Synthesis and Characterization of K and Eu Binary Phosphides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Single-Crystal X-ray Diffraction
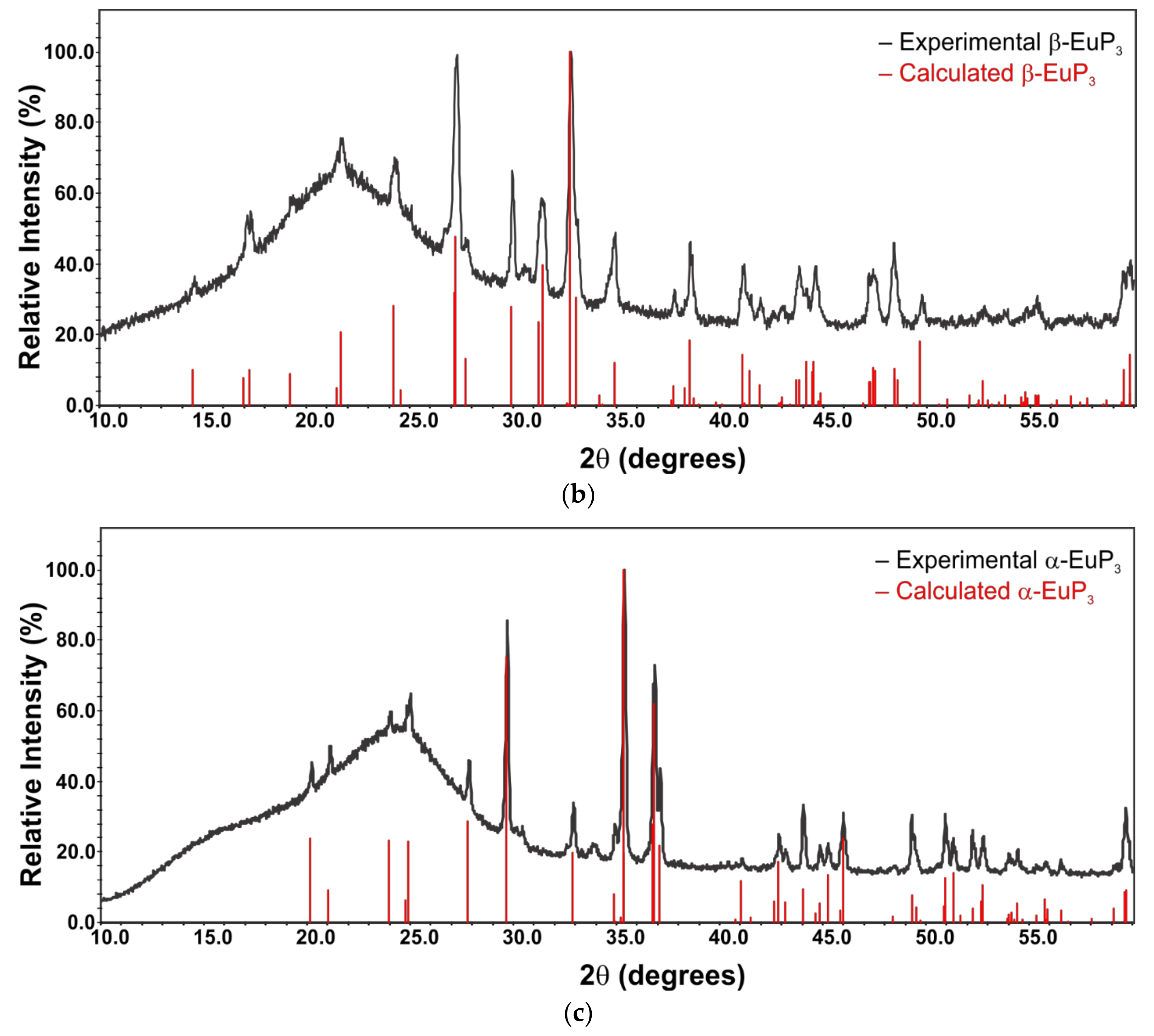
2.3. X-ray Powder Diffraction
2.4. Differential Scanning Calorimetry
2.5. Solid-State Diffuse Reflectance Spectroscopy
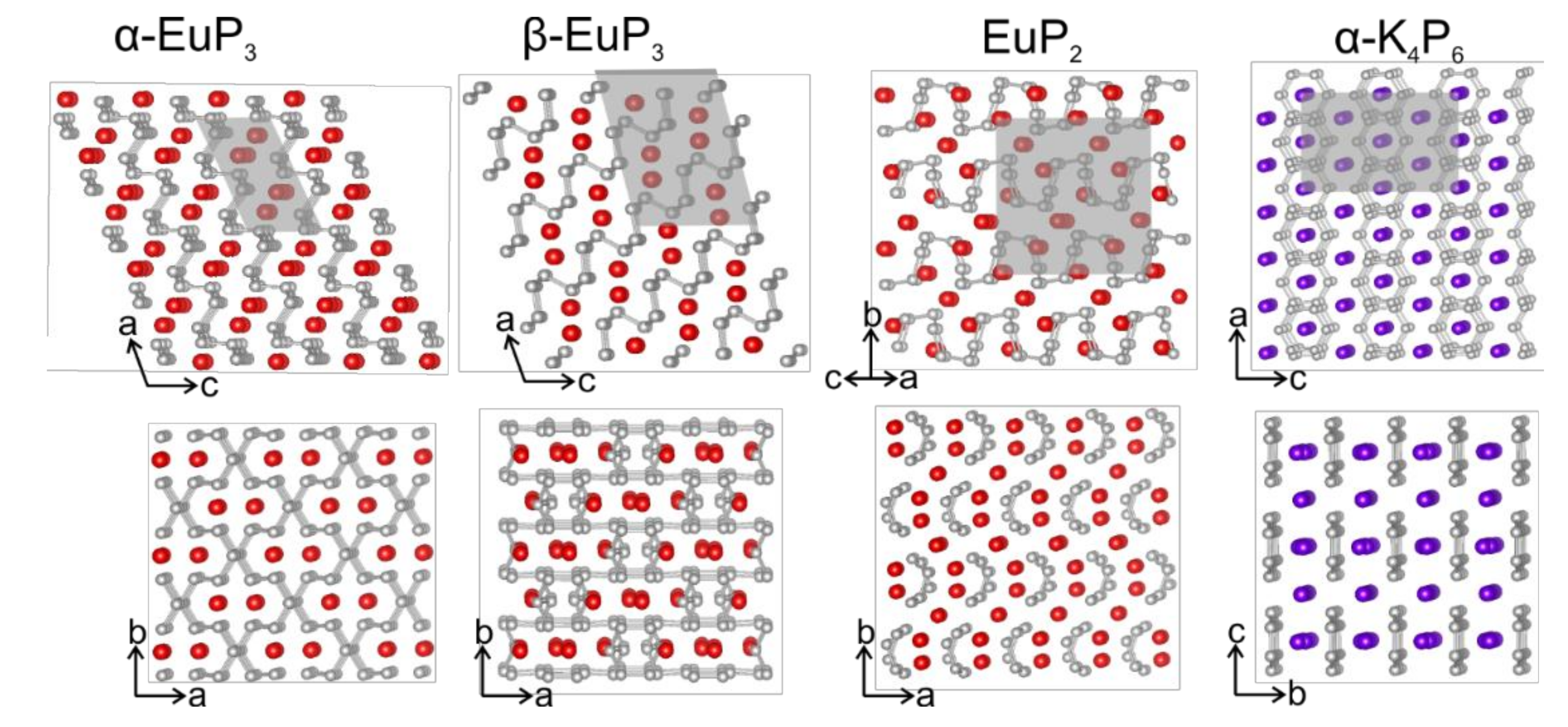
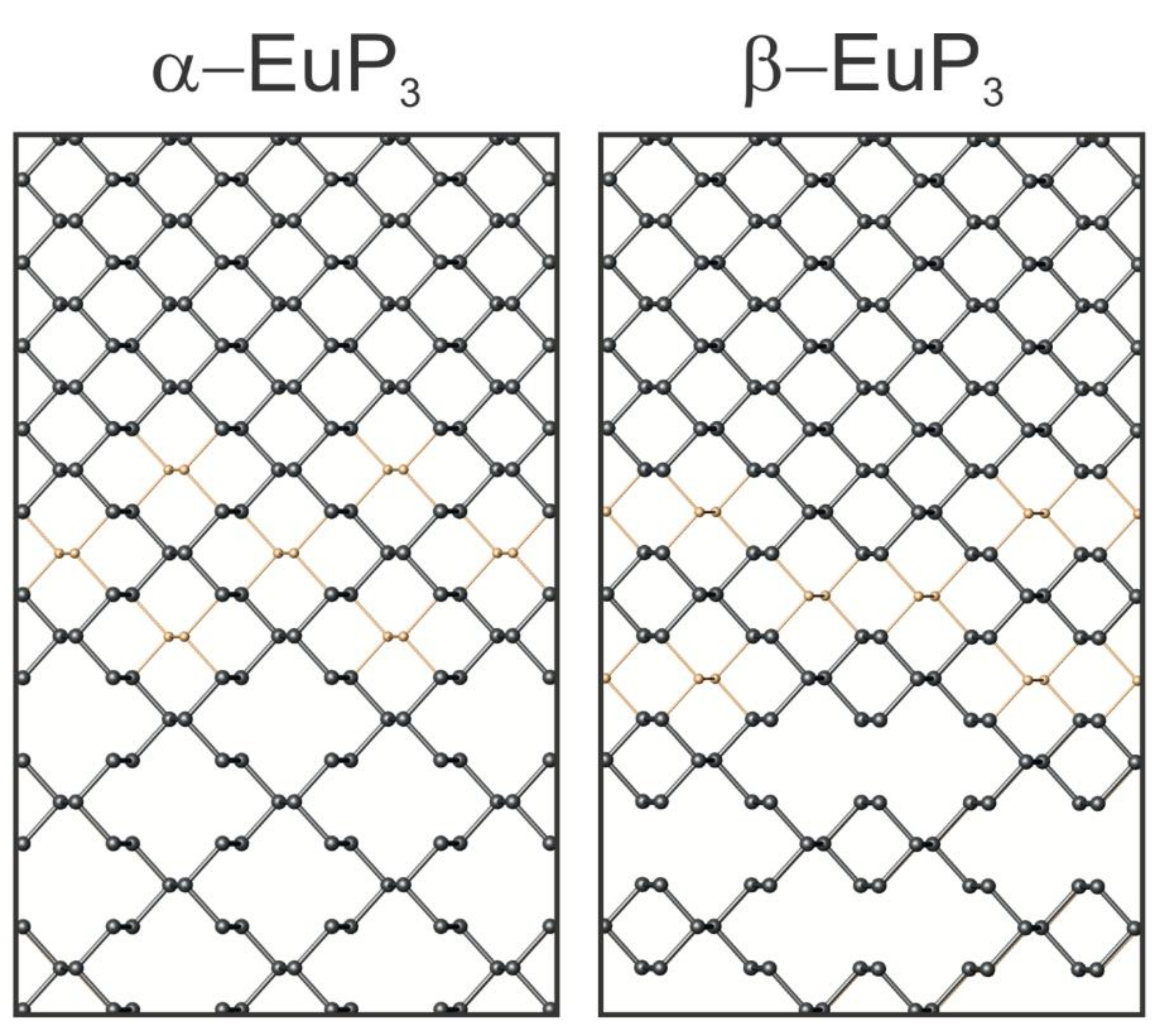
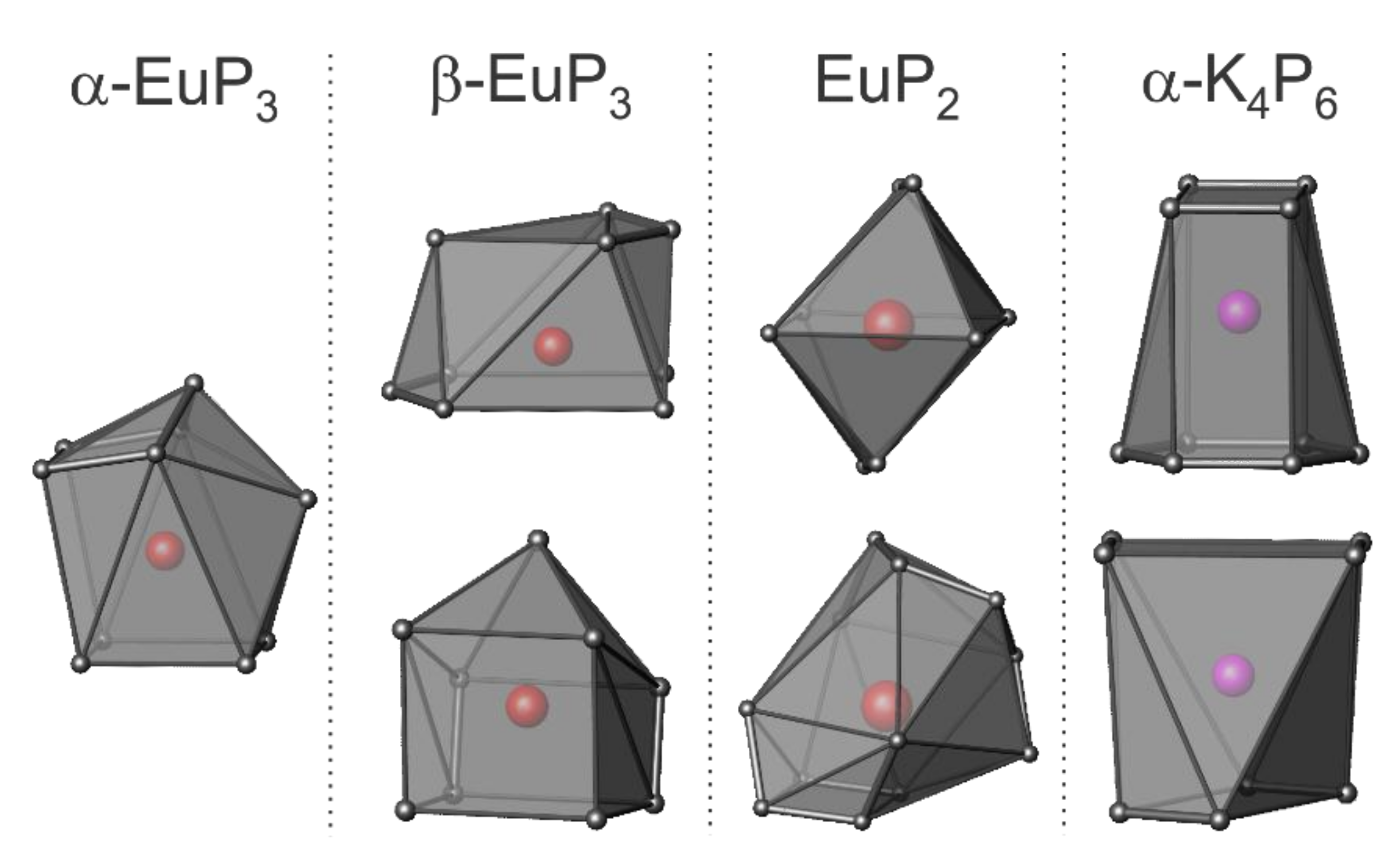
3. Results and Discussion
3.1. Synthesis and Thermal Stability
3.2. Crystal Structures
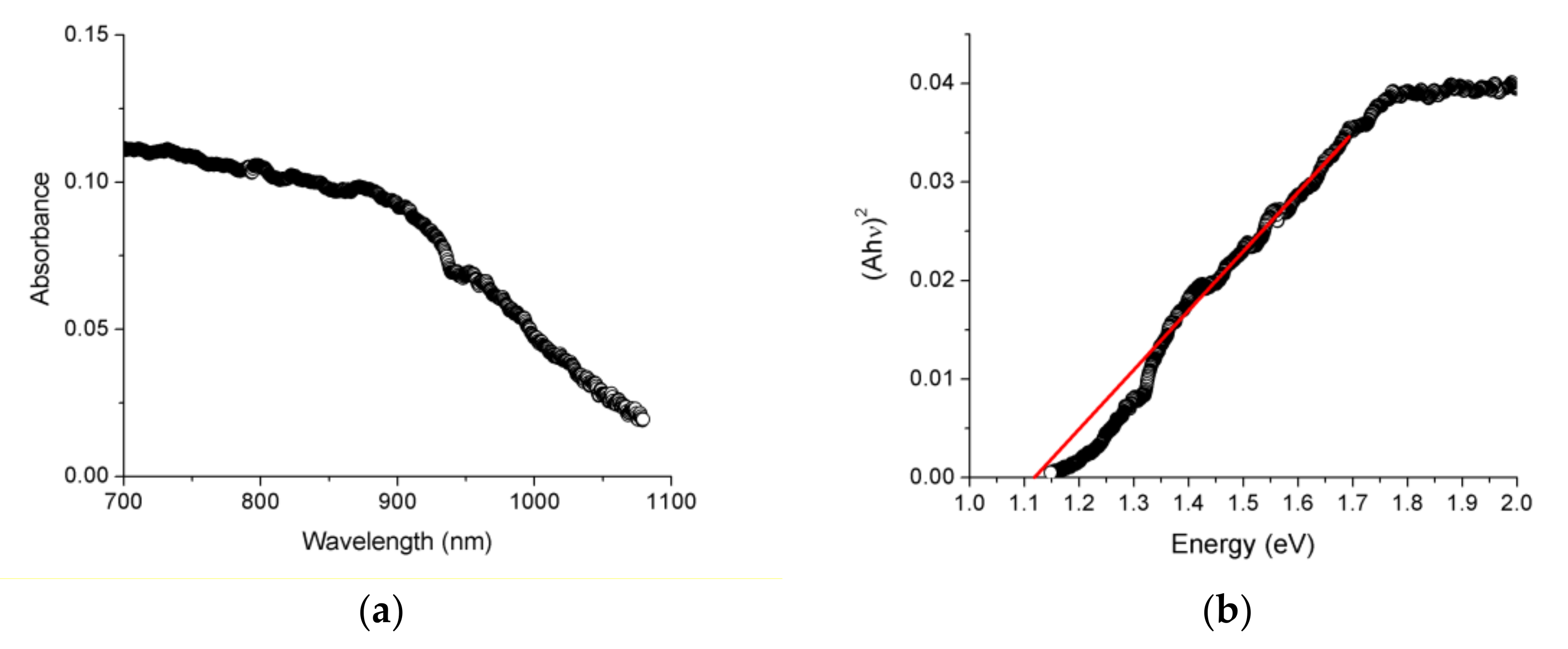
3.3. Solid-State Diffuse Reflectance Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hönle, W.; von Schnering, H.G. Chemistry and Structural Chemistry of Phosphides and Polyphosphides. 48. Bridging Chasms with Polyphosphides. Chem. Rev. 1988, 88, 243–273. [Google Scholar]
- Pöttgen, R.; Hönle, W.; von Schnering, H.G. Phosphides: Solid State Chemistry. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Von Schnering, H.G.; Wittman, M. Europium (II) Heptaphosphide EuP7. Zeitschrift für Naturforschung B 1980, 35, 824–831. [Google Scholar] [CrossRef]
- Schmettow, W.; Mensing, C.; von Schnering, H.-G. Zur Chemie und Strukturchemie der Phosphide und Polyphosphide. 34 [1] Dampfdruckuntersuchungen am System Europium–Phosphor. Z. Anorg. Allg. Chem. 1984, 510, 51–60. [Google Scholar] [CrossRef]
- Abicht, H.-P.; Hönle, W.; von Schnering, H.G. Zur Chemie und Strukturchemie von Phosphiden und Polyphosphiden. 36. Tetrakaliumhexaphosphid: Darstellung, Struktur und Eigenschaften von α-K4P6 und β-K4P6. Z. Anorg. Allg. Chem. 1984, 519, 7–23. [Google Scholar] [CrossRef]
- Dolyniuk, J.; Kaseman, D.C.; Sen, S.; Zhou, J.; Kovnir, K. mP-BaP3: A New Phase from an Old Binary System. Chem. Eur. J. 2014, 20, 10829–10837. [Google Scholar] [CrossRef] [PubMed]
- Dolyniuk, J.; He, H.; Ivanov, A.; Boldyrev, A.; Bobev, S.; Kovnir, K. Ba and Sr Binary Phosphides: Synthesis, Crystal Structures, and Bonding Analysis. Inorg. Chem. 2015, 54, 8608–8616. [Google Scholar] [CrossRef]
- Fulmer, J.; Kaseman, D.; Dolyniuk, J.; Lee, K.; Sen, S.; Kovnir, K. BaAu2P4: Layered Zintl Polyphosphide with Infinite ∞1(P–) Chains. Inorg. Chem. 2013, 52, 7061–7067. [Google Scholar] [CrossRef]
- Shatruk, M.M.; Kovnir, K.A.; Shevelkov, A.V.; Popovkin, B.A. Ag3SnP7: A Polyphosphide with a Unique (P7) Chain and a Novel Ag3Sn Heterocluster. Angew. Chem. Int. Ed. 2000, 39, 2508–2509. [Google Scholar] [CrossRef]
- Fässler, T.F. Relationships between Soluble Zintl Anions, Ligand-Stabilized Cage Compounds, and Intermetalloid Clusters of Tetrel (Si-Pb) and Pentel (P-Bi) Elements. In Zintl Ions: Principles and Recent Developments; Fässler, T.F., Ed.; Springer: Berlin, Germany, 2011; Volume 140, pp. 91–131. [Google Scholar]
- Scharfe, S.; Kraus, F.; Stegmaier, S.; Schier, A.; Fässler, T.F. Zintl Ions, Cage Compounds, and Intermetalloid Clusters of Group 14 and Group 15 Elements. Angew. Chem. Int. Ed. 2011, 50, 3630–3670. [Google Scholar] [CrossRef]
- Lange, S.; Sebastian, C.P.; Zhang, L.; Eckert, H.; Nilges, T. Ag3SnCuP10: Ag3Sn Tetrahedra Embedded Between Adamantane-Type P-10 Cages. Inorg. Chem. 2006, 45, 5878–5885. [Google Scholar] [CrossRef]
- Lange, S.; Sebastian, C.P.; Nilges, T. A New Preparative Approach To HgPbP14 Structure Type Materials: Crystal Structure of Cu0.73(1)Sn1.27(1)P14 And Characterization of M1-XSn1+XP14 (M = Cu, Ag) and AgSbP14. Z. Anorg. Allg. Chem. 2006, 632, 195–203. [Google Scholar] [CrossRef]
- Dolyniuk, J.; Zaikina, J.V.; Kaseman, D.C.; Sen, S.; Kovnir, K. Breaking the Tetra-Coordinated Framework Rule: New Clathrate Ba8M24P28+δ (M = Cu/Zn). Angew. Chem. Int. Ed. 2017, 56, 2418–2422. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Bawohl, M.; Weihrich, R.; Nilges, T. Mineralization Routes to Polyphosphides: Cu2P20 and Cu5InP16. Angew. Chem. Int. Ed. 2008, 47, 5654–5657. [Google Scholar] [CrossRef] [PubMed]
- Lincke, H.; Nilges, T.; Johrendt, D.; Pöttgen, R. Crystal and Electronic Structure of La3Zn2-xP4—New Phosphide with Isolated P3− species. Solid State Sci. 2008, 10, 1006–1011. [Google Scholar] [CrossRef]
- Wang, J.; Yox, P.; Voyles, J.; Kovnir, K. Synthesis, Crystal Structure, and Properties of Three La-Zn-P Compounds with Different Dimensionality of Zn-P Framework. Cryst. Growth Des. 2018, 18, 4076–4083. [Google Scholar] [CrossRef]
- Kovnir, K.; Zaikina, J.V.; Reshetova, L.N.; Olenev, A.V.; Dikarev, E.V.; Shevelkov, A.V. Unusually High Chemical Compressibility of Normally Rigid Type-I Clathrate Framework: Synthesis and Structural Study of Sn24P19.3BrxI8-x Solid Solution, the Prospective Thermoelectric Material. Inorg. Chem. 2004, 43, 3230–3236. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.; Dolyniuk, J.; Tran, N.; Kovnir, K. Crystal and Electronic Structure and Optical Properties of AE2SiP4 (AE = Sr, Eu, Ba) and Ba4Si3P8. Z. Anorg. Allg. Chem. 2018. [Google Scholar] [CrossRef]
- Bawohl, M.; Schmidt, P.; Nilges, T. Temperature Initiated P-Polymerization in Solid [Cd3Cu]CuP10. Inorg. Chem. 2013, 52, 11895–11901. [Google Scholar] [CrossRef]
- Eckstein, N.; Hohmann, A.; Weihrich, R.; Nilges, T.; Schmidt, P. Synthesis and Phase Relations of Single-Phase Fibrous Phosphorus. Z. Anorg. Allg. Chem. 2013, 639, 2741–2743. [Google Scholar] [CrossRef]
- Dewalsky, M.V.; Jeitschko, W.; Wortmann, U. The Metallic Polyphosphide Ti2NiP5. Chem. Mater. 1991, 3, 316–319. [Google Scholar] [CrossRef]
- Eschen, M.; Wallinda, J.; Jeitschko, W. Preparation and Crystal Structure of Au1-xSn1+yP14 and Other Polyphosphides with HgPbP14-Type Structure. Z. Anorg. Allg. Chem. 2002, 628, 2764–2771. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Mordvinova, N.E.; Lebedev, O.I.; Kovnir, K. The smaller the better: Hosting trivalent rare-earth guests in Cu-P clathrate cages. Chem 2018, 4, 1465–1475. [Google Scholar] [CrossRef]
- Eschen, M.; Jeitschko, W. Au2PbP2, Au2TlP2, and Au2HgP2: Ternary Gold Polyphosphides with Lead, Thallium, and Mercury in the Oxidation State Zero. J. Solid State Chem. 2002, 165, 238–246. [Google Scholar] [CrossRef]
- Bartsch, T.; Wiegand, T.; Ren, J.J.; Eckert, H.; Johrendt, D.; Niehaus, O.; Eul, M.; Pöttgen, R. Phosphide Oxides RE2AuP2O (RE = La, Ce, Pr, Nd): Synthesis, Structure, Chemical Bonding, Magnetism, and 31P and 139La Solid State NMR. Inorg. Chem. 2013, 52, 2094–2102. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dolyniuk, J.; Kovnir, K. Unconventional Clathrates with Transition Metal-Phosphorus Frameworks. Acc. Chem. Res. 2018, 51, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Pfannenschmidt, U.; Behrends, F.; Lincke, H.; Eul, M.; Schäfer, K.; Eckert, H.; Pöttgen, R. CaBe2Ge2 Type Phosphides REIr2P2 (RE = La-Nd, Sm) and Arsenides REIr2As2 (RE = La-Nd): Synthesis, Structure, and Solid State NMR Spectroscopy. Dalton Trans. 2012, 41, 14188–14196. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, B.; Röβler, U. A Polyphosphide Of Unusual Composition: The Crystal Structure of Ba5P9. Z. Anorg. Allg. Chem. 2003, 629, 459–462. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, L.P.; Yamanaka, S. High-Pressure Synthesis and Structural Characterization of Three New Polyphosphides, α-SrP3, BaP8, and LaP5. J. Solid State Chem. 2003, 173, 449–455. [Google Scholar] [CrossRef]
- Ivanov, A.S.; Morris, A.J.; Bozhenko, K.V.; Pickard, C.J.; Boldyrev, A.I. Inorganic Double-Helix Structures of Unusually Simple Lithium-Phosphorus Species. Angew. Chem. Int. Ed. 2012, 51, 8330–8333. [Google Scholar] [CrossRef]
- Wang, J.; Lebedev, O.I.; Lee, K.; Dolyniuk, J.; Klavins, P.; Bux, S.; Kovnir, K. A high-efficiency thermoelectric Ba8Cu14Ge6P26: Bridging the gap between tetrel-based and tetrel-free clathrates. Chem. Sci. 2017, 8, 8030–8038. [Google Scholar] [CrossRef]
- Dahlmann, W.; von Schnering, H.G. CaP3, A New Calcium Phosphide. Naturwissenschaften 1973, 60, 518. [Google Scholar] [CrossRef]
- Bauhofer, W.; Gmelin, E.; Mollendorf, M.; Nesper, R.; von Schnering, H.G. Electrical and Magnetic Properties of Single Crystalline EuAs3, β-EuP3 and Their Mixed Crystals. J. Phys. C Solid State Phys. 1985, 18, 3017–3035. [Google Scholar] [CrossRef]
- Chattopadhyay, T.; Voiron, J.; Bartholin, H. Magnetization investigations of the magnetic (H, T) phase diagrams of EuAs3, Eu(As1−xPx)3 and β-EuP3: Effects of hydrostatic pressure up to 15 kbar. J. Magn. Magn. Mater. 1988, 72, 35–44. [Google Scholar] [CrossRef]
- Kraus, F.; Hanauer, T.; Korber, N. Chemical Bond in the Cyclic Anions P64− and As64−: Synthesis, Crystal Structure, and Electron Localization Function of {Rb[18]crown-6)}2-Rb2As6-6NH3. Angew. Chem. Int. Ed. 2005, 44, 7200–7204. [Google Scholar] [CrossRef]
- Ystenes, M. Quantum Chemical Vibrational Analysis of Clusters Providing an Explanation for the Planarity of the P64− Ring. J. Mol. Struct. THEOCHEM 1996, 369, 23–28. [Google Scholar] [CrossRef]
- Scherer, O.J.; Sitzmann, H.; Wolmershauser, G. Hexaphosphabenzene as Complex Ligand. Angew. Chem. Int. Ed. 1985, 24, 351–353. [Google Scholar] [CrossRef]
- Kauzlarich, S.M. (Ed.) Chemistry, Structure and Bonding of Zintl Phases and Ions; VCH: New York, NY, USA, 1996. [Google Scholar]
- Miller, G.J.; Schmidt, M.W.; Wang, F.; You, T.-S. Struct. Bonding; Springer: Berlin, Germany, 2011; Volume 139, pp. 1–55. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, A64, 112–122. [Google Scholar] [CrossRef]


| α-EuP3 | β-EuP3 | EuP2 | α-K4P6 | |
|---|---|---|---|---|
| Space Group | C2/m | C2/m | P21/c | Fmmm |
| Temp [K] | 100(2) | |||
| λ [Å] | Mo Kα, 0.71073 | |||
| a [Å] | 9.044(1) | 11.272(1) | 6.1040(8) | 8.5508(8) |
| b [Å] | 7.2087(9) | 7.3390(6) | 11.684(2) | 9.2899(8) |
| c [Å] | 5.5710(7) | 8.4242(7) | 7.352(1) | 14.148(1) |
| β [degree] | 113.117(4) | 103.304(1) | 127.680(5) | |
| V [Å3] | 334.06(7) | 678.2(1) | 415.0(1) | 1123.9(2) |
| Z | 4 | 8 | 6 | 4 |
| ρ [g cm−3] | 4.869 | 4.796 | 5.135 | 2.023 |
| μ [mm−1] | 19.91 | 19.62 | 23.45 | 2.37 |
| θ [degree] | 3.74 < θ < 24.71 | 2.48 < θ < 29.98 | 3.49 < θ < 26.30 | 2.88 < θ < 29.45 |
| data/parameters | 259/22 | 1062/44 | 844/43 | 432/18 |
| R1 (I > 2σ(I)) | 0.020 | 0.013 | 0.042 | 0.022 |
| wR2 (I > 2σ(I)) | 0.041 | 0.029 | 0.109 | 0.033 |
| Goodness-of-fit | 1.10 | 0.96 | 1.12 | 1.06 |
| Diff. peak and hole, e/Å3 | 0.91 and −1.13 | 0.93 and −0.80 | 3.71 and −2.36 | 0.48 and −0.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolyniuk, J.-A.; Mark, J.; Lee, S.; Tran, N.; Kovnir, K. Synthesis and Characterization of K and Eu Binary Phosphides. Materials 2019, 12, 251. https://doi.org/10.3390/ma12020251
Dolyniuk J-A, Mark J, Lee S, Tran N, Kovnir K. Synthesis and Characterization of K and Eu Binary Phosphides. Materials. 2019; 12(2):251. https://doi.org/10.3390/ma12020251
Chicago/Turabian StyleDolyniuk, Juli-Anna, Justin Mark, Shannon Lee, Nhon Tran, and Kirill Kovnir. 2019. "Synthesis and Characterization of K and Eu Binary Phosphides" Materials 12, no. 2: 251. https://doi.org/10.3390/ma12020251





