Optimum Bloating-Activation Zone of Artificial Lightweight Aggregate by Dynamic Parameters
Abstract
:1. Introduction
2. Experimental Method
2.1. Raw Material Analysis
2.2. Aggregate Molding and Firing
3. Results and Discussion
3.1. Raw Material
3.2. Rapid Sintering
3.3. Normal Sintering
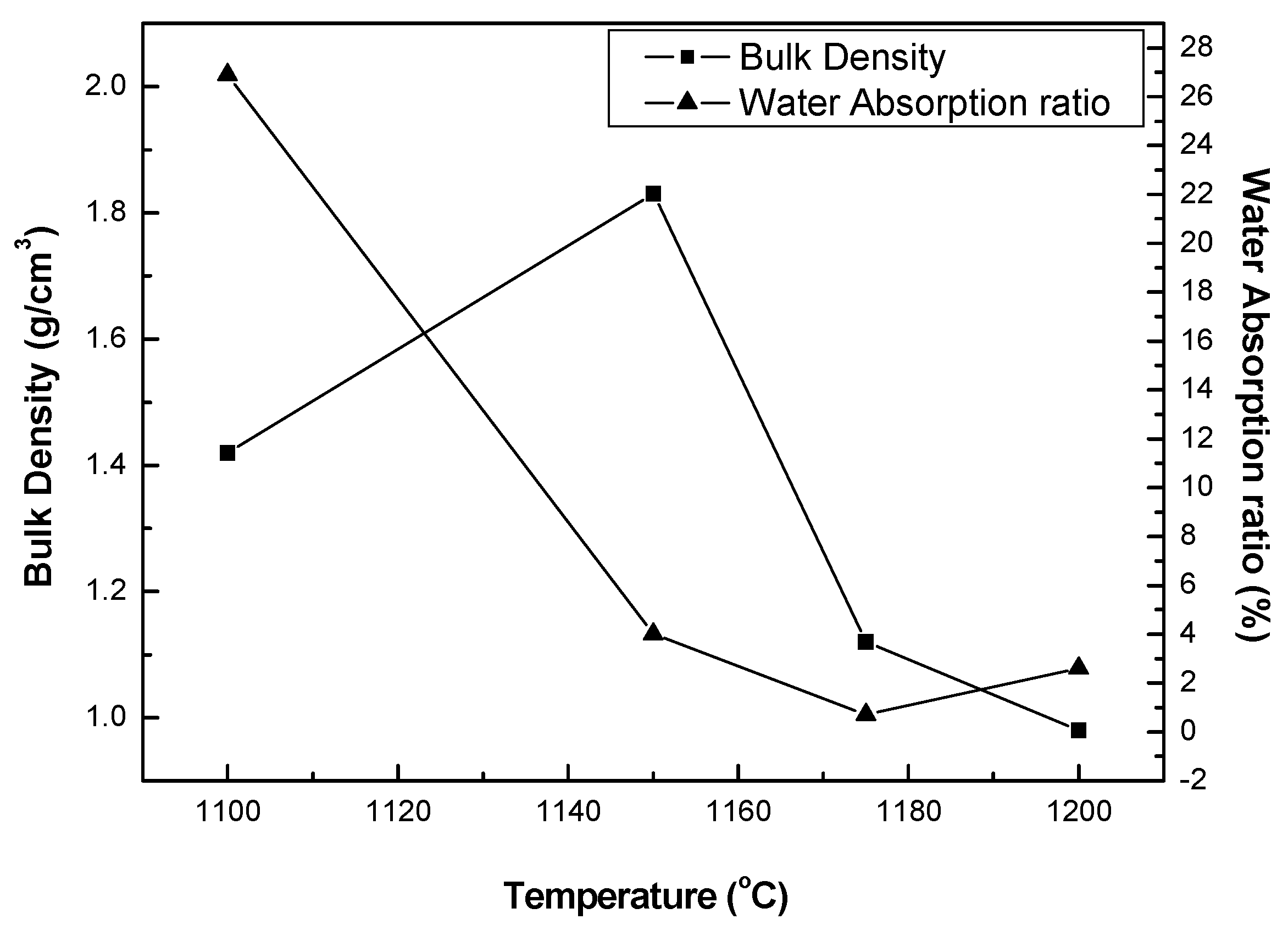
3.3.1. Bulk Density and Water Absorption Ratio
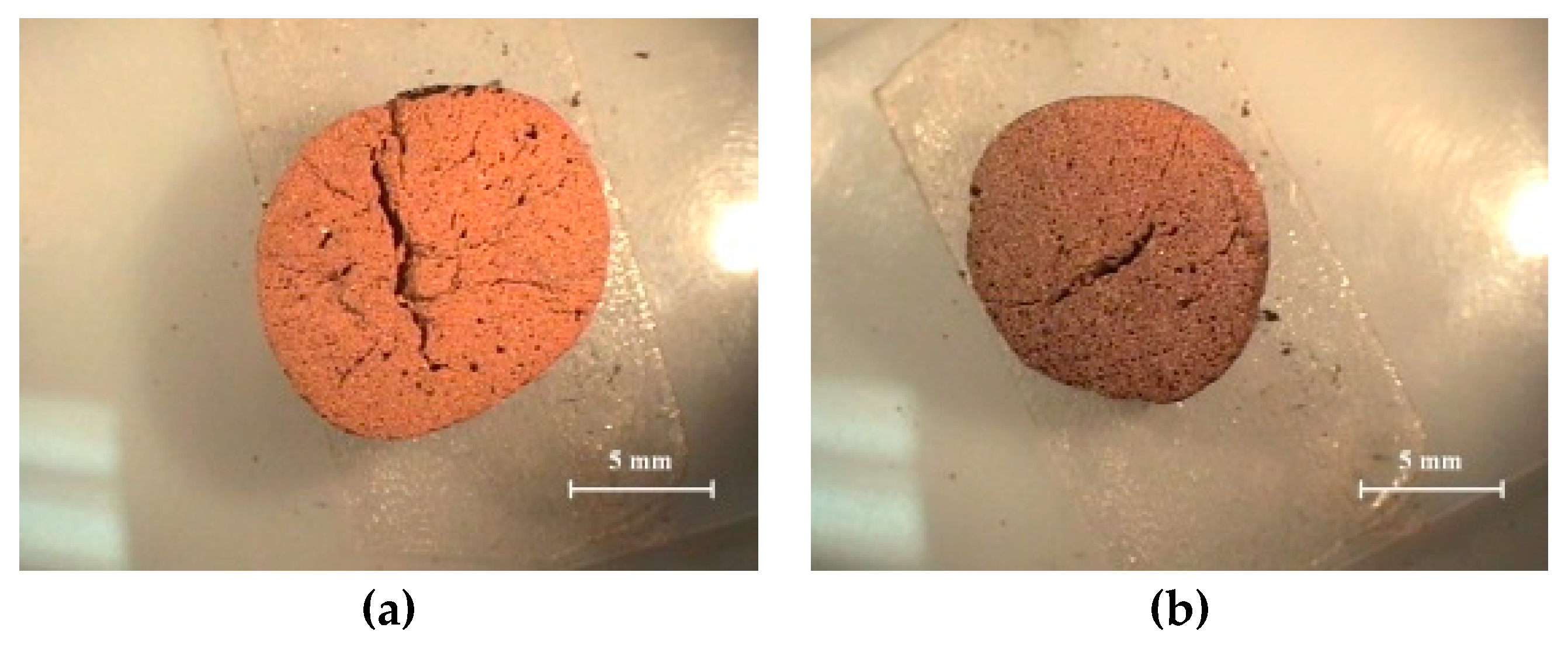
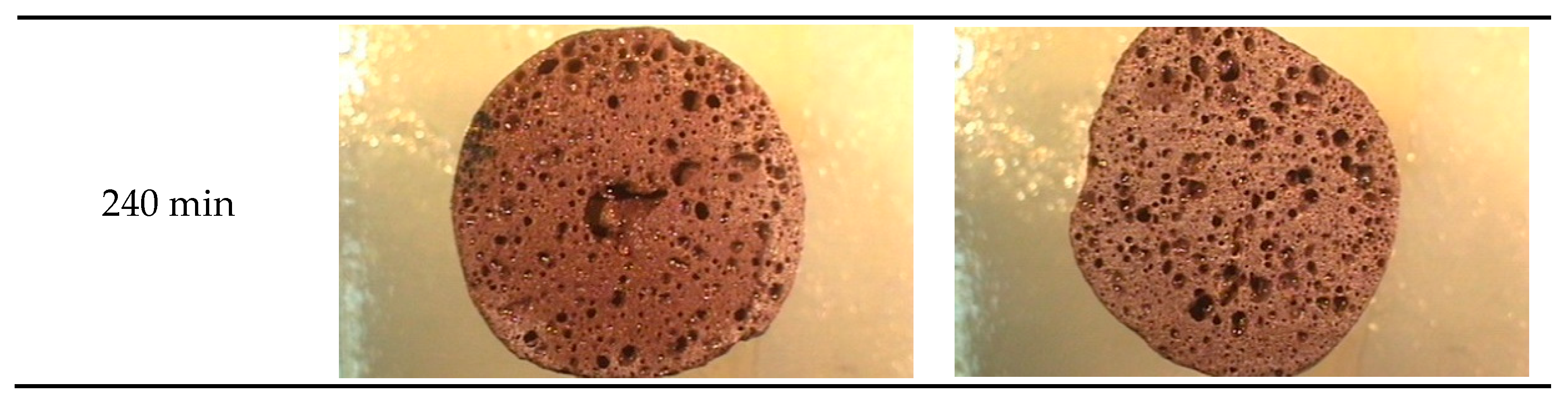
3.3.2. Characteristics of the Pores
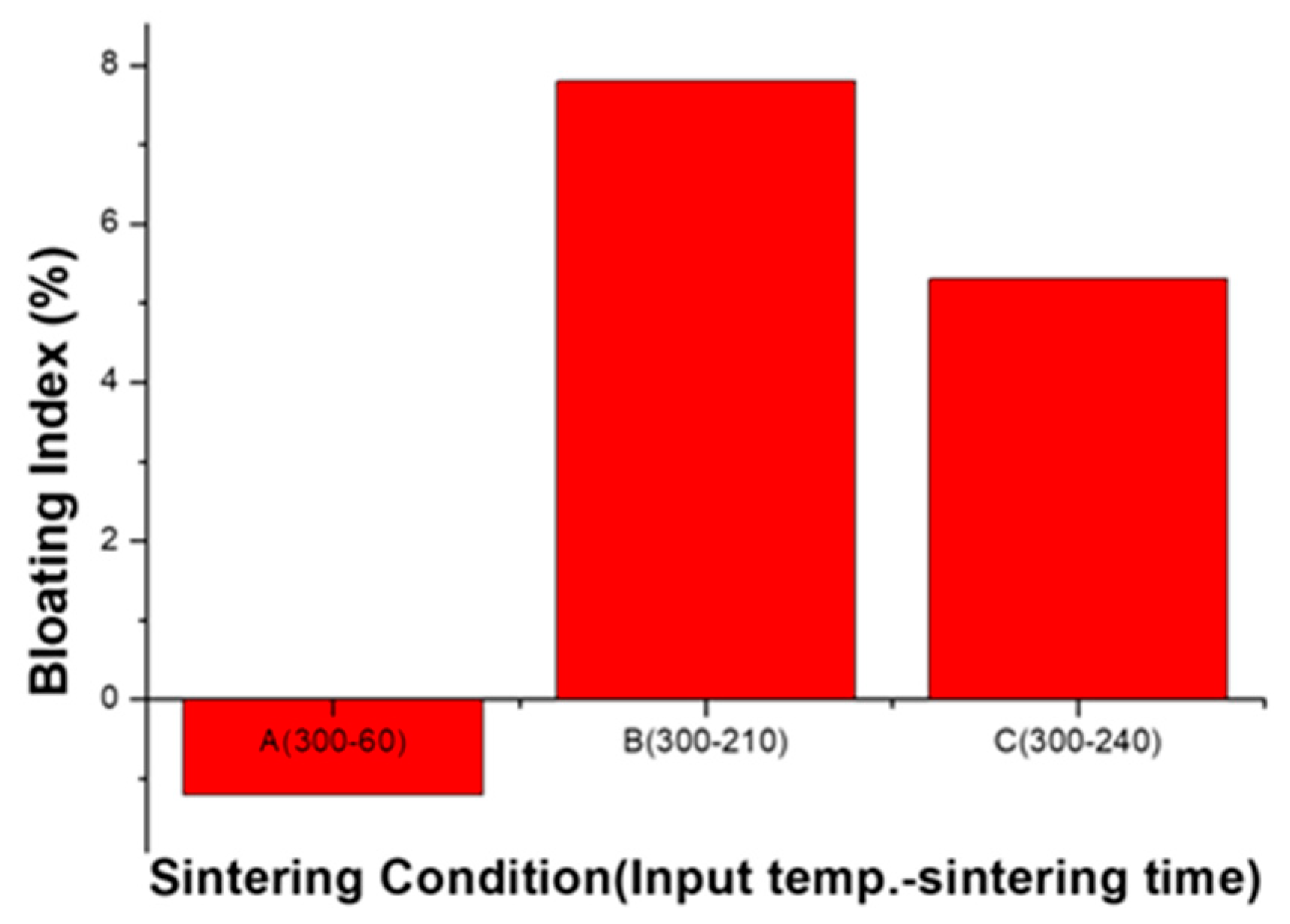
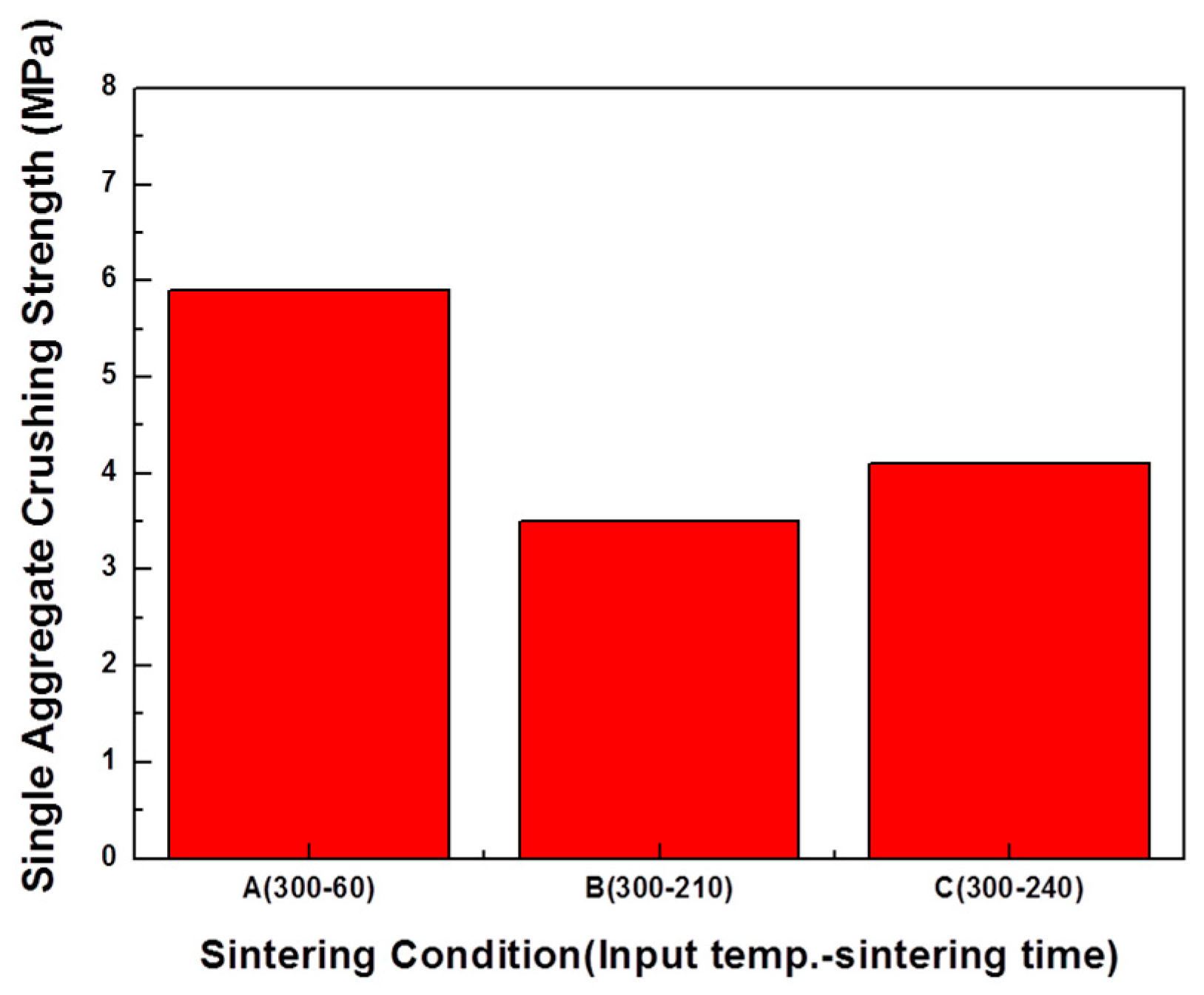
3.3.3. Bloating Index and Single Aggregate Crushing Strength
4. Conclusions
- (1)
- The bloating mechanism of the acid clay is not related to the typical reactions of lightweight aggregates in which a black core is formed. It is a combination of Ostwald ripening and detachment of the crystalline water of montmorillonite minerals. Detachment of crystalline water of montmorillonite is continuous at high temperature.
- (2)
- When the sintering rate is very rapid and the supplied amount of energy is low, the aggregate does not show sufficient viscous behavior and bloating does not occur. (A: Sintering zone.)
- (3)
- In order to bloat the aggregate, sufficient viscous behavior and gas generation should be properly combined, so there is an optimal bloating-activation zone because the two conditions are in conflict with each other. (B: Bloating-activation zone.)
- (4)
- When the sintering rate is low, the viscous behavior for bloating is sufficient. However, bloating is not adequate because the calcination section becomes longer and the internal pressure is lowered. The aggregate is over-sintered in this section. (C: Over-sintering zone.)
Author Contributions
Funding
Conflicts of Interest
References
- Liu, P.; Farzana, R.; Rajarao, R.; Sahajwalla, V. Lightweight expanded aggregates from the mixture of waste automotive plastics and clay. Constr. Build. Mater. 2017, 145, 283–291. [Google Scholar] [CrossRef]
- Li, B.; Ling, T.C.; Qu, L.; Wang, Y. Effects of a two-step heating process on the properties of lightweight aggregate prepared with sewage sludge and saline clay. Constr. Build. Mater. 2016, 114, 119–126. [Google Scholar] [CrossRef]
- González-Corrochano, B.; Alonso-Azcárate, J.; Rodas, M. Effect of thermal treatment on the retention of chemical elements in the structure of lightweight aggregates manufactured from contaminated mine soil and fly ash. Constr. Build. Mater. 2012, 35, 497–507. [Google Scholar] [CrossRef]
- Han, M.C.; Han, D.Y.; Shin, J. Use of bottom ash and stone dust to make lightweight aggregate. Constr. Build. Mater. 2015, 99, 192–199. [Google Scholar] [CrossRef]
- Ayati, B.; Molineux, C.; Newport, D.; Cheeseman, C. Manufacture and performance of lightweight aggregate from waste drill cuttings. J. Clean. Prod. 2019, 208, 252–260. [Google Scholar] [CrossRef]
- Moreno-Maroto, J.M.; Gonzalez-Corrochano, B.; Alonso-Azcarate, J.; Garcia, C.M. A study on the valorization of a metallic ore mining tailing and its combination with polymeric wastes for lightweight aggregates production. J. Clean. Prod. 2019, 212, 997–1007. [Google Scholar] [CrossRef]
- Moreno-Maroto, J.M.; Gonzalez-Corrochano, B.; Alonso-Azcarate, J.; Rodriguez, L.; Acosta, A. Manufacturing of lightweight aggregates with carbon fiber and mineral wastes. Cem. Concr. Compos. 2017, 83, 335–348. [Google Scholar] [CrossRef]
- Riley, C.M. Relation of Chemical Properties to the Bloating of Clays. J. Am. Ceram. Soc. 1951, 34, 121–128. [Google Scholar] [CrossRef]
- Cougny, G. Specifications for clayey raw materials used to produce expanded lightweight aggregates. Bull. Int. Assoc. Eng. Geol. 1990, 41, 47–55. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, Y.T.; Lee, K.G.; Kang, S.G.; Kim, J.H. The Mechanism of black core formation. J. Korean Cryst. Grow. Cryst. Technol. 2005, 15, 208–215. [Google Scholar]
- Lee, K.G. Bloating Mechanism of Lightweight Aggregate with the Size. J. Korean Ceram. Soc. 2016, 53, 241–245. [Google Scholar] [CrossRef]
- Kang, M.A.; Kang, S.G.; Lee, K.G.; Kim, Y.T. Fabrication of Artificial Light-weight Aggregates of Uniform Bloating Properties Using a Temperature-raising Sintering Method. J. Korean Ceram. Soc. 2012, 49, 161–166. [Google Scholar] [CrossRef]
- Dondi, M.; Cappelletti, P.; D’Amore, M.; de Gennaro, R.; Graziano, S.F.; Langella, A.; Raimondo, M.; Zanelli, C. Lightweight aggregates from waste materials: Reappraisal of expansion behavior and prediction schemes for bloating. Constr. Build. Mater. 2016, 127, 394–409. [Google Scholar] [CrossRef]
- KS F 2304: Method of Test for Plastic Limit of Soil; Korean Standards Association: Seoul, Korea, 2000.
- KS F 2503: Testing Method for Density and Absorption of Coarse Aggregate; Korean Standards Association: Seoul, Korea, 2007.
- JIS A5002: Lightweight Aggregate for Structural Concrete; Japanese Standards Association: Tokyo, Japan, 2003.
- Fakhfakh, E.; Hajjaji, W.; Medhioub, M.; Rocha, F.; Lopez-Galindo, A.; Setti, M.; Kooli, F.; Zargouni, F.; Jamoussi, F. Effects of sand addition on production of lightweight aggregates from Tunisian smectite-rich clayey rocks. Appl. Clay Sci. 2007, 35, 228–237. [Google Scholar] [CrossRef]
- Gonzalez-Corrochano, B.; Alonso-Azcarate, J.; Rodas, M. Effect of prefiring and firing dwell times on the properties of artificial lightweight aggregates. Constr. Build. Mater. 2014, 53, 91–101. [Google Scholar] [CrossRef]
- Yashima, S.; Kanda, Y.; Sano, S. Relationships between particle size and fracture energy or impact velocity required to fracture as estimated from single particle crushing. Powder Technol. 1987, 51, 277–282. [Google Scholar] [CrossRef]
- Li, Y.; Wu, D.; Zhang, J.; Chang, L.; Wu, D.; Fang, Z.; Shi, Y. Measurement and statistics of single pellet mechanical strength of differentely shaped catalysts. Powder Technol. 2000, 113, 176–184. [Google Scholar] [CrossRef]
- Lee, J.G.; Kim, J.W.; Pac, Y.H.; Lee, J.S.; Lee, B.H.; Shin, D.W. Ceramics Raw Materials; Bando: Seoul, Korea, 1996; pp. 41–43. [Google Scholar]
- Moreno-Maroto, J.M.; Alonso-AzCarate, J. Wat is clay? A new definition of “clay” based on plasticity and its impact on the most widespread soil classification systems. Appl. Clay Sci. 2018, 161, 57–63. [Google Scholar] [CrossRef]
- Liao, Y.C.; Huang, C.Y.; Chen, Y.M. Lightweight aggregates from water reservoir sediment with added sodium hydroxide. Constr. Build. Mater. 2013, 127, 79–85. [Google Scholar] [CrossRef]
- Lee, W.J.; Cho, W.S.; Hwang, K.T.; Kim, J.H.; Hwang, H.J.; Lee, Y.O. Characterization of Lightweight Earthenware Tiles using Foaming Agents. J. Korean Ceram. Soc. 2015, 52, 473–748. [Google Scholar] [CrossRef]
- Kaz’mina, O.V.; Vereshchangin, V.I.; Abiyaka, A.N.; Popletneva, Y.V. Viscosity evaluation for glass and glass crystal compositions in their foaming temperature range. Glass Ceram. 2009, 66, 236–239. [Google Scholar] [CrossRef]
- Kose, S.; Bayer, G. Schaumbildung im system altglas-SiC und die eigenschaften derartiger schaumgläser. Glastechnik Ber 1982, 55, 151–160. [Google Scholar]
- Chiang, Y.M.; Birnie, D.P.; Kingery, W.D. Physical Ceramics (Korean Version), 2nd ed.; ITC: Paju, Korea, 2003; pp. 459–462. [Google Scholar]
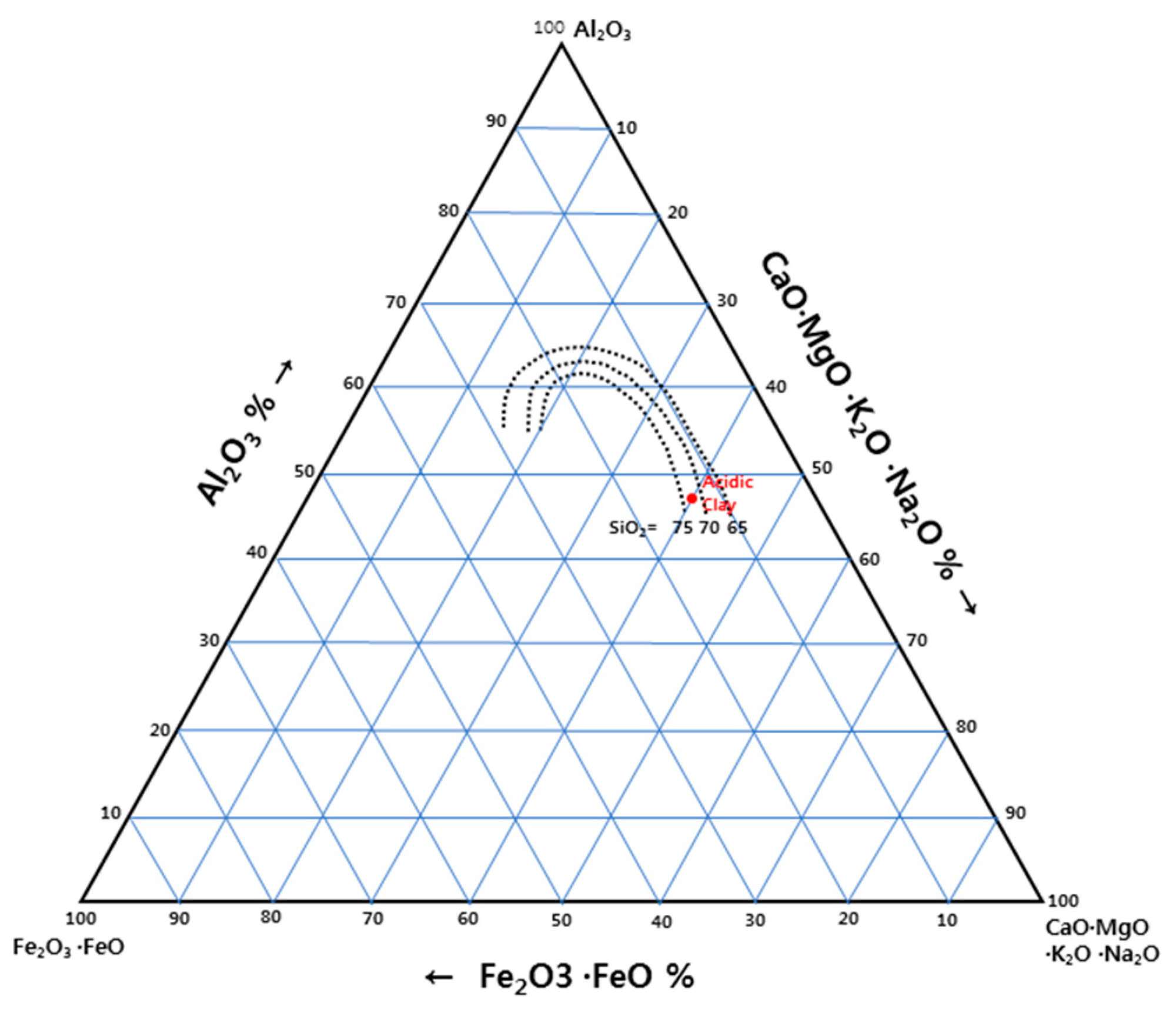
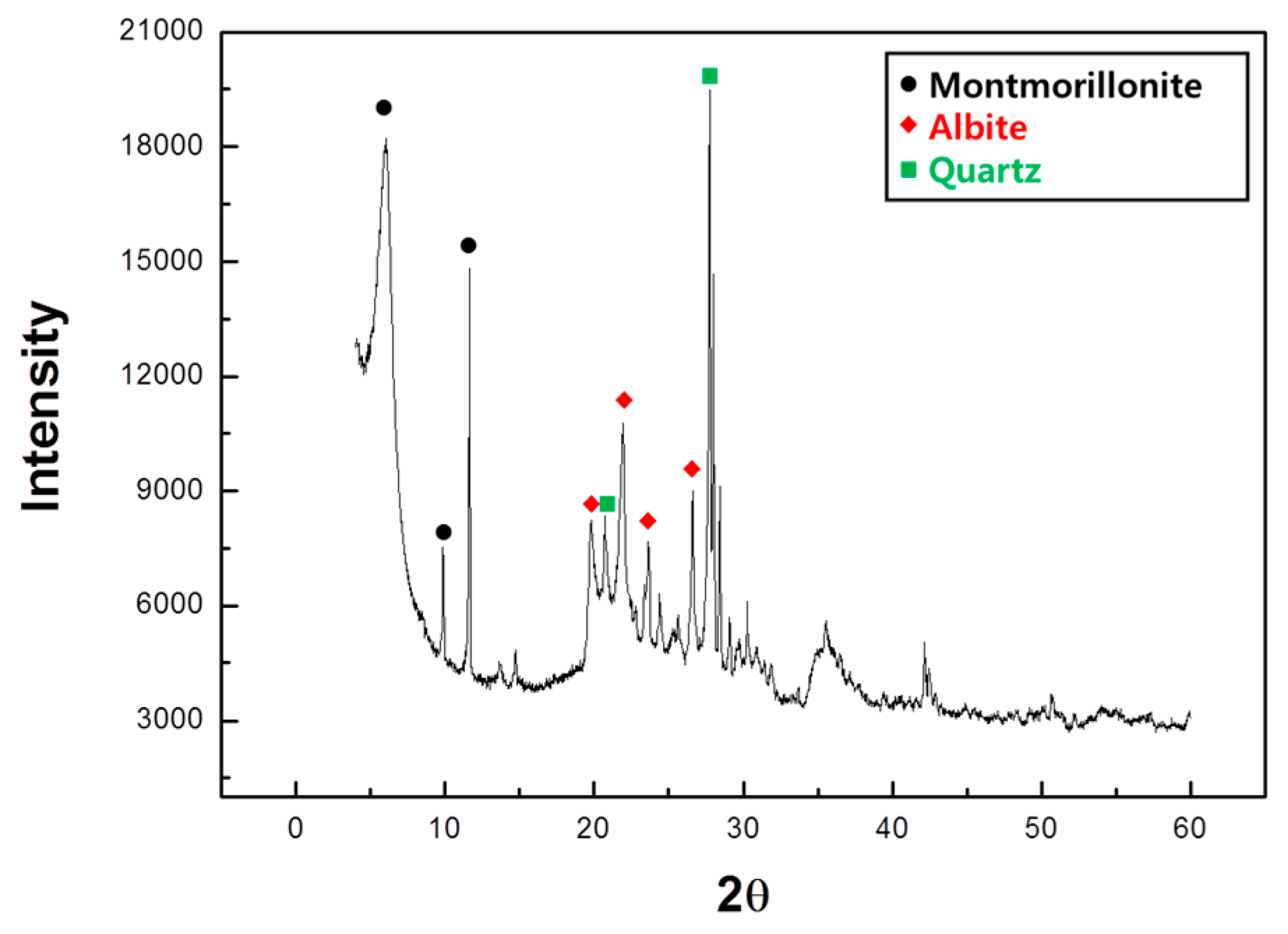
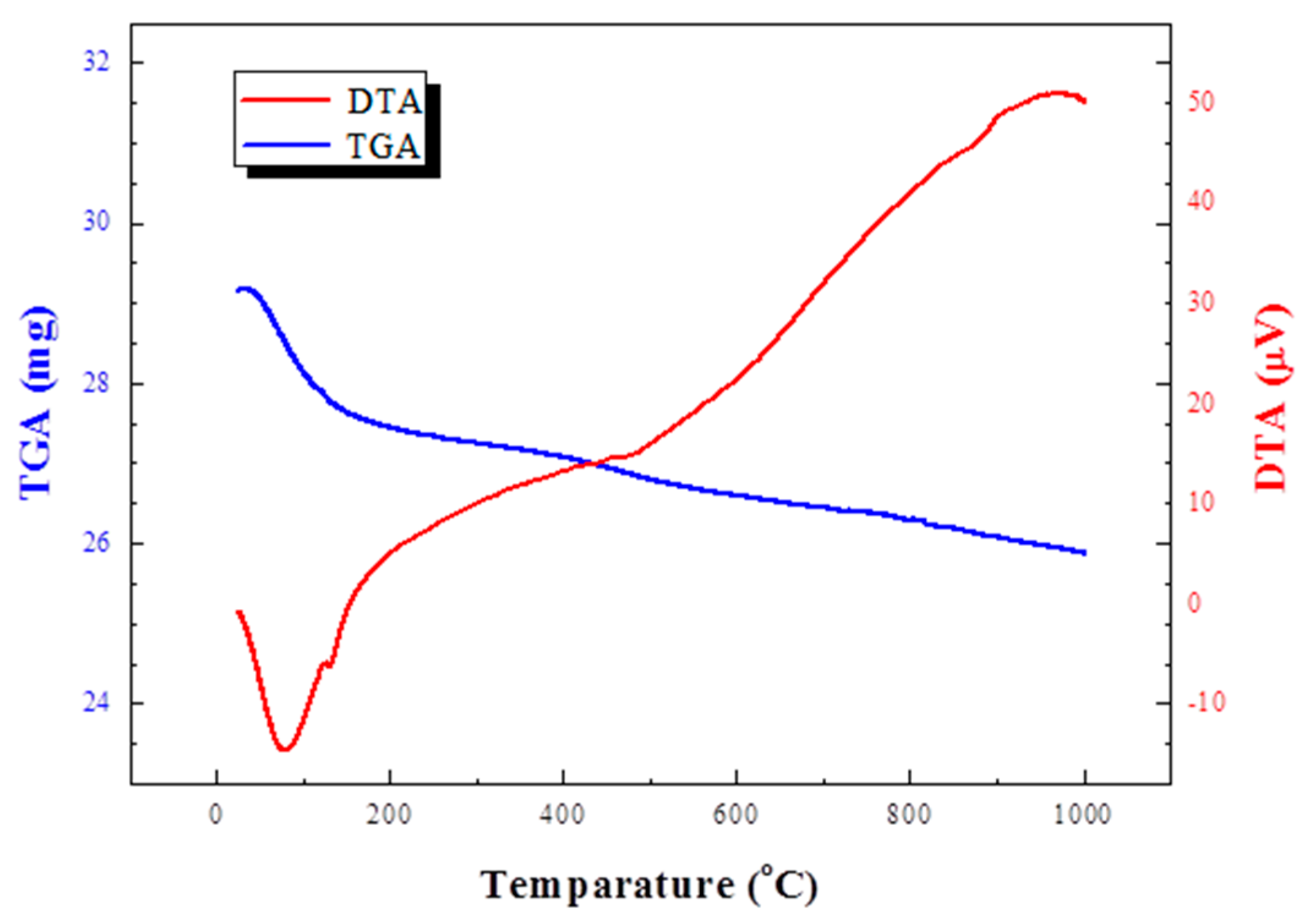
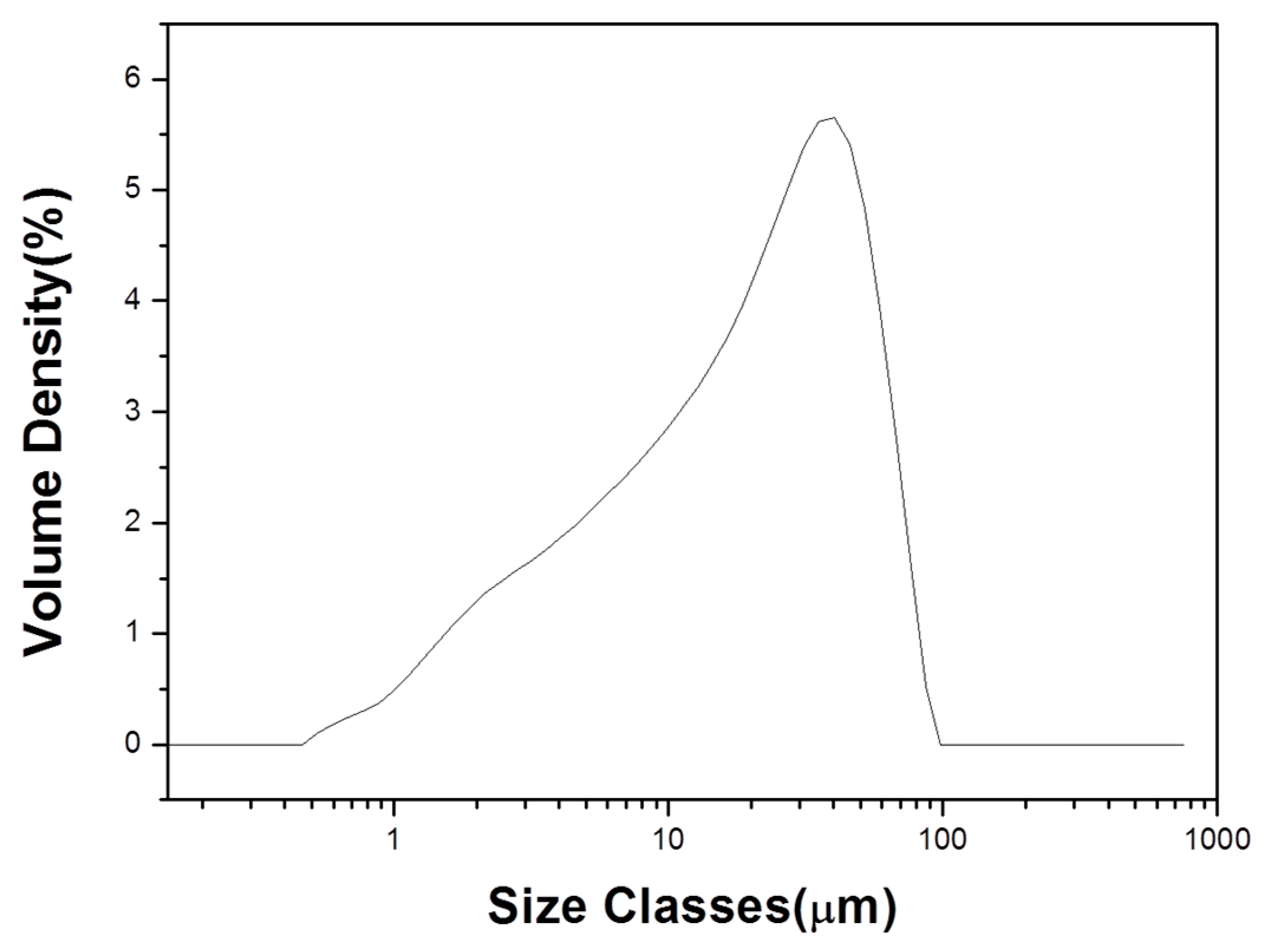





| Items | Acid Clay |
|---|---|
| SiO2 | 67.3 |
| Al2O3 | 12.9 |
| Fe2O3 | 2.9 |
| CaO | 2.8 |
| MgO | 2.4 |
| Na2O | 1.6 |
| K2O | 1.8 |
| TiO2 | 0.6 |
| P2O5 | 0 |
| Ig-Loss | 7.7 |
| Total | 100 |
| Input Temperature (°C) | Maximum Temperature (°C) | Sintering Time (min) |
|---|---|---|
| 300 | 1200 | 30, 40, 50, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330 |
| 600 | 30, 40, 50, 60, 90, 120, 150, 180, 210, 240, 270 |
| Liquid Limit (LL) | Plastic Limit (PL) | Plasticity Index (PI) |
|---|---|---|
| 52 | 38 | 14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wie, Y.M.; Lee, K.G. Optimum Bloating-Activation Zone of Artificial Lightweight Aggregate by Dynamic Parameters. Materials 2019, 12, 267. https://doi.org/10.3390/ma12020267
Wie YM, Lee KG. Optimum Bloating-Activation Zone of Artificial Lightweight Aggregate by Dynamic Parameters. Materials. 2019; 12(2):267. https://doi.org/10.3390/ma12020267
Chicago/Turabian StyleWie, Young Min, and Ki Gang Lee. 2019. "Optimum Bloating-Activation Zone of Artificial Lightweight Aggregate by Dynamic Parameters" Materials 12, no. 2: 267. https://doi.org/10.3390/ma12020267




