Femtosecond Laser Fabrication of Stable Hydrophilic and Anti-Corrosive Steel Surfaces
Abstract
:1. Introduction
2. Materials & Methods
2.1. Material
2.2. fs Laser Surface Structuring
2.3. Corrosion Test
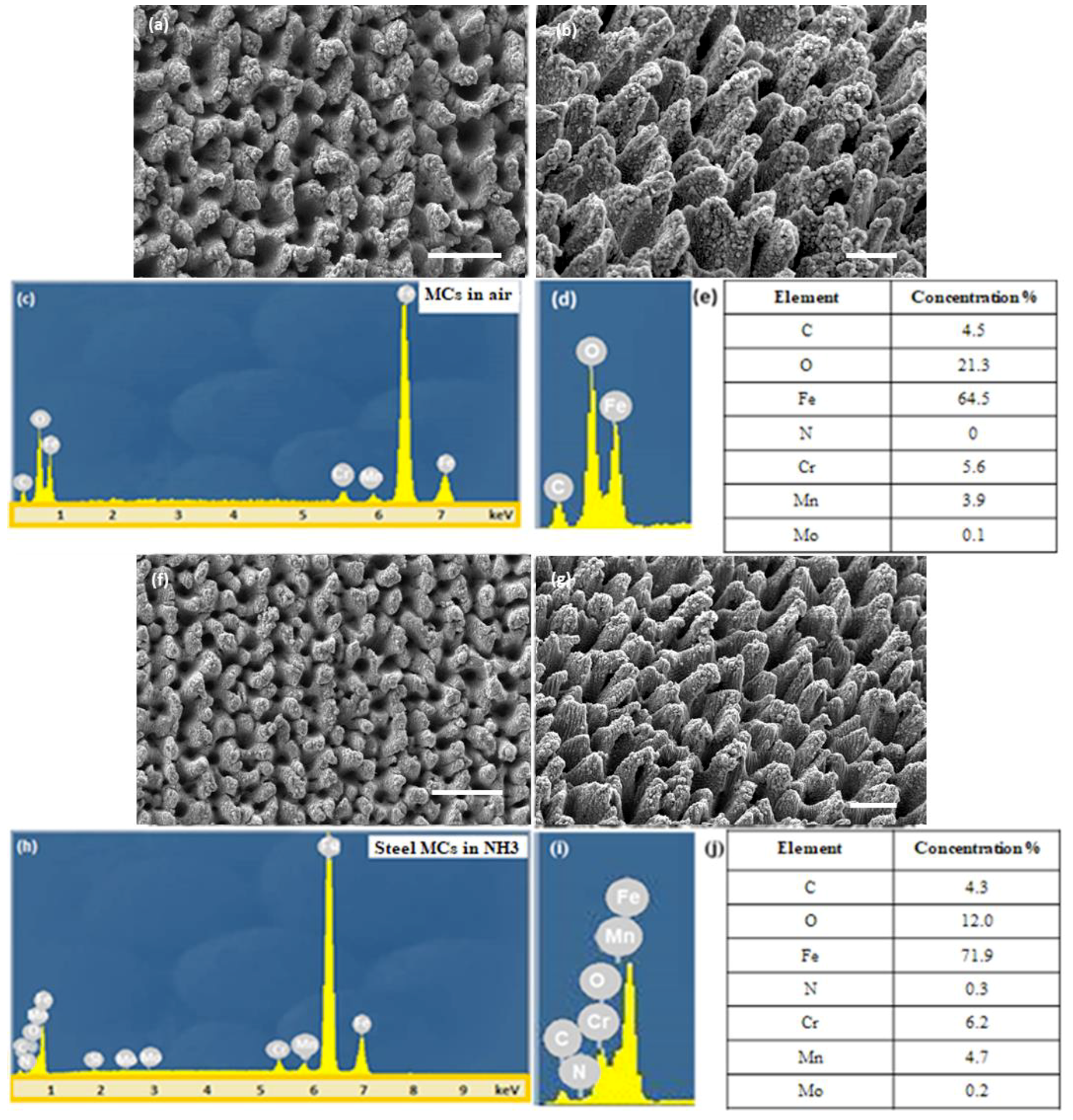
2.4. Characterization of Micropatterned Steel Substrates
3. Results
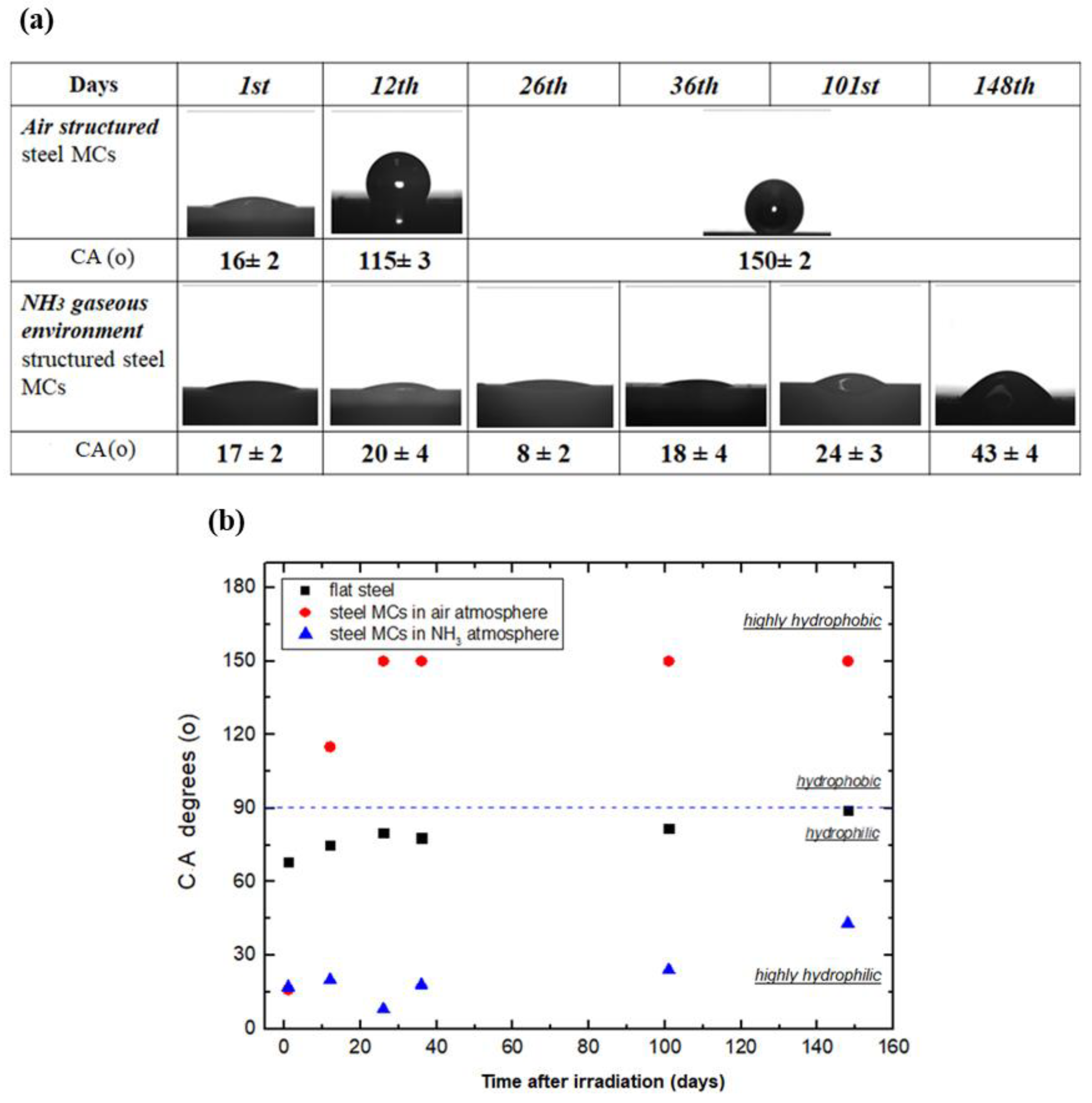
3.1. Surface Wettability
3.2. Surface Corrosion Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hermens, U.; Kirner, S.V.; Emonts, C.; Comanns, P.; Skoulas, E.; Mimidis, A.; Meschedera, H.; Winandsa, K.; Krügerb, J.; Stratakis, E.; et al. Applied Surface Science. Mimicking lizard-like surface structures upon ultrashort laser pulse irradiation of inorganic materials. Appl. Surf. Sci. 2017, 418, 499–507. [Google Scholar] [CrossRef]
- Bizi-bandoki, P.; Valette, S.; Audouard, E.; Benayoun, S. Time dependency of the hydrophilicity and hydrophobicity of metallic alloys subjected to femtosecond laser irradiations. Appl. Surf. Sci. 2013, 273, 399–407. [Google Scholar] [CrossRef]
- Stratakis, E.; Ranella, A.; Fotakis, C. Biomimetic micro/nanostructured functional surfaces for microfluidic and tissue engineering applications. Biomicrofluidics 2011, 5, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Zorba, V.; Stratakis, E.; Barberoglou, M.; Spanakis, E.; Tzanetakis, P.; Anastasiadis, S.H.; Fotakis, C. Biomimetic artificial surfaces quantitatively reproduce the water repellency of a lotus leaf. Adv. Mater. 2008, 20, 4049–4054. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Skoulas, E.; Mimidis, A.; Perrakis, G.; Kenanakis, G.; Tsibidis, G.D.; Stratakis, E. Biomimetic Omnidirectional Antireflective Glass via Direct Ultrafast Laser Nanostructuring. Adv. Mater. 2019, 31, 1901123. [Google Scholar] [CrossRef]
- Zhu, Q.; Shen, M. Femtosecond laser irradiation-induced infrared absorption on silicon surfaces. Int. J. Smart Nano Mater. 2015, 6, 113–123. [Google Scholar] [CrossRef]
- Bonse, J.; Kirner, S.; Griepentrog, M.; Spaltmann, D.; Krüger, J. Femtosecond Laser Texturing of Surfaces for Tribological Applications. Materials 2018, 11, 801. [Google Scholar] [CrossRef]
- Kirner, S.V.; Hermens, U.; Mimidis, A.; Skoulas, E.; Florian, C.; Hischen, F.; Plamadeala, C.; Baumgartner, W.; Winands, K.; Mescheder, H.; et al. Mimicking bug-like surface structures and their fluid transport produced by ultrashort laser pulse irradiation of steel. Appl. Phys. A 2017, 123, 1–13. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, N.; Xu, J. Fabrication and application of superhydrophilic surfaces: A review. J. Adhes. Sci. Technol. 2014, 28, 769–790. [Google Scholar] [CrossRef]
- Zorba, V.; Chen, X.; Mao, S.S. Superhydrophilic TiO2 surface without photocatalytic activation. Appl. Phys. Lett. 2010, 96, 093702. [Google Scholar] [CrossRef]
- Kim, P.; Wong, T.-S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-Infused Nanostructured Surfaces with Extreme Anti-Ice and Anti-Frost Performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef] [PubMed]
- Adera, S.; Raj, R.; Enright, R.; Wang, E.N. Non-wetting droplets on hot superhydrophilic surfaces. Nat. Commun. 2013, 4, 2518. [Google Scholar] [CrossRef] [Green Version]
- Paradisanos, I.; Fotakis, C.; Anastasiadis, S.H.; Stratakis, E. Gradient induced liquid motion on laser structured black Si surfaces. Appl. Phys. Lett. 2015, 107, 111603. [Google Scholar] [CrossRef] [Green Version]
- Ranella, A.; Barberoglou, M.; Bakogianni, S.; Fotakis, C.; Stratakis, E. Tuning cell adhesion by controlling the roughness and wettability of 3D micro/nano silicon structures. Acta Biomater. 2010, 6, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.Y.; Cui, X.G.; Ren, X.D.; Qi, M.J.; Hu, J.D.; Wang, Y.M. Surface oxidation phenomenon and mechanism of AISI304 stainless steel induced by Nd: YAG pulsed laser. Appl. Surf. Sci. 2014, 305, 817–824. [Google Scholar] [CrossRef]
- Kam, D.H.; Bhattacharya, S.; Mazumder, J. Control of the wetting properties of an AISI316L stainless steel surface by femtosecond laser-induced surface modification. J. Micromech. Microeng. 2012, 22, 105019–105025. [Google Scholar] [CrossRef]
- Florian Baron, C.; Mimidis, A.; Puerto, D.; Skoulas, E.; Stratakis, E.; Solis, J.; Siegel, J. Biomimetic surface structures in steel fabricated with femtosecond laser pulses: influence of laser rescanning on morphology and wettability. Beilstein J. Nanotechnol. 2018, 9, 2802–2812. [Google Scholar] [CrossRef] [Green Version]
- Kietzig, A.M.; Hatzikiriakos, S.G.; Englezos, P. Patterned superhydrophobic metallic surfaces. Langmuir 2009, 25, 4821–4827. [Google Scholar] [CrossRef]
- Chang, F.M.; Cheng, S.L.; Hong, S.J.; Sheng, Y.J.; Tsao, H.K. Superhydrophilicity to superhydrophobicity transition of CuO nanowire films. Appl. Phys. Lett. 2010, 96, 114101. [Google Scholar] [CrossRef]
- Rajab, F.H.; Liu, Z.; Li, L. Applied Surface Science Production of stable superhydrophilic surfaces on 316L steel by simultaneous laser texturing and SiO2 deposition. Appl. Surf. Sci. 2018, 427, 1135–1145. [Google Scholar] [CrossRef]
- Sygletou, M.; Petridis, C.; Kymakis, E.; Stratakis, E. Advanced Photonic Processes for Photovoltaic and Energy Storage Systems. Adv. Mater. 2017, 29, 1700335. [Google Scholar] [CrossRef] [PubMed]
- Pou, P.; Del Val, J.; Riveiro, A.; Comesaña, R.; Arias-González, F.; Lusquiños, F.; Bountinguiza, M.; Quintero, F.; Pou, J. Laser texturing of stainless steel under different processing atmospheres: From superhydrophilic to superhydrophobic surfaces. Appl. Surf. Sci. 2019, 475, 896–905. [Google Scholar] [CrossRef]
- Shugaev, M.V.; Shih, C.Y.; Karim, E.T.; Wu, C.; Zhigilei, L.V. Generation of nanocrystalline surface layer in short pulse laser processing of metal targets under conditions of spatial confinement by solid or liquid overlayer. Appl. Surf. Sci. 2017, 417, 54–63. [Google Scholar] [CrossRef]
- Stratakis, E.; Zorba, V.; Barberoglou, M.; Fotakis, C.; Shafeev, G.A. Laser writing of nanostructures on bulk Al via its ablation in liquids. Nanotechnology 2009, 20, 105303. [Google Scholar] [CrossRef]
- Iqbal, M.H.; Bashir, S.; Rafique, M.S.; Dawood, A.; Akram, M.; Mahmood, K.; Hayat, A.; Ahmad, R.; Hussain, T.; Mahmood, A. Pulsed laser ablation of Germanium under vacuum and hydrogen environments at various fluences. Appl. Surf. Sci. 2015, 344, 146–158. [Google Scholar] [CrossRef]
- Guidoni, A.G.; Mele, A.; Di Palma, T.M.; Flamini, C.; Orlando, S.; Teghil, R. AIN thin film deposition by pulsed laser ablation of Al in NH3. Thin Solid Films 1997, 295, 77–82. [Google Scholar] [CrossRef]
- Melling, J. Treatment of ammoniacal copper etchants. Resour. Conserv. 1986, 12, 113–124. [Google Scholar] [CrossRef]
- Kokawa, H. Nitrogen absorption and desorption by steels during arc and laser welding. Weld. Int. 2004, 18, 277–287. [Google Scholar] [CrossRef]
- Zumdahl, S.S.; DeCoste, D.J. Chemical Principles, 8th ed.; Cengage Learning: Boston, MA, USA, 2016. [Google Scholar]
- Burns, R.M. Chemical reactions in the corrosion of metals. N. Engl. Assoc. Chem. Teach. 1953, 8, 318–321. [Google Scholar] [CrossRef]
- Hasan, B.O. Effect of Salt Content on The Corrosion Rate of Steel Pipe in Turbulently Flowing Solutions. Coll. Eng. J. 2010, 13, 66–73. [Google Scholar]
- Baba, H.; Kodama, T.; Katada, Y. Role of nitrogen on the corrosion behavior of austenitic stainless steels. Corros. Sci. 2002, 44, 2393–2407. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanara, C.; Mimidis, A.; Stratakis, E. Femtosecond Laser Fabrication of Stable Hydrophilic and Anti-Corrosive Steel Surfaces. Materials 2019, 12, 3428. https://doi.org/10.3390/ma12203428
Lanara C, Mimidis A, Stratakis E. Femtosecond Laser Fabrication of Stable Hydrophilic and Anti-Corrosive Steel Surfaces. Materials. 2019; 12(20):3428. https://doi.org/10.3390/ma12203428
Chicago/Turabian StyleLanara, Christina, Alexandros Mimidis, and Emmanuel Stratakis. 2019. "Femtosecond Laser Fabrication of Stable Hydrophilic and Anti-Corrosive Steel Surfaces" Materials 12, no. 20: 3428. https://doi.org/10.3390/ma12203428





