A Comparison of Ethylene-Tar-Derived Isotropic Pitches Prepared by Air Blowing and Nitrogen Distillation Methods and Their Carbon Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Spinnable Pitches
2.3. Preparation of Carbon Fibers
2.4. Characterization of Pitches and Carbon Fibers
3. Results and Discussion
3.1. Characterization of NDP and ABP
3.2. Spinning Properties of NDP and ABP
3.3. Stabilization and Carbonization of NDP-PF and ABP-PF
3.4. Morphology and Mechanical Properties of NDP-CF and ABP-CF
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frank, E.; Steudle, L.M.; Ingildeev, D. Carbon Fibers: Precursor Systems, Processing, Structure, and Properties. Angew. Chem. Int. Ed. 2014, 53, 5262–5298. [Google Scholar] [CrossRef] [PubMed]
- Mochida, I.; Toshima, H.; Korai, Y. Blending mesophase pitch to improve its properties as a precursor for carbon fibre I. Blending of PVC pitch into coal tar and petroleum-derived mesophase pitches. J. Mater. Sci. 1988, 23, 670–677. [Google Scholar] [CrossRef]
- Kim, B.; Eom, Y.; Kato, O. Preparation of carbon fibers with excellent mechanical properties from isotropic pitches. Carbon 2014, 77, 747–755. [Google Scholar] [CrossRef]
- Mora, E.; Blanco, C.; Prada, V. A study of pitch-based precursors for general purpose carbon fibers. Carbon 2002, 40, 2719–2725. [Google Scholar] [CrossRef]
- Xu, Y.; Chung, D.D.L. Silane-treated carbon fiber for reinforcing cement. Carbon 2001, 39, 1995–2001. [Google Scholar] [CrossRef]
- Fu, X.; Lu, W.; Chung, D.D.L. Ozone treatment of carbon fiber for reinforcing cement. Carbon 1998, 36, 1337–1345. [Google Scholar] [CrossRef]
- Chand, S. Review carbon fibers for composites. J. Mater. Sci. 2000, 35, 1303–1313. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Hamim, S.U.; Karbalaei, A.M. Carbon fiber reinforced metal matrix composites: Fabrication processes and properties. Compos. Part A-Appl. Sci. 2017, 92, 70–96. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, C.; Choi, Y.O. Preparations of pitch-based CF/ACF webs by electrospinning. Carbon 2013, 41, 2655–2657. [Google Scholar] [CrossRef]
- Yang, J.; Nakabayashi, K.; Miyawaki, J. Preparation of pitch based carbon fibers using Hyper-coal as a raw material. Carbon 2016, 106, 28–36. [Google Scholar] [CrossRef]
- Kim, J.G.; Kim, J.H.; Song, B. Synthesis and its characterization of pitch from pyrolyzed fuel oil (PFO). J. Ind. Eng. Chem. 2016, 36, 293–297. [Google Scholar] [CrossRef]
- Barr, J.B.; Lewis, I.C. Chemical changes during the mild air oxidation of pitch. Carbon 1978, 16, 439–444. [Google Scholar] [CrossRef]
- Mochida, I.; Inaba, T.; Korai, Y. Carbonization properties of carbonaceous substances oxidized by air blowing—I: Carbonization behaviors and chemical structure of residual oils oxidized by air blowing. Carbon 1983, 21, 543–552. [Google Scholar] [CrossRef]
- Mochida, I.; Inaba, T.; Korai, Y. Carbonization properties of carbonaceous substances oxidized by air blowing—II: Acid-catalyzed modification of oxidized residual oil for better anisotropic development. Carbon 1983, 21, 553–558. [Google Scholar] [CrossRef]
- Blanco, C.; Santamar, A.R.; Bermejo, J. A comparative study of air-blown and thermally treated coal-tar pitches. Carbon 2000, 38, 517–523. [Google Scholar] [CrossRef]
- Menendez, R.; Blanco, C.; Santamaria, R. On the chemical composition of thermally treated coal-tar pitches. Energy Fuels 2001, 15, 214–223. [Google Scholar] [CrossRef]
- Prada, V.; Granda, M.; Bermejo, J. Preparation of novel pitches by tar air-blowing. Carbon 1999, 37, 97–106. [Google Scholar] [CrossRef]
- Maeda, T.; Zeng, S.M.; Tokumitsu, K. Preparation of isotropic pitch precursors for general purpose carbon fibers (GPCF) by air blowing—I. Preparation of spinnable isotropic pitch precursor from coal tar by air blowing. Carbon 1993, 31, 407–412. [Google Scholar] [CrossRef]
- Zeng, S.M.; Maeda, T.; Tokumitsu, K. Preparation of isotropic pitch precursors for general purpose carbon fibers (GPCF) by air blowing—II. Air blowing of coal tar, hydrogenated coal tar, and petroleum pitches. Carbon 1993, 31, 413–419. [Google Scholar] [CrossRef]
- Fernández, J.J.; Figueiras, A.; Granda, M. Modification of coal-tar pitch by air-blowing—I. Variation of pitch composition and properties. Carbon 1995, 33, 295–307. [Google Scholar] [CrossRef]
- Fernández, A.L.; Granda, M.; Bermejo, J. Air-blowing of anthracene oil for carbon precursors. Carbon 2000, 38, 1315–1322. [Google Scholar] [CrossRef]
- Mishra, A.; Saha, M.; Bhatia, G. A comparative study on the development of pitch precursor for general-purpose carbon fibres. J. Mater. Process. Tech. 2005, 168, 316–320. [Google Scholar] [CrossRef]
- Wu, B.; Hu, H.; Zhao, Y. XPS analysis and combustibility of residues from two coals extraction with sub- and supercritical water. J. Fuel. Chem. Technol. 2009, 37, 385–392. [Google Scholar] [CrossRef]
- Matsumoto, T.; Mochida, I. A structural study on oxidative stabilization of mesophase pitch fibers derived from coal tar. Carbon 1992, 30, 1041–1046. [Google Scholar] [CrossRef]
- Andersen, S.I.; Jensen, J.O.; Speight, J.G. X-ray Diffraction of subfractions of petroleum asphaltenes. Energy Fuels 2005, 19, 2371–2377. [Google Scholar] [CrossRef]
- Korai, Y.; Mochida, I. Molecular assembly of mesophase and isotropic pitches at their fused states. Carbon 1992, 30, 1019–1024. [Google Scholar] [CrossRef]
- Drbohlav, J.; Stevenson, W.T.K. The oxidative stabilization and carbonization of a synthetic mesophase pitch, part I: The oxidative stabilization process. Carbon 1995, 33, 693–711. [Google Scholar] [CrossRef]


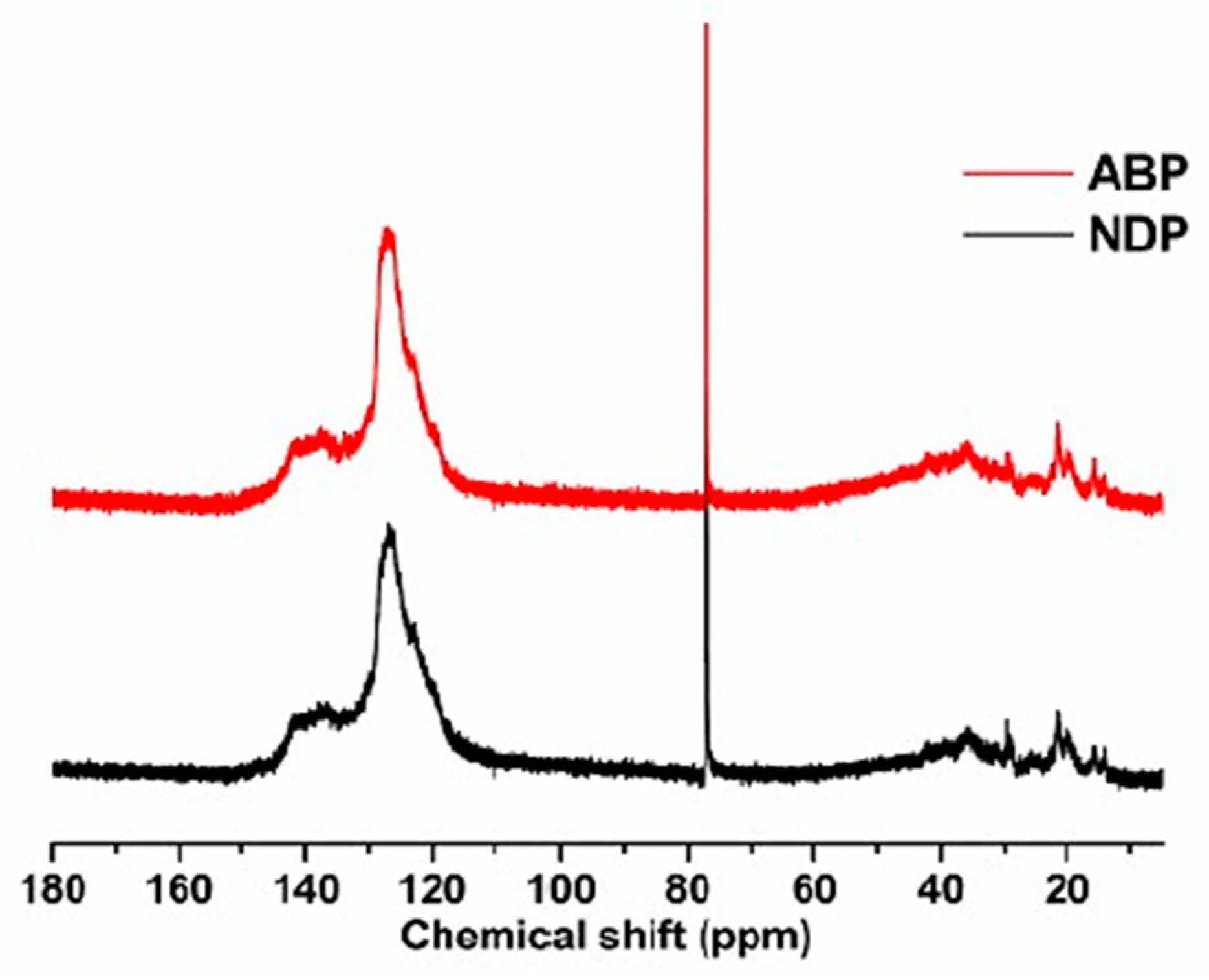
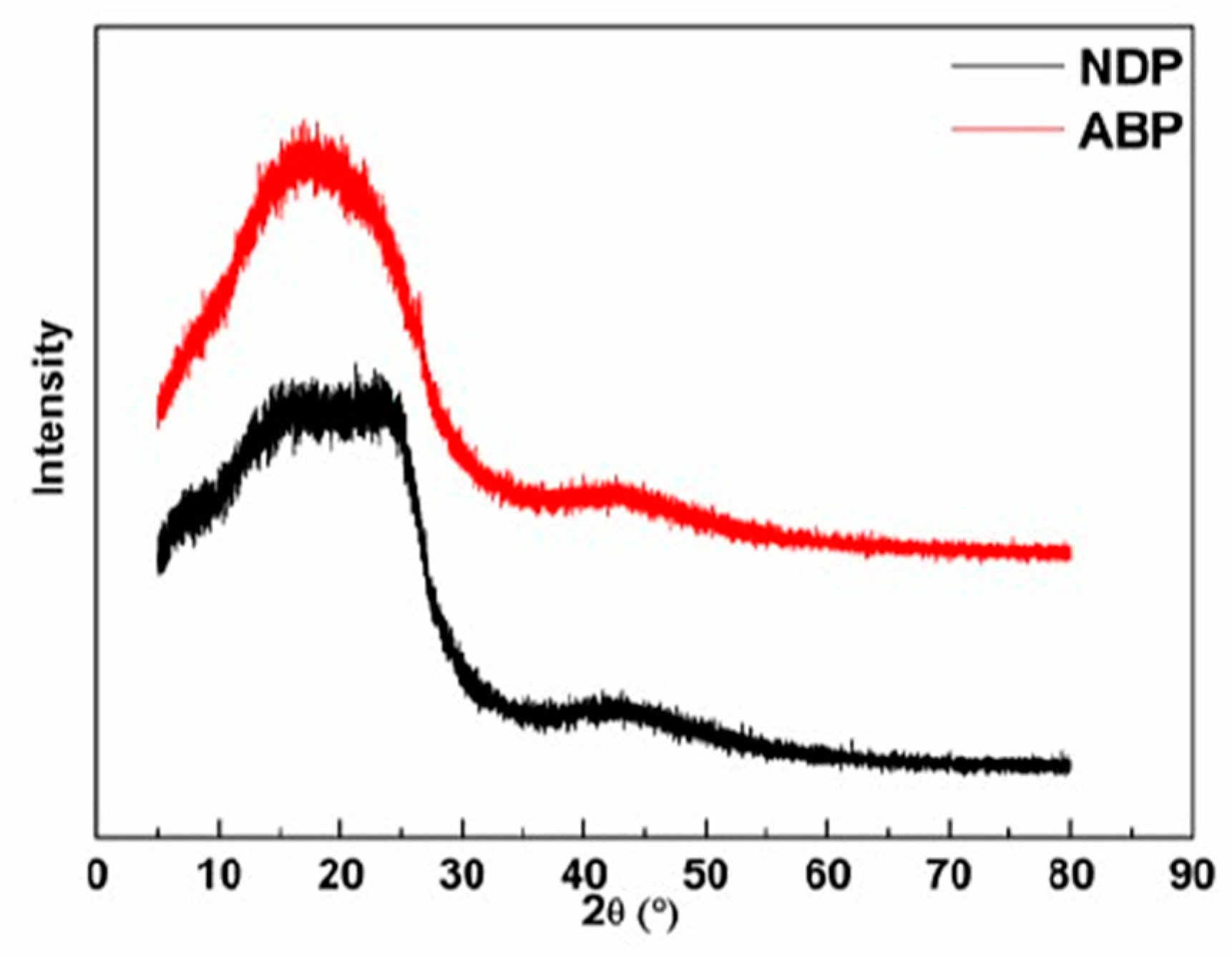




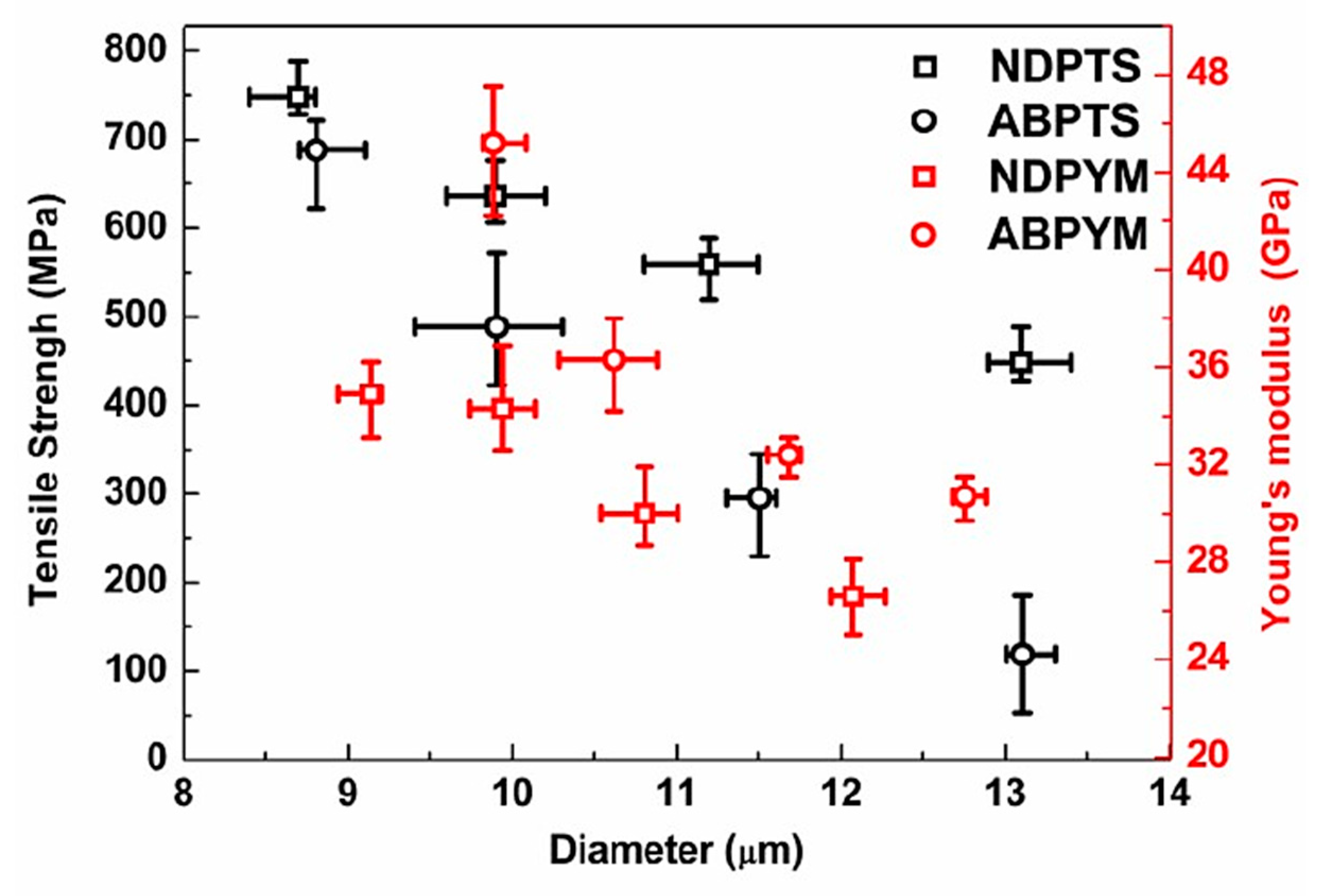
| Sample | SP (°C) | Yield (%) | Elemental Analysis (%) | |||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | C/H | |||
| NDP | 253 | 22 | 93.53 | 5.56 | 0.02 | 0.04 | 0.78 | 1.40 |
| ABP | 252 | 28 | 91.55 | 6.06 | 0.05 | 0.10 | 2.31 | 1.26 |
| Samples | Cal | Car | Cal | Car | Car/Cal | |||
|---|---|---|---|---|---|---|---|---|
| CH3 | CH2 | Cα2 | Car1,3 | Car1,2 | ||||
| NDP | 0.04 | 0.08 | 0.04 | 0.60 | 0.24 | 0.16 | 0.84 | 5.29 |
| ABP | 0.03 | 0.14 | 0.09 | 0.52 | 0.22 | 0.26 | 0.74 | 2.85 |
| Samples | Tmax (°C) | Wmax (%) | Ea (kJ/mol) | Oxygen Content (%) | Yield (%) |
|---|---|---|---|---|---|
| NDP-SF | 286 | 12.8 | 124.5 | 25.46 | 114.0 |
| ABP-SF | 278 | 11.6 | 120.5 | 25.72 | 112.5 |
| NDP-CF | - | - | - | 8.96 | 71.3 |
| ABP-CF | - | - | - | 10.29 | 71.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, K.; Yang, J.; Ye, C.; Liu, H.; Li, X. A Comparison of Ethylene-Tar-Derived Isotropic Pitches Prepared by Air Blowing and Nitrogen Distillation Methods and Their Carbon Fibers. Materials 2019, 12, 305. https://doi.org/10.3390/ma12020305
Shi K, Yang J, Ye C, Liu H, Li X. A Comparison of Ethylene-Tar-Derived Isotropic Pitches Prepared by Air Blowing and Nitrogen Distillation Methods and Their Carbon Fibers. Materials. 2019; 12(2):305. https://doi.org/10.3390/ma12020305
Chicago/Turabian StyleShi, Kui, Jianxiao Yang, Chong Ye, Hongbo Liu, and Xuanke Li. 2019. "A Comparison of Ethylene-Tar-Derived Isotropic Pitches Prepared by Air Blowing and Nitrogen Distillation Methods and Their Carbon Fibers" Materials 12, no. 2: 305. https://doi.org/10.3390/ma12020305
APA StyleShi, K., Yang, J., Ye, C., Liu, H., & Li, X. (2019). A Comparison of Ethylene-Tar-Derived Isotropic Pitches Prepared by Air Blowing and Nitrogen Distillation Methods and Their Carbon Fibers. Materials, 12(2), 305. https://doi.org/10.3390/ma12020305






