | 0.0204 | ||||||
| 0.18 thickness Cr/Cr2N | −0.51 | 0.0651 | ||||||
| NC Ni–W alloy films | 30–56 µm | Mild steel | 0.2 M H2SO4 | Best with 100-ppm concentration of the additive | No additive | 0.481 | 434 | [47] |
| 50-ppm additive | −0.298 | 10.8 | ||||||
| 100-ppm additive | −0.302 | 7.02 | ||||||
| 250-ppm additive | −0.322 | 37.96 | ||||||
| NC zinc deposits (59 nm avg. grain size) | _ | Without a substrate | Deaerated 0.5 N NaOH | Corrosion rate for NC zinc deposits was 60% lower than that for electrogalvanised (EG) steel samples | NC Zn | −1.47 | 90 | [32] |
| Electrogalvanised (EG) steel | −1.455 | 229 | ||||||
| NC Ni–Cu alloy (grain size 2–30 nm) | 20 µm | Mild steel | Deaerated 3 wt.% NaCl Solution | Icorr values were lowest for pulse current electrodeposited Ni–Cu alloy of the 35.8 wt.% Cu and 12.7-nm avg. crystalline size | Monel-400 (67Ni-30Cu-2Fe-0.03C) | −0.314 | 0.807 | [44] |
| DC NC Ni-30.6 wt.% Cu | −0.322 | 0.312 | ||||||
| PC NC Ni-26.0 wt.% Cu | −0.305 | 0.251 | ||||||
| PC NC Ni-38.5 wt.% Cu | −0.294 | 0.113 | ||||||
| NC Ni–Co coating | 30 µm | Carbon steel (AISI 1045) | 10 w/w% NaOH solution | Addition of saccharin achieved better resistance than sodium lauryl sulphate | - | No potentiodynamic test performed. Only EIS | [65] | |
| NC Zn–Ni alloy | _ | Carbon steel | 3 wt.% NaCl Solution | Best with NC Zn–Ni alloy coating of 17.62 wt.% and a 37-nm grain size. | NC Zn-12Ni alloy | −0.912 | 29.9 | [48] |
| NC Zn-17Ni alloy | −0.927 | 23.2 | ||||||
| Microcrystalline Zn-18Ni alloy | −1.08 | 47.6 | ||||||
| NC Co and Co–P (grain sizes 67 nm and 50 nm, respectively) | 15–20 µm | - | 0.25-M Na2SO4 solution | Resistance order: NC Co > polycrystalline Co > NC Co–P | Nanocrystalline Co (67 grain size) | −0.574 * | 0.86 | [54] |
| Polycrystalline Co 100 micron | −0.546 * | 1.847 | ||||||
| NC Co–P | 20 ± 2 µm | Mild steel | 3.5 wt.% NaCl solution | Best with 9 wt.% P of the alloy in pulse and in direct current electrodeposition | DC-Plain Co | −0.597 * | 3.3 | [49] |
| DC-90%Co-10%P | −0.541 * | 2.0 | ||||||
| DC-91%Co-9%P | −0.451 * | 1.1 | ||||||
| PC-91%Co-9%P | −0.476 * | 0.8 | ||||||
| NC Co and Co-1.1 wt.% P (grain sizes 20 nm and 10 nm, respectively) | 0.2 mm | Titanium | Deaerated 0.1 M H2SO4 solution | Resistance order: C Co-1.1P > microCo >~= microCo | - | No values provided. Only graph | [58] | |
| NC Ni coating (250 nm, 54 nm, 16 nm grain size) | _ | _ | 10 wt.% NaOH solution | Best with the lowest grain size (16 nm) | Ni 3 micon | −0.312 | 0.5759 | [42] |
| Ni 250 nm | −0.418 | 0.3456 | ||||||
| Ni 16 nm | −0.591 | 0.1095 | ||||||
| NC Fe coating (grain size 45 nm) | 8 µm | Low carbon steel | 10 wt.% NaOH solution | Resistance order: NC Fe > as cast Fe > annealed Fe | NC Fe deposit | −0.35 | 0.289 | [53] |
| Annealed Fe | −0.63 | 5.36 | ||||||
| As-cast Fe | −0.5 | 0.613 | ||||||
| NC Zn–Ni coating (grain size 28 nm with single gamma-phase) | _ | Carbon Steel | 3.5 wt.% NaCl solution | Best with 13 wt.% Ni content | Pure Zn | −1.039 | 144.2 | [52] |
| Zn-9.62 wt.% Ni | −0.935 | 52.73 | ||||||
| Zn-13.31 wt.% Ni | −0.792 | 40.14 | ||||||
| Zn-15.91 wt.% Ni | −0.826 | 50.99 | ||||||
| Ni–P (amorphous and crystalline structure) | _ | Carbon Steel | 3 wt.% NaCl, 1-N H2SO4, and 1-N NaOH solutions | Amorphous structure resists better than the crystalline one in neutral and acidic media, but has the same resistance in alkaline media. Higher P content had better resistance. | - | No values provided. Only graph | [57] | |
| nano Co (67 nm grain size) and micro Co | 50 µm | AISI_1045 steel | 10 wt.% NaCl, 10 wt.% H2SO4, 3.5 wt.% NaCl and 0.1-M H2SO4 solutions | Icorr from highest to lowest: HCl, NaOH, NaCl, H2SO4 | NC Co, 3.5 wt.% NaCl | −0.736 | 11.18 | [43] |
| NC Co, 0.1 M H2SO4 | −0.343 | 9.837 | ||||||
| NC Co, 10% NaOH | −1.022 | 18.91 | ||||||
| NC Co, 10% HCl | −0.409 | 31.58 | ||||||
| NC Cu-70Zr (10–20 nm grain size) | 20 µm | _ | Deaerated 0.1-M and 0.5-M HCl solutions | Grain refinement has a stabilisation effect on the corrosion process | - | No values provided. Only graph | [45] | |
| Coating | Nanomaterial (Particle Size in nm) | Coating Thickness (µm) | Substrate | Electrolyte | Ecorr (V vs. SCE) | Icorr (μA/cm2) | Corrosion Resistance | Ref. |
|---|---|---|---|---|---|---|---|---|
| Al2O3–Ni | Al2O3 (13) | 50 | Steel | 0.5 M potassium and sulphate solution | −0.1588 | 0.5 | [155] | |
| 0.5 M NaCl solution | −0.3592 | 0.43 | ||||||
| Al2O3–Ni | Al2O3 (100) | 25 | Mild steel | 3.5 wt.% NaCl solutions | −0.253 | 0.011 | Highest value with sediment co-deposition technique (SCD) at 7.58 wt.% Al2O3 | [154] |
| Al2O3–Ni | Al2O3 (40) | _ | Steel | 0.5 M Na2SO4 solution | −0.150 | 1.42 | [147] | |
| SiC–Ni | SiC (45) | −0.170 | 2.81 | |||||
| Al2O3 + SiC–Ni | Al2O3 + SiC (40–45) | −0.130 | 1.02 | |||||
| SiC–Ni | SiC (50) | _ | Copper | 3.5 wt.% NaCl solution | No values provided. Only graph | [148] | ||
| SiC–Ni | SiC (20) | 50 | 0.5 M K2SO4 solution | No values provided. Only graph | [149] | |||
| SiC–Ni | SiC (20) | 200 | Carbon-steel | 0.5 M Na2SO4 | −0.2605 | 1.9 | [150] | |
| SiC–Ni | SiC (40) | Copper | 3.5 wt.% NaCl solution | −0.248 | 0.6645 | [151] | ||
| SiC–Ni–W | SiC (80) | Copper | 3.5 wt.% NaCl solution | No values provided. Only graph | - | [152] | ||
| SiC–Ni–Co | SiC (50) | 20 | Copper | 3.5 wt.% NaCl solution | - | 7900 | Highest at 3.2 wt.% of SiC in Ni-Co matrix | [153] |
| TiO2–Ni | TiO2 (10) | _ | Sintered NdFeB magnet | 3.5 wt.% NaCl solution | - | 0.214 | - | [51] |
| Coating | Nanomaterial (Particle Size in nm) | Coating Thickness (µm) | Substrate | Electrolyte | Ecorr (V vs. SCE) | Icorr (μA/cm2) | Corrosion Resistance | Ref. |
|---|---|---|---|---|---|---|---|---|
| Al2O3–Ni–P | Al2O3 (50) | 8–12 | Low carbon steel | 3.5 wt.% NaCl solution | No values provided. Only graph | The highest surface resistance was with the 75 g/l alumina (Al3) coatings (100 times higher than the as-polished samples). The surface resistance decreased sharply after heat treatment. | [169] | |
| TiO2–Ni–P | TiO2 (30–60) | 0.038 | Copper | 3.5 wt.% NaCl solution | −0.26 | 0.34 | Optimum conditions: concentration of nickel source solution: 50 g/L, concentration of reducing agent: 10 g/L, concentration of TiO2 powder: 10 g/L, and bath temperature: 85 °C | [170] |
| TiO2–Ni–P | TiO2 (25) | _ | Low carbon steel | 3.5 wt.% NaCl solution | −0.318 | 5.38 | The corrosion resistance was the highest with 4.347 g/l concentrations of dodecyl trimethyl ammonium bromide (DTAB) surfactant, with 86.13 wt.% Ni, 6.92 wt.% P, and 6.95 wt.% TiO2 | [171] |
| TiO2–Ni–Zn–P | TiO2 (100–200) | _ | Low carbon steel | 3.5 wt.% NaCl solution | −0.404 | 0.364 | Highest corrosion resistance for heat-treated coating at 6.18 wt.% Zn, 10.56 wt.% P, and 2.30 wt.% TiO2 | [172] |
| SiC–Ni–P | SiC (40) | _ | X70 steel | CO2 containing media in the presence of acetic acid | −0.440 | 1.1 | Optimum concentration is 2.45 wt.% of SiC in the coating | [173] |
| SiO2–Ni–P | SiO2 (20) | 29 | API-5L X65 steel substrates | 3.5 wt.% NaCl solution | −0.336 | 0.308 | Optimum concentration at 2 wt.% of SiC | [174] |
| SiC–Ni–P | SiC (40) | 20 ± 1 | St37 tool steel substrate | 3 wt.% NaCl solution | −0.255 | 1.58 | Highest corrosion resistance for heat-treated nanocomposite at 4 wt.% of SiC in Ni–P | [175] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdeen, D.H.; El Hachach, M.; Koc, M.; Atieh, M.A. A Review on the Corrosion Behaviour of Nanocoatings on Metallic Substrates. Materials 2019, 12, 210. https://doi.org/10.3390/ma12020210
Abdeen DH, El Hachach M, Koc M, Atieh MA. A Review on the Corrosion Behaviour of Nanocoatings on Metallic Substrates. Materials. 2019; 12(2):210. https://doi.org/10.3390/ma12020210
Chicago/Turabian StyleAbdeen, Dana H., Mohamad El Hachach, Muammer Koc, and Muataz A. Atieh. 2019. "A Review on the Corrosion Behaviour of Nanocoatings on Metallic Substrates" Materials 12, no. 2: 210. https://doi.org/10.3390/ma12020210
APA StyleAbdeen, D. H., El Hachach, M., Koc, M., & Atieh, M. A. (2019). A Review on the Corrosion Behaviour of Nanocoatings on Metallic Substrates. Materials, 12(2), 210. https://doi.org/10.3390/ma12020210