Tuning the Photophysical Features of Self-Assembling Photoactive Polypeptides for Light-Harvesting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culturing of Bacteria and Isolation of Chromatophores
2.2. Isolation of Pigments
2.3. Synthesis of Ni-bacteriopheophytin a
2.4. Isolation of Native LH1
2.5. Preparation of LH1 Subunits
2.6. Pigment Exchange in LH1
2.7. Spectroscopic Measurements
3. Results
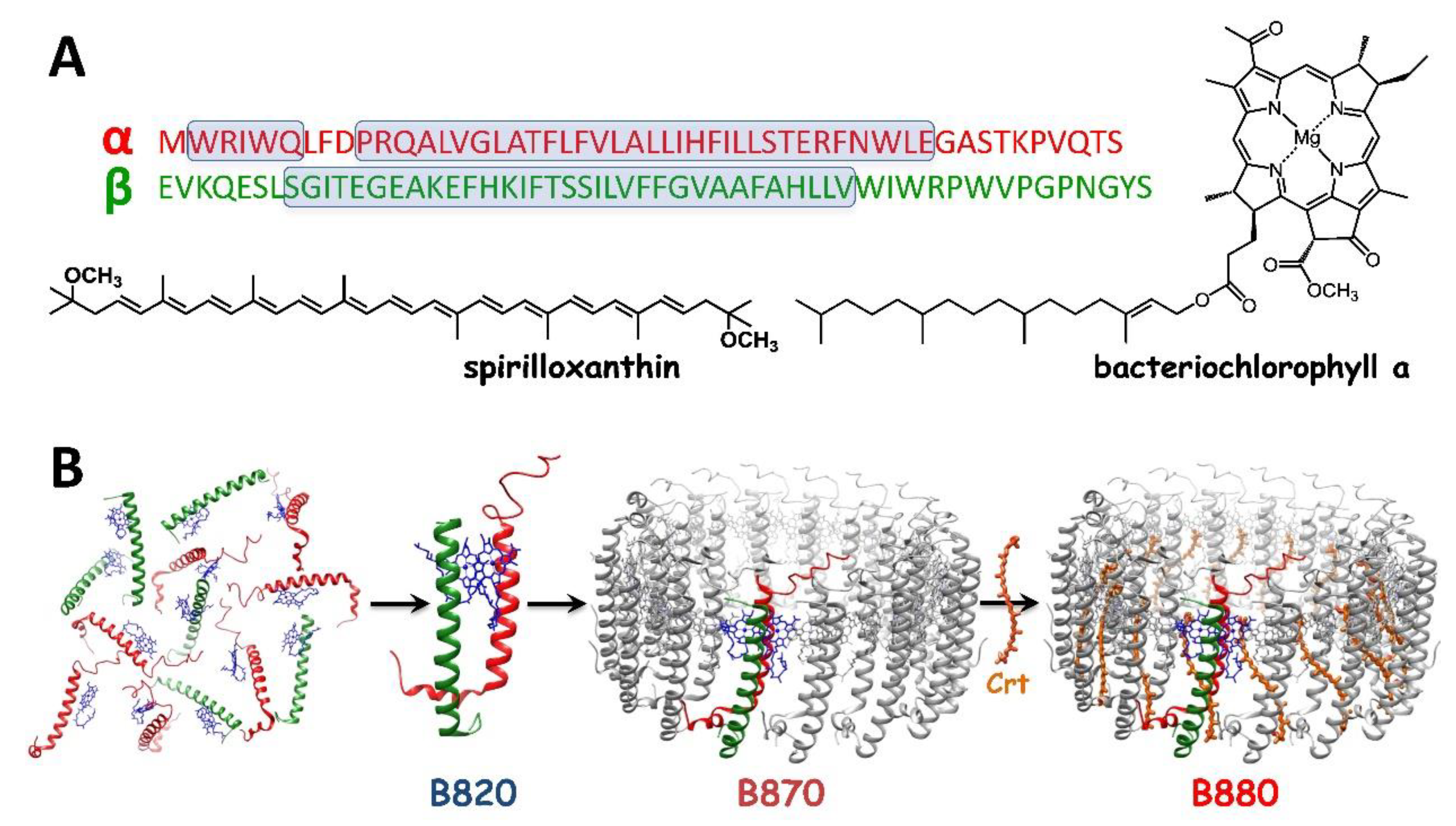
3.1. Isolation of Various Oligomeric Forms of LH1
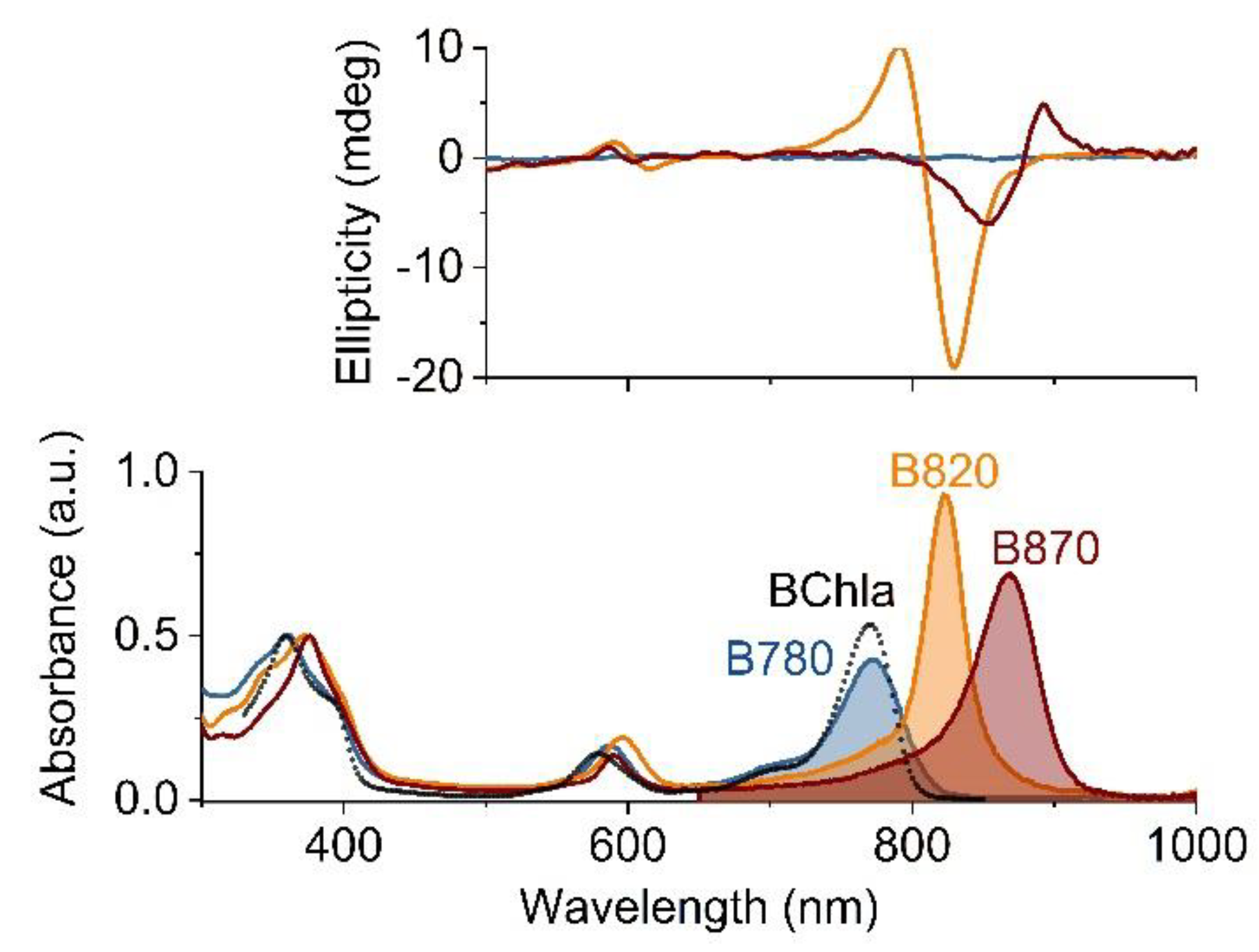
3.2. Electronic Properties
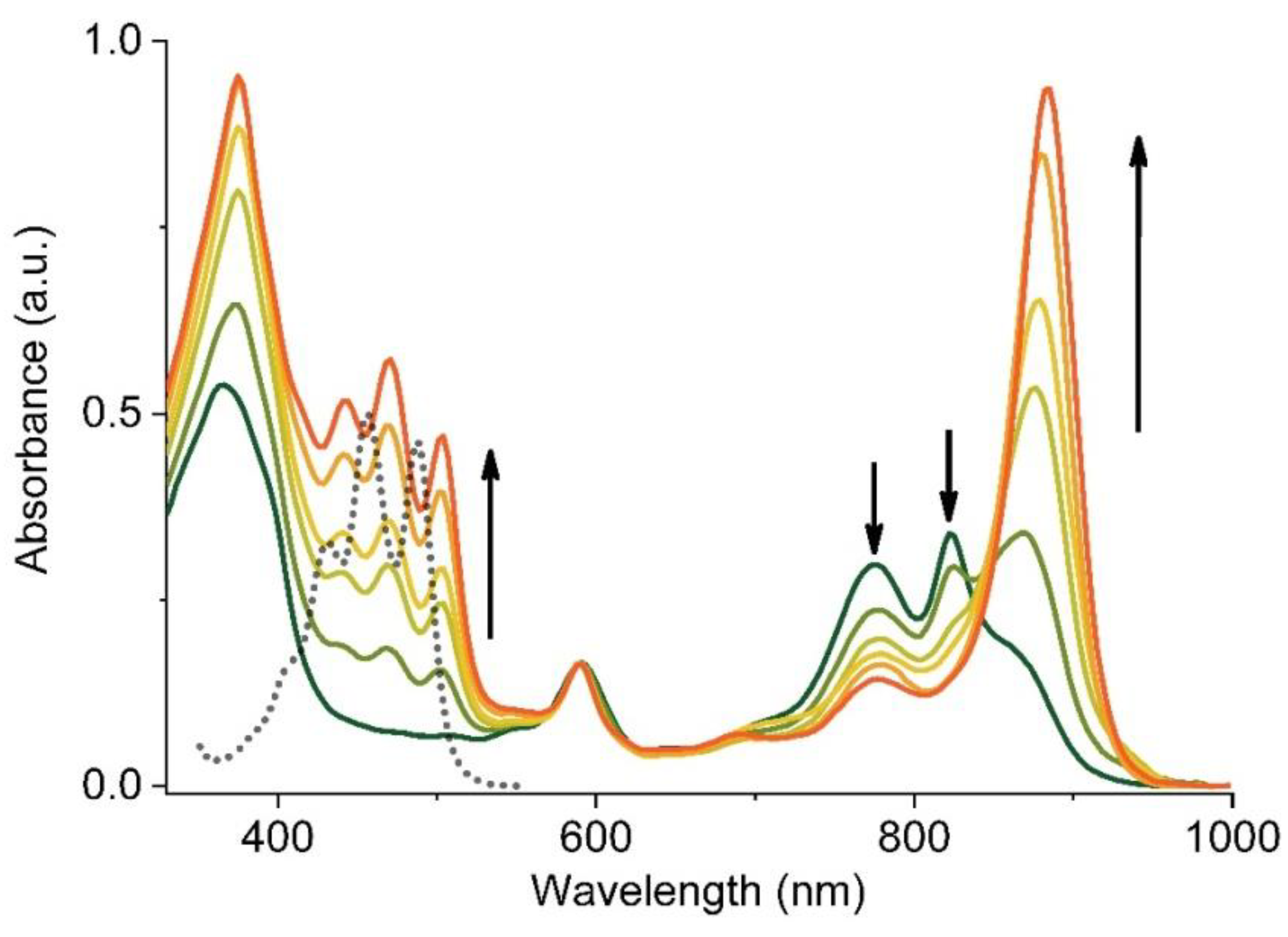
3.3. LH1 Assembly
3.4. Intra-complex Energy Transfer
3.5. Excited-State Properties
4. Discussion
4.1. Tuning of the Oligomeric State
4.2. Tuning the Light-Harvesting and Excited-State Features
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hohmann-Marriott, M.F.; Blankenship, R.E. Evolution of Photosynthesis. Annu. Rev. Plant Biol. 2011, 62, 515–548. [Google Scholar] [CrossRef] [Green Version]
- Orf, G.S.; Gisriel, C.; Redding, K.E. Evolution of photosynthetic reaction centers: Insights from the structure of the heliobacterial reaction center. Photosynth. Res. 2018, 138, 11–37. [Google Scholar] [CrossRef]
- Jones, M.R. Reaction centers: Structure and mechanism. In Light Harvesting in Photosynthesis; Croce, R., van Grondelle, R., van Amerongen, H., van Stokkum, I., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 181–205. [Google Scholar]
- Cogdell, R.J.; Gardiner, A.T.; Roszak, A.W.; Law, C.J.; Southall, J.; Isaacs, N.W. Rings, ellipses and horseshoes: How purple bacteria harvest solar energy. Photosynth. Res. 2004, 81, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsen, M.; Gardiner, A.T.; Cogdell, R.J. Peripheral Complexes of Purple Bacteria. In The Purple Phototrophic Bacteria; Hunter, C.N., Daldal, F., Thurnauer, M.C., Beatty, J.T., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2009; pp. 135–153. [Google Scholar]
- Mothersole, D.J.; Farmer, D.A.; Hitchcock, A.; Hunter, C.N. Photosynthetic apparatus in purple bacteria. In Light Harvesting in Photosynthesis; Croce, R., van Grondelle, R., van Amerongen, H., van Stokkum, I., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 95–120. [Google Scholar]
- Croce, R.; van Amerongen, H. Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 2014, 10, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, L.; Zbyradowski, M.; Pilch, M. Tetrapyrrole pigments of photosynthetic antennae and reaction centers of higher plants: Structures, biophysicis, functions, biochemistry, mechanisms of regulation, applications. In Metabolism, Structure and Function of Plant Tetrapyrroles: Introduction, Microbial and Eukaryotic Chlorophyll Synthesis and Catabolism, 1st ed.; Grimm, B., Ed.; Academic Press: London, UK, 2019; Volume 90, pp. 1–33. [Google Scholar]
- Mirkovic, T.; Ostroumov, E.E.; Anna, J.M.; Van Grondelle, R.; Scholes, G.D. Light absorption and energy transfer in the antenna complexes of photosynthetic organisms. Chem. Rev. 2017, 117, 249–293. [Google Scholar] [CrossRef] [PubMed]
- Roszak, A.W.; Howard, T.D.; Southall, J.; Gardiner, A.T.; Law, C.J.; Isaacs, N.W.; Cogdell, R.J. Crystal structure of the RC-LH1 core complex from Rhodopseudomonas palustris. Science 2003, 302, 1969–1972. [Google Scholar] [CrossRef] [PubMed]
- Niwa, S.; Yu, L.J.; Takeda, K.; Hirano, Y.; Kawakami, T.; Wang-Otomo, Z.Y.; Miki, K. Structure of the LH1-RC complex from Thermochromatium tepidum at 3.0 angstrom. Nature 2014, 508, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Karrasch, S.; Bullough, P.A.; Ghosh, R. The 8.5 A projection map of the light-harvesting complex from Rhodospirillum rubrum reveals a ring composed of 16 subunits. EMBO J. 1995, 14, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, L.; Leupold, D.; Teuchner, K.; Voigt, B.; Hunter, C.N.; Scherz, A.; Scheer, H. Excitation trap approach to analyze size and pigment-pigment coupling: Reconstitution of LH1 antenna of Rhodobacter sphaeroides with Ni-substituted bacteriochlorophyll. Biochemistry 2001, 40, 3737–3747. [Google Scholar] [CrossRef]
- Loach, P.A.; Sekura, D.L. Primary photochemistry and electron transport in Rhodospirillum rubrum. Biochemistry 1968, 7, 2642–2649. [Google Scholar] [CrossRef]
- Picorel, R.; Belanger, G.; Gingras, G. Antenna holochrome B880 of Rhodospirillum rubrum S1. Pigment, phospholipid, and polypeptide composition. Biochemistry 1983, 22, 2491–2497. [Google Scholar] [CrossRef]
- Akahane, J.; Rondonuwu, F.S.; Fiedor, L.; Watanabe, Y.; Koyama, Y. Dependence of singlet-energy transfer on the conjugation length of carotenoids reconstituted into the LH1 complex from Rhodospirillum rubrum G9. Chem. Phys. Lett. 2004, 393, 184–191. [Google Scholar] [CrossRef]
- Davis, C.M.; Bustamante, P.L.; Loach, P.A. Reconstitution of the bacterial core light-harvesting complexes of Rhodobacter sphaeroides and Rhodospirillum rubrum with isolated α- and β-polypeptides, bacteriochlorophyll a, and carotenoid. J. Biol. Chem. 1995, 270, 5793–5804. [Google Scholar] [CrossRef]
- Fiedor, J.; Pilch, M.; Fiedor, L. Tuning the thermodynamics of association of transmembrane helices. J. Phys. Chem. B 2009, 113, 12831–12838. [Google Scholar] [CrossRef] [PubMed]
- Polivka, T.; Frank, H. Light harvesting by carotenoids. Acc. Chem. Res. 2010, 43, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Parkes-Loach, P.S.; Sprinkle, J.R.; Loach, P.A. Reconstitution of the B873 light-harvesting complex of Rhodospirillum rubrum from separately isolated α- and β-polypeptides and bacteriochlorophyll a. Biochemistry 1988, 27, 2718–2727. [Google Scholar] [CrossRef] [PubMed]
- Popot, J.-L.; Gerchman, S.-E.; Engelman, D.M. Refolding of bacteriorhodopsin in lipid bilayers. A thermodynamically controlled two-stage process. J. Mol. Biol. 1987, 198, 655–676. [Google Scholar] [CrossRef]
- Fiedor, L.; Scheer, H. Trapping of an intermediate of LH1 complex assembly beyond the B820 subunit. J. Biol. Chem. 2005, 280, 20921–20926. [Google Scholar] [CrossRef]
- Fiedor, L.; Akahane, J.; Koyama, Y. Carotenoid-induced cooperative formation of bacterial photosynthetic LH1 complex. Biochemistry 2004, 43, 16487–16496. [Google Scholar] [CrossRef]
- Cohen-Bazire, G.; Sistrom, W.R.; Stanier, R.Y. Kinetic studies of pigment synthesis by non-sulphur purple bacteria. J. Cell. Comp. Physiol. 1957, 49, 25–58. [Google Scholar] [CrossRef]
- Fujii, R.; Onaka, K.; Kuki, M.; Koyama, Y.; Watanabe, Y. The 2Ag- energies of all-trans-neurosporene and spheroidene as determined by fluorescence spectroscopy. Chem. Phys. Lett. 1998, 288, 847–853. [Google Scholar] [CrossRef]
- Omata, T.; Murata, N. Preparation of chlorophyll a, chlorophyll b and bacteriochlorophyll a by column chromatography with DEAE-Sepharose CL-6B and Sepharose CL-6B. Plant Cell Physiol. 1983, 24, 1093–1100. [Google Scholar]
- Hartwich, G.; Fiedor, L.; Simonin, I.; Cmiel, E.; Schäfer, W.; Noy, D.; Scherz, A.; Scheer, H. Metal-Substituted Bacteriochlorophylls. 1. Preparation and Influence of Metal and Coordination on Spectra. J. Am. Chem. Soc. 1998, 120, 3675–3683. [Google Scholar] [CrossRef]
- Fiedor, L. Hexacoordination of bacteriochlorophyll in photosynthetic antenna LH1. Biochemistry 2006, 45, 1910–1918. [Google Scholar] [CrossRef]
- Lambrev, P.H.; Miloslavina, Y.; Van Stokkum, I.H.M.; Stahl, A.D.; Michalik, M.; Susz, A.; Tworzydło, J.; Fiedor, J.; Huhn, G.; Groot, M.-L.; et al. Excitation energy trapping and dissipation by Ni-substituted bacteriochlorophyll a in reconstituted LH1 complexes from Rhodospirillum rubrum. J. Phys. Chem. B 2013, 117, 11260–11271. [Google Scholar] [CrossRef]
- Chang, M.C.; Callahan, P.M.; Parkes-Loach, P.S.; Cotton, T.M.; Loach, P.A. Spectroscopic characterization of the light-harvesting complex of Rhodospirillum rubrum and its structural subunit. Biochemistry 1990, 29, 421–429. [Google Scholar] [CrossRef]
- Karcz, D.; Boron, B.; Matwijczuk, A.; Furso, J.; Staron, J.; Ratuszna, A.; Fiedor, L. Lessons from chlorophylls: Modifications of porphyrinoids towards optimized solar energy conversion. Molecules 2014, 19, 15938–15954. [Google Scholar] [CrossRef]
- Nakagawa, K.; Suzuki, S.; Fujii, R.; Gardiner, A.T.; Cogdell, R.J.; Nango, M.; Hashimoto, H. Probing the effect of the binding site on the electrostatic behavior of a series of carotenoids reconstituted into the light-harvesting 1 complex from purple photosynthetic bacterium Rhodospirillum rubrum detected by Stark spectroscopy. J. Phys. Chem. B 2008, 112, 9467–9475. [Google Scholar] [CrossRef]
- Ashikhmin, A.; Makhneva, Z.; Bolshakov, M.; Moskalenko, A. Incorporation of spheroidene and spheroidenone into light-harvesting complexes from purple sulfur bacteria. J. Photochem. Photobio. B 2017, 170, 99–107. [Google Scholar] [CrossRef]
- Cogdell, R.J.; Andersson, P.O.; Gillbro, T. Carotenoid singlet-states and their involvement in photosynthetic light-harvesting. J. Photochem. Photobiol. B 1992, 15, 105–112. [Google Scholar] [CrossRef]
- Kramer, H.J.M.; Pennoyer, J.D.; van Grondelle, R.; Westerhuis, W.H.J.; Niederman, R.A.; Amesz, J. Low-temperature optical properties and pigment organization of the B875 light-harvesting bacteriochlorophyll-protein complex of purple photosynthetic bacteria. Biochim. Biophys. Acta 1984, 767, 335–344. [Google Scholar] [CrossRef]
- Monshouwer, R.; Abrahamsson, M.; van Mourik, F.; van Grondelle, R. Superradiance and exciton delocalization in bacterial photosynthetic light-harvesting systems. J. Phys. Chem. B 1997, 101, 7241–7248. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Muraoka, Y.; Shimonaga, M.; Kobayashi, M.; Nozawa, T. Selective detection and assignment of the solution NMR signals of bacteriochlorophyll a in a reconstituted subunit of a light-harvesting complex. J. Am. Chem. Soc. 2002, 124, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Sturgis, J.N.; Robert, B. Thermodynamics of membrane polypeptide oligomerization in light-harvesting complexes and associated structural changes. J. Mol. Biol. 1994, 238, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Visschers, R.W.; Chang, M.C.; van Mourik, F.; Parkes-Loach, P.S.; Heller, B.A.; Loach, P.A.; van Grondelle, R. Fluorescence polarization and low-temperature absorption spectroscopy of a subunit form of light-harvesting complex I from purple photosynthetic bacteria. Biochemistry 1991, 30, 5734–5742. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Hayashi, H.; Tasumi, M. Factors controlling the efficiency of energy transfer from carotenoids to bacteriochlorophyll in purple photosynthetic bacteria. Biochim. Biophys. Acta 1990, 1017, 280–290. [Google Scholar] [CrossRef]
- Van Amerongen, H.; Valkunas, L.; Van Grondelle, R. Photosynthetic Excitons; Farrer Road 912805; World Scientific: Singapore, 2000. [Google Scholar] [CrossRef]
- Hashimoto, H.; Uragami, C.; Yukihira, N.; Gardiner, A.T.; Cogdell, R.J. Understanding/unravelling carotenoid excited singlet states. Interface 2018, 15, 20180026. [Google Scholar] [CrossRef]
- Meadows, K.A.; Iida, K.; Tsuda, K.; Recchia, P.A.; Heller, B.A.; Antonio, B.; Nango, M.; Loach, P.A. Enzymatic and chemical cleavage of the core light-harvesting polypeptides of photosynthetic bacteria—Determination of the minimal polypeptide size and structure required for subunit and light-harvesting complex-formation. Biochemistry 1995, 34, 1559–1574. [Google Scholar] [CrossRef]
- Parkes-Loach, P.S.; Majeed, A.P.; Law, C.J.; Loach, P.A. Interactions stabilizing the structure of the core light-harvesting complex (LH1) of photosynthetic bacteria and its subunit (B820). Biochemistry 2004, 43, 7003–7016. [Google Scholar] [CrossRef]
- Dracheva, T.V.; Novoderezhkin, V.I.; Razjivin, A.P. Exciton delocalization in the light-harvesting LH2 complex of photosynthetic purple bacteria. Photochem. Photobiol. 1997, 66, 605–610. [Google Scholar] [CrossRef]
- Drews, G. Formation of the light-harvesting complex I (B870) of anoxygenic phototrophic purple bacteria. Arch. Microbiol. 1996, 166, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Pilch, M.; Dudkowiak, A.; Jurzyk, B.; Łukasiewicz, J.; Susz, A.; Stochel, G.; Fiedor, L. Molecular symmetry determines the mechanism of a very efficient ultrafast excitation-to-heat conversion in Ni-substituted chlorophylls. Biochim. Biophys. Acta 2013, 1827, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kania, A.; Pilch, M.; Rutkowska-Zbik, D.; Susz, A.; Stochel, G.; Fiedor, L. High-pressure and theoretical studies reveal significant differences in the electronic structure and bonding of magnesium, zinc and nickel ions in metalloporphyrinoids. Inorg. Chem. 2014, 53, 8473–8484. [Google Scholar] [CrossRef] [PubMed]
- Kotkowiak, M.; Dudkowiak, A.; Fiedor, L. Intrinsic photoprotective mechanisms in chlorophylls. Angew. Chem. Int. Ed. 2017, 56, 10457–10461. [Google Scholar] [CrossRef]
- Noy, D.; Fiedor, L.; Hartwich, G.; Scheer, H.; Scherz, A. Metal-substituted bacteriochlorophylls. 2. Changes in redox potentials and electronic transition energies are dominated by intramolecular electrostatic interactions. J. Am. Chem. Soc. 1998, 120, 3684–3693. [Google Scholar] [CrossRef]
- Musewald, C.; Hartwich, G.; Lossau, H.; Gilch, P.; Pöllinger-Dammer, F.; Scheer, H.; Michel-Beyerle, M.E. Ultrafast photophysics and photochemistry of [Ni]-bacteriochlorophyll a. J. Phys. Chem. B 1999, 103, 7055–7060. [Google Scholar] [CrossRef]
- Drzewiecka-Matuszek, A.; Skalna, A.; Karocki, A.; Stochel, G.; Fiedor, L. Effects of heavy central metal on the ground and excited states of chlorophyll. J. Biol. Inorg. Chem. 2005, 10, 453–462. [Google Scholar] [CrossRef]
- Fiedor, L.; Scheer, H.; Hunter, C.N.; Tschirschwitz, F.; Voigt, B.; Ehlert, J.; Nibbering, E.; Leupold, D.; Elsaesser, T. Introduction of a 60 fs deactivation channel in the photosynthetic antenna LH1 by Ni-Bacteriopheophytin a. Chem. Phys. Lett. 2000, 319, 145–152. [Google Scholar] [CrossRef]
- Hartwich, G.; Scheer, H.; Aust, V.; Angerhofer, A. Absorption and ADMR studies on bacterial photosynthetic reaction centres with modified pigments. Biochim. Biophys. Acta 1995, 1230, 97–113. [Google Scholar] [CrossRef] [Green Version]



| Major Absorption Maxima (nm, cm−1) | Fluorescence Maximum (nm) | τfl (ns) | |||
|---|---|---|---|---|---|
| Soret | QX | QY | |||
| BChla | 357, 28011 | 579, 17271 | 770, 12987 | 775 | 2.9 a |
| B780 | 361, 27701 | 586, 17065 | 780, 12821 | 791 | 2.30 ± 0.003 |
| B820 | 370, 27027 | 595, 16807 | 821, 12180 | 833 | 0.77 ± 0.02 |
| B870 | 375, 26667 | 590, 16950 | 869, 11507 | 887 | 0.61 ± 0.003 |
| Sph-LH1 | 377, 26525 | 591, 16920 | 883, 11325 | 904 | 0.79 ± 0.001 |
| native LH1 | 377, 26525 | 590, 16950 | 881, 11351 | 902 | 0.85 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalik, M.; Zbyradowski, M.; Heriyanto; Fiedor, L. Tuning the Photophysical Features of Self-Assembling Photoactive Polypeptides for Light-Harvesting. Materials 2019, 12, 3554. https://doi.org/10.3390/ma12213554
Michalik M, Zbyradowski M, Heriyanto, Fiedor L. Tuning the Photophysical Features of Self-Assembling Photoactive Polypeptides for Light-Harvesting. Materials. 2019; 12(21):3554. https://doi.org/10.3390/ma12213554
Chicago/Turabian StyleMichalik, Maciej, Mateusz Zbyradowski, Heriyanto, and Leszek Fiedor. 2019. "Tuning the Photophysical Features of Self-Assembling Photoactive Polypeptides for Light-Harvesting" Materials 12, no. 21: 3554. https://doi.org/10.3390/ma12213554





