In Vitro Degradation of Absorbable Zinc Alloys in Artificial Urine
Abstract
:1. Introduction
2. Material and Methods
2.1. Material and Specimen Preparation
2.2. In Vitro Degradation Testing
2.3. Surface and Corrosion Layer Characterization
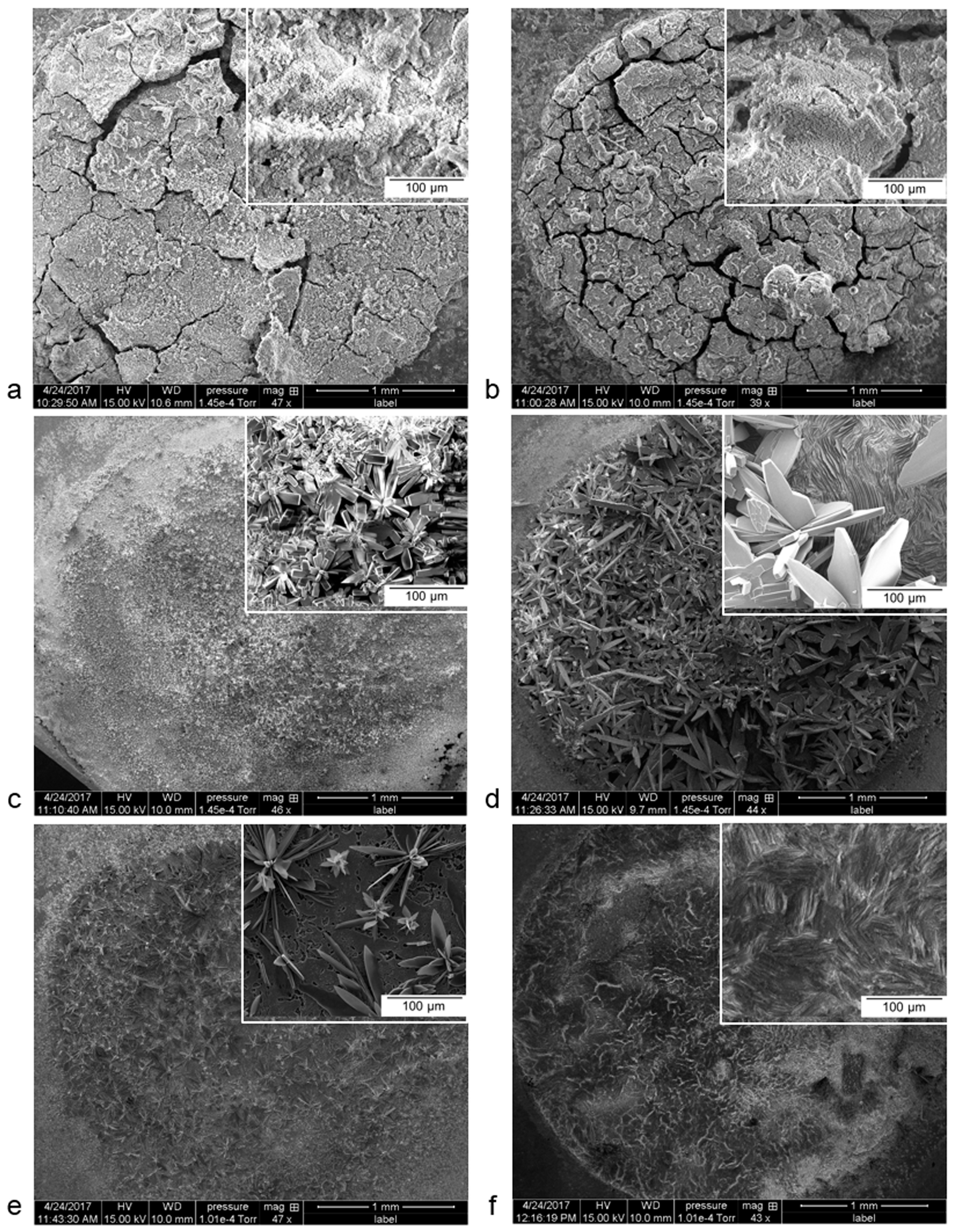
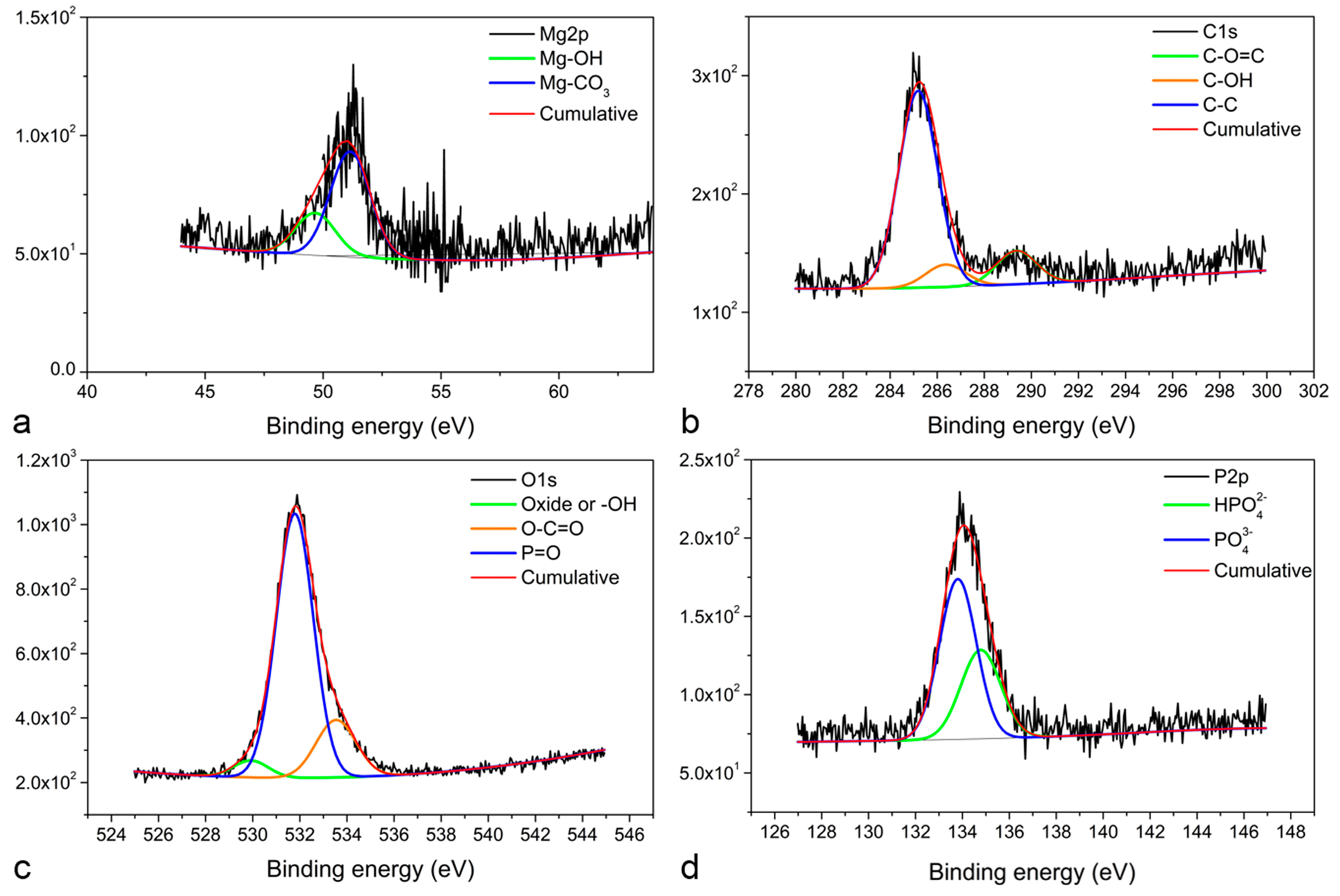
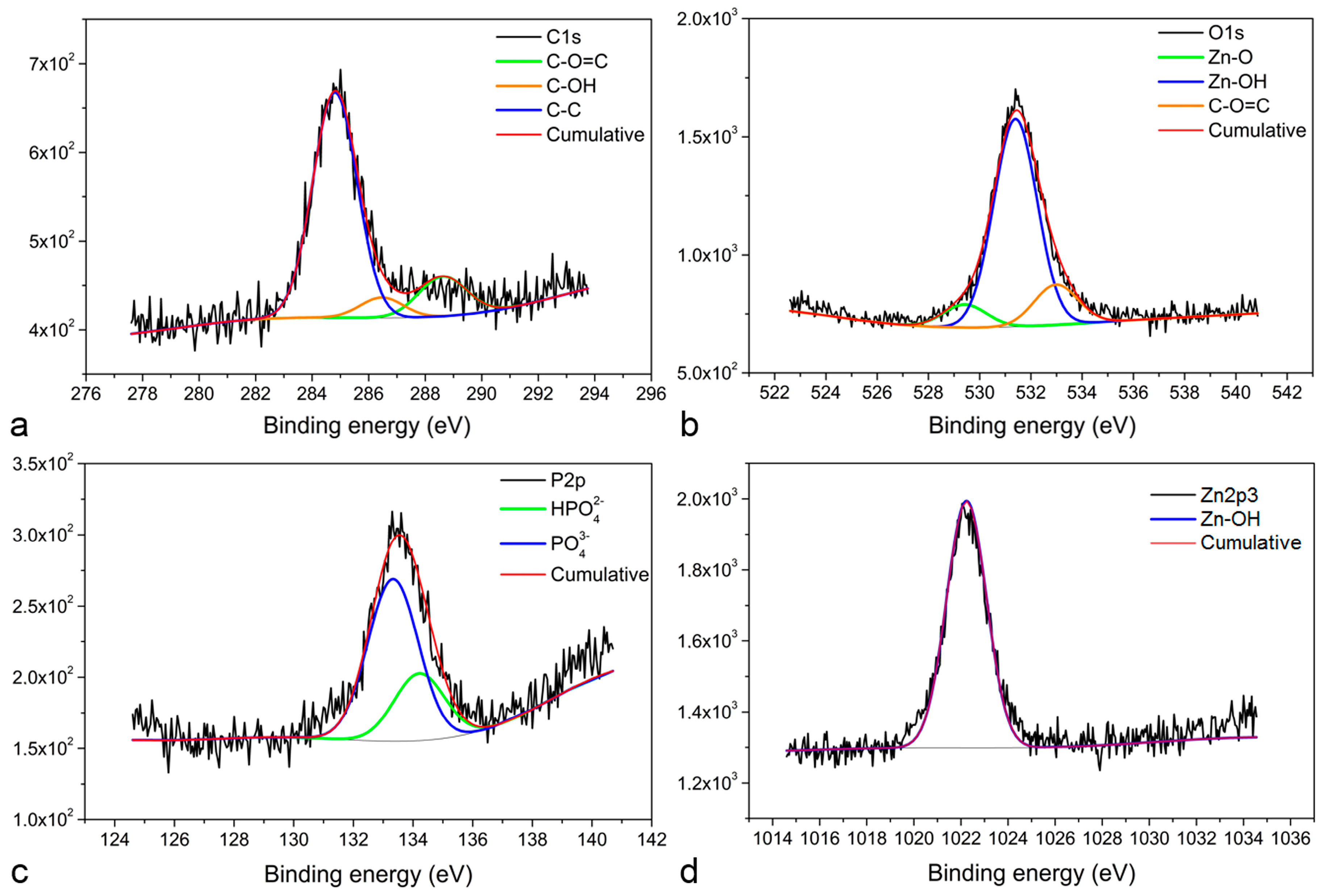
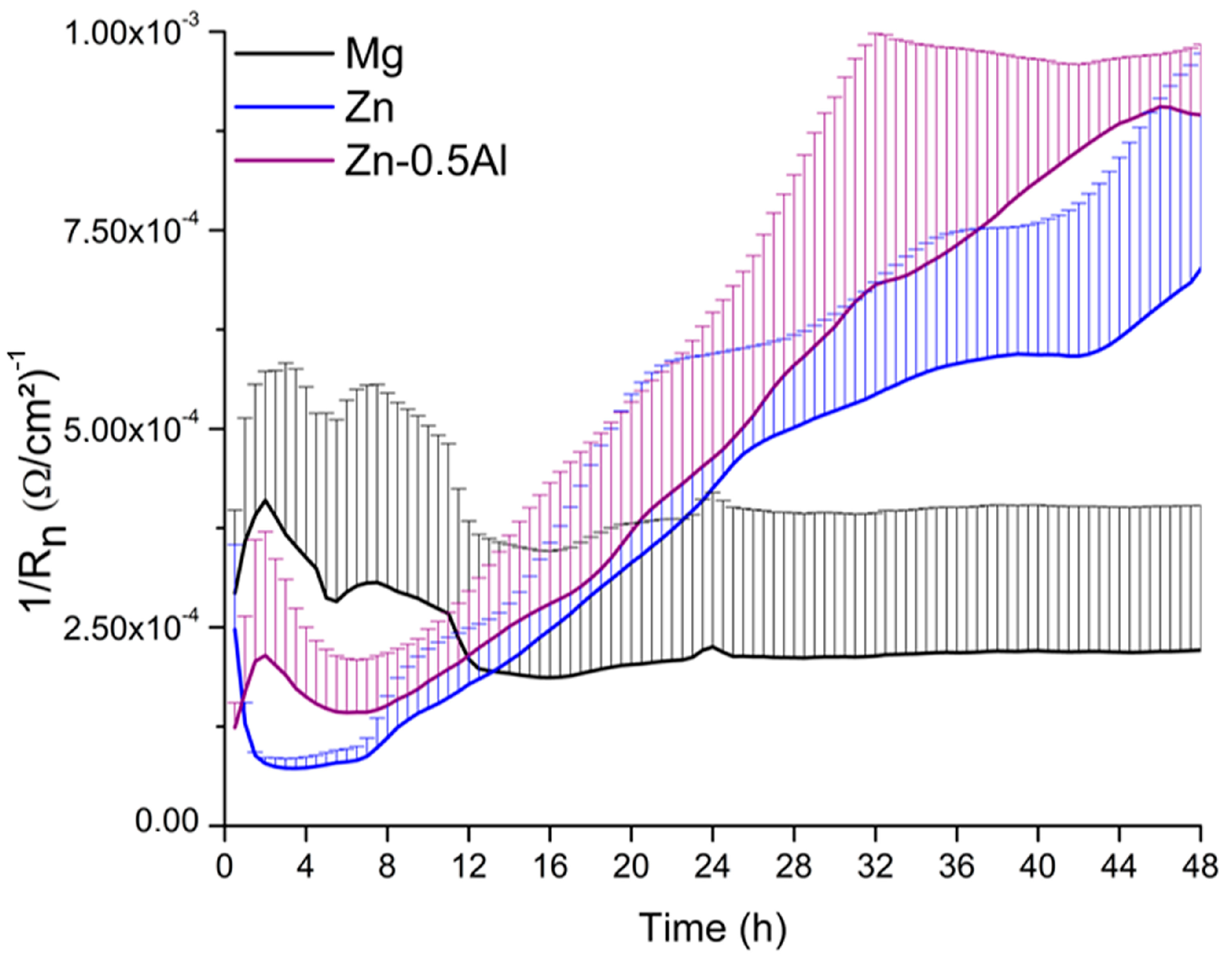
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- ADAM Medical Encyclopedia. Acute Bilateral Obstructive Uropathy; ADAM Inc.: Atlanta, GA, USA, 2010. [Google Scholar]
- Public Health Agency Canada. Economic Burden of Illness in Canada 2005–2008; Public Health Agency Canada: Ottawa, ON, USA, 2014.
- Tseng, T.Y.; Stoller, M.L. Obstructive Uropathy. Clin. Geriatr. Med. 2009, 25, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Lange, D.; Bidnur, S.; Hoag, N.; Chew, B.H. Ureteral stent-associated complications—Where we are and where we are going. Nat. Rev. Urol. 2015, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Auge, B.K.; Sarvis, J.A.; L’Esperance, J.O.; Preminger, G.M. Practice Patterns of Ureteral Stenting after Routine Ureteroscopic Stone Surgery: A Survey of Practicing Urologists. J. Endourol. 2007, 21, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Lange, D.; Chew, B.H. Update on ureteral stent technology. Ther. Adv. Urol. 2009, 1, 143–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talja, M.; Välimaa, T.; Tammela, T.; Petas, A.; Törmälä, P. Bioabsorbable and biodegradable stents in urology. J. Endourol. 1997, 11, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shan, H.; Wang, J.; Hou, Y.; Ding, J.; Chen, Q.; Guan, J.; Wang, C.; Chen, X. Characterization of nanostructured ureteral stent with gradient degradation in a porcine model. Int. J. Nanomed. 2015, 10, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Pedro, R.N.; Hendlin, K.; Kriedberg, C.; Monga, M. Wire-Based Ureteral Stents: Impact on Tensile Strength and Compression. Urology 2007, 70, 1057–1059. [Google Scholar] [CrossRef]
- Hendlin, K.; Korman, E.; Monga, M. New Metallic Ureteral Stents: Improved Tensile Strength and Resistance to Extrinsic Compression. J. Endourol. 2011, 26, 271–274. [Google Scholar] [CrossRef]
- Hermawan, H. Updates on the research and development of absorbable metals for biomedical applications. Prog. Biomater. 2018, 7, 93–110. [Google Scholar] [CrossRef]
- Francis, A.; Yang, Y.; Virtanen, S.; Boccaccini, A.R. Iron and iron-based alloys for temporary cardiovascular applications. J. Mater. Sci. Mater. Med. 2015, 26, 138. [Google Scholar] [CrossRef]
- Haude, M.; Ince, H.; Kische, S.; Abizaid, A.; Tölg, R.; Alves, L.P.; van Mieghem, N.; Verheye, S.; von Birgelen, C.; Christiansen, E. Sustained safety and clinical performance of a drug-eluting absorbable metal scaffold up to 24 months: Pooled outcomes of BIOSOLVE-II and BIOSOLVE-III. EuroIntervention 2017, 20, 432–439. [Google Scholar]
- Drelich, A.J.; Zhao, S.; Guillory, R.J.; Drelich, J.W.; Goldman, J. Long-term surveillance of zinc implant in murine artery: Surprisingly steady biocorrosion rate. Acta Biomater. 2017, 58, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, C.; Liu, C.; Chen, H.; Wu, Y.; Han, J.; Jia, Z.; Lin, W.; Zhang, D.; Li, W.; et al. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials 2017, 145, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.Y.; Wyatt, E.; Upadhyayula, S.; Whall, A.; Nuñez, V.; Vullev, V.I.; Liu, H. Degradation and antibacterial properties of magnesium alloys in artificial urine for potential resorbable ureteral stent applications. J. Biomed. Mater. Res. A 2014, 102, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, Y.; Zhang, L.; Bi, Y.; Li, J.; Liu, J.; Yu, Y.; Guo, H.; Li, Y. In vitro and in vivo corrosion and histocompatibility of pure Mg and a Mg-6Zn alloy as urinary implants in rat model. Mater. Sci. Eng. C 2016, 68, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Bi, Y.; Li, J.; Wang, Z.; Yan, J.; Song, J.; Sheng, H.; Guo, H.; Li, Y. Biodegradation behavior of magnesium and ZK60 alloy in artificial urine and rat models. Bioact. Mater. 2017, 2, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Mostaed, E.; Sikora-Jasinska, M.; Mostaed, A.; Loffredo, S.; Demir, A.G.; Previtali, B.; Mantovani, D.; Beanland, R.; Vedani, M. Novel Zn-based alloys for biodegradable stent applications: Design, development and in vitro degradation. J. Mech. Behav. Biomed. Mater. 2016, 60, 581–602. [Google Scholar] [CrossRef] [PubMed]
- Kirchmann, H.; Pettersson, S. Human urine—Chemical composition and fertilizer use efficiency. Fertil. Res. 1994, 40, 149–154. [Google Scholar] [CrossRef]
- ASTM International. ASTM F2129-17 Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Ghali, E. Corrosion Resistance of Aluminum and Magnesium Alloys: Understanding, Performance, and Testing; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Jamesh, M.; Kumar, S.; Narayanan, T.S.N.S. Corrosion behavior of commercially pure Mg and ZM21 Mg alloy in Ringer’s solution—Long term evaluation by EIS. Corros. Sci. 2011, 53, 645–654. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Dambatta, M.S.; Izman, S.; Kurniawan, D.; Farahany, S.; Yahaya, B.; Hermawan, H. Influence of thermal treatment on microstructure, mechanical and degradation properties of Zn–3Mg alloy as potential biodegradable implant material. Mater. Des. 2015, 85, 431–437. [Google Scholar] [CrossRef]
- Zhang, X.G. Corrosion and Electrochemistry of Zinc; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [Green Version]
- Rosalbino, F.; Negri, S.D.; Saccone, A.; Angelini, E.; Delfino, S. Bio-corrosion characterization of Mg–Zn–X (X = Ca, Mn, Si) alloys for biomedical applications. J. Mater. Sci. Mater. Med. 2010, 21, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Xu, L.; Yu, G.; Zhang, E.; Pan, F.; Yang, K. In vivo corrosion behavior of Mg-Mn-Zn alloy for bone implant application. J. Biomed. Mater. Res. A 2007, 83A, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Abidin, N.I.Z.; Rolfe, B.; Owen, H.; Malisano, J.; Martin, D.; Hofstetter, J.; Uggowitzer, P.J.; Atrens, A. The in vivo and in vitro corrosion of high-purity magnesium and magnesium alloys WZ21 and AZ91. Corros. Sci. 2013, 75, 354–366. [Google Scholar] [CrossRef]
- Törne, K.; Larsson, M.; Norlin, A.; Weissenrieder, J. Degradation of zinc in saline solutions, plasma, and whole blood. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Kubásek, J.; Vojtěch, D.; Jablonská, E.; Pospíšilová, I.; Lipov, J.; Ruml, T. Structure, mechanical characteristics and in vitro degradation, cytotoxicity, genotoxicity and mutagenicity of novel biodegradable Zn–Mg alloys. Mater. Sci. Eng. C 2016, 58, 24–35. [Google Scholar] [CrossRef]
- Kirejczyk, J.K.; Porowski, T.; Filonowicz, R.; Kazberuk, A.; Stefanowicz, M.; Wasilewska, A.; Debek, W. An association between kidney stone composition and urinary metabolic disturbances in children. J. Pediatr. Urol. 2014, 10, 130–135. [Google Scholar] [CrossRef]
- Selvaraju, R.; Raja, A.; Thiruppathi, G. FT-IR spectroscopic, thermal analysis of human urinary stones and their characterization. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 1397–1402. [Google Scholar] [CrossRef]
- Hosking, N.C.; Ström, M.A.; Shipway, P.H.; Rudd, C.D. Corrosion resistance of zinc–magnesium coated steel. Corros. Sci. 2007, 49, 3669–3695. [Google Scholar] [CrossRef]
- Liu, M.; Zanna, S.; Ardelean, H.; Frateur, I.; Schmutz, P.; Song, G.; Atrens, A.; Marcus, P. A first quantitative XPS study of the surface films formed, by exposure to water, on Mg and on the Mg–Al intermetallics: Al3Mg2 and Mg17Al12. Corros. Sci. 2009, 51, 1115–1127. [Google Scholar] [CrossRef]
- Reid, G.; Davidson, R.; Denstedt, J.D. XPS, SEM and EDX analysis of conditioning film deposition onto ureteral stents. Surf. Interface Anal. 1994, 21, 581–586. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, L.; Dong, J.; Ke, W.; Chen, N. Potentiostatic Conversion of Phosphate Mineral Coating on AZ31 Magnesium Alloy in 0.1 M K2HPO4 Solution. Electrochim. Acta 2014, 145, 71–80. [Google Scholar] [CrossRef]
- Santamaria, M.; di Quarto, F.; Zanna, S.; Marcus, P. Initial surface film on magnesium metal: A characterization by X-ray photoelectron spectroscopy (XPS) and photocurrent spectroscopy (PCS). Electrochim. Acta 2007, 53, 1314–1324. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Shan, D.; Chen, R.; Zhang, F.; Han, E.-H. A novel phosphate conversion film on Mg–8.8Li alloy. Surf. Coat. Technol. 2009, 203, 1107–1113. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Yan, Y.L.; Helfand, M.A.; Clayton, C.R. Evaluation of the effect of surface roughness on thin film thickness measurements using variable angle XPS. Appl. Surf. Sci. 1989, 37, 395–405. [Google Scholar] [CrossRef]
- Tamilselvi, S.; Raman, V.; Rajendran, N. Corrosion behaviour of Ti–6Al–7Nb and Ti–6Al–4V ELI alloys in the simulated body fluid solution by electrochemical impedance spectroscopy. Electrochim. Acta 2006, 52, 839–846. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef]
- Macdonald, D.D. Reflections on the history of electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1376–1388. [Google Scholar] [CrossRef]
- Jorcin, J.-B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef] [Green Version]
- Scully, J.R.; Silverman, D.C. Electrochemical Impedance: Analysis and Interpretation; ASTM International: West Conshohocken, PA, USA, 1993. [Google Scholar]
- Alves, M.M.; Prošek, T.; Santos, C.F.; Montemor, M.F. Evolution of the in vitro degradation of Zn–Mg alloys under simulated physiological conditions. RSC Adv. 2017, 7, 28224–28233. [Google Scholar] [CrossRef] [Green Version]
- Gusmano, G.; Montesperelli, G.; Pacetti, S.; Petitti, A.; D’Amico, A. Electrochemical Noise Resistance as a Tool for Corrosion Rate Prediction. Corrosion 1997, 53, 860–868. [Google Scholar] [CrossRef]



| Chemical | NaCl | NaH2PO4 | Na3C6H5O7 | MgSO4 | Na2SO4 | KCl | CaCl2 | Na2C2O4 |
|---|---|---|---|---|---|---|---|---|
| Mass (g) | 6.17 | 4.59 | 0.944 | 0.463 | 2.408 | 4.75 | 0.638 | 0.043 |
| Sample | OCP (V vs. SCE) | icorr (µA/cm²) | Corrosion Rate (mm/year) |
|---|---|---|---|
| Mg | −1.58 ± 0.02 | 94 ± 37 | 2.16 ± 0.84 |
| Mg–2Zn–1Mn | −1.48 ± 0.07 | 84 ± 17 | 1.90 ± 0.38 |
| Zn | −1.11 ± 0.06 | 58 ± 6 | 0.87 ± 0.09 |
| Zn–0.5Mg | −1.18 ± 0.01 | 92 ± 5 | 1.39 ± 0.07 |
| Zn–1Mg | −1.17 ± 0.01 | 99 ± 5 | 1.50 ± 0.08 |
| Zn–0.5Al | −1.15 ± 0.01 | 77 ± 2 | 1.14 ± 0.03 |
| Sample | Element | ||||||
|---|---|---|---|---|---|---|---|
| EDS (wt %) | C | O | Mg | P | Ca | Zn | Al |
| Mg | 4.9 | 50.6 | 11.9 | 19.7 | 13.0 | - | - |
| Mg–2Zn–1Mn | 2.3 | 47.2 | 13.3 | 22.1 | 15.3 | - | - |
| Zn | 16.4 | 17.5 | - | 8.8 | - | 57.3 | - |
| Zn–0.5Mg | 13.9 | 11.7 | - | 7.9 | - | 66.6 | - |
| Zn–1Mg | 22.3 | 14.2 | - | 5.6 | - | 57.9 | - |
| Zn–0.5Al | 9.2 | 6.7 | - | 4.0 | - | 65.5 | 14.6 |
| XPS (at %) | C1s | O1s | Mg2p | P2p | Ca2p | Zn3p2 | Al2p |
| Mg | 31 ± 10 | 40 ± 8 | 15 ± 2 | 5 ± 4 | 9 ± 1 | - | - |
| Mg–2Zn–1Mn | 23 ± 3 | 46 ± 3 | 14 ± 4 | 9 ± 12 | 8 ± 3 | - | - |
| Zn | 25 ± 3 | 45 ± 2 | - | 18 ± 2 | - | 12 ± 2 | - |
| Zn–0.5Mg | 28 ± 5 | 42 ± 5 | - | 19 ± 1 | - | 11.1 ± 1 | - |
| Zn–1Mg | 31 ± 6 | 42 ± 2 | - | 17 ± 3 | - | 10 ± 2 | - |
| Zn–0.5Al | 26 ± 2 | 44 ± 2 | - | 9 ± 7 | - | 10 ± 2 | 5 ± 2 |
| Specimen | Mg | Mg–2Zn–1Mn | Zn | Zn–Mg | Zn–0.5Al | |
|---|---|---|---|---|---|---|
| Model 1 R(QR) | Rct | 634 ± 141 | 1357 ± 321 | - | - | - |
| CPEdl | 56 ± 8 | 277 ± 49 | - | - | - | |
| Model 2 R(Q(QR)) | Rct | - | - | 9 ± 4 | 5 ± 5 | 13 ± 2 |
| CPEdl | - | - | 8480 ± 2770 | 1220 ± 1700 | 2740 ± 153 | |
| CPEol | - | - | 23 ± 15 | 2030 ± 1520 | 29 ± 10 | |
| Model 3 R(QR)(QR) | Rct | 45 ± 1 | 104 ± 47 | 20 ± 3 | 25 ± 4 | 495 ± 108 |
| Rol | 716 ± 178 | 583 ± 47 | 95 ± 27 | 242 ± 51 | 14 ± 2 | |
| CPEdl | 85 ± 11 | 84 ± 11 | 2970 ± 1060 | 871 ± 119 | 74 ± 17 | |
| CPEol | 63 ± 19 | 317 ± 101 | 77 ± 48 | 82 ± 49 | 1850 ± 170 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Champagne, S.; Mostaed, E.; Safizadeh, F.; Ghali, E.; Vedani, M.; Hermawan, H. In Vitro Degradation of Absorbable Zinc Alloys in Artificial Urine. Materials 2019, 12, 295. https://doi.org/10.3390/ma12020295
Champagne S, Mostaed E, Safizadeh F, Ghali E, Vedani M, Hermawan H. In Vitro Degradation of Absorbable Zinc Alloys in Artificial Urine. Materials. 2019; 12(2):295. https://doi.org/10.3390/ma12020295
Chicago/Turabian StyleChampagne, Sébastien, Ehsan Mostaed, Fariba Safizadeh, Edward Ghali, Maurizio Vedani, and Hendra Hermawan. 2019. "In Vitro Degradation of Absorbable Zinc Alloys in Artificial Urine" Materials 12, no. 2: 295. https://doi.org/10.3390/ma12020295
APA StyleChampagne, S., Mostaed, E., Safizadeh, F., Ghali, E., Vedani, M., & Hermawan, H. (2019). In Vitro Degradation of Absorbable Zinc Alloys in Artificial Urine. Materials, 12(2), 295. https://doi.org/10.3390/ma12020295






