Thermogravimetry and Mass Spectrometry of Extractable Organics from Manufactured Nanomaterials for Identification of Potential Coating Components
Abstract
:1. Introduction
2. Materials and Methods
2.1. Manufactured Nanomaterials
2.2. Chemicals
2.3. Thermogravimetric Analysis
2.4. Water Loss and Loss on Ignition (LOI) by the Laboratory Furnace Method
2.5. Pressurized Liquid Extraction
2.6. Gas Chromatography-Mass Spectrometry
2.7. Liquid Chromatography-Mass Spectrometry
2.8. Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry
3. Results and Discussion
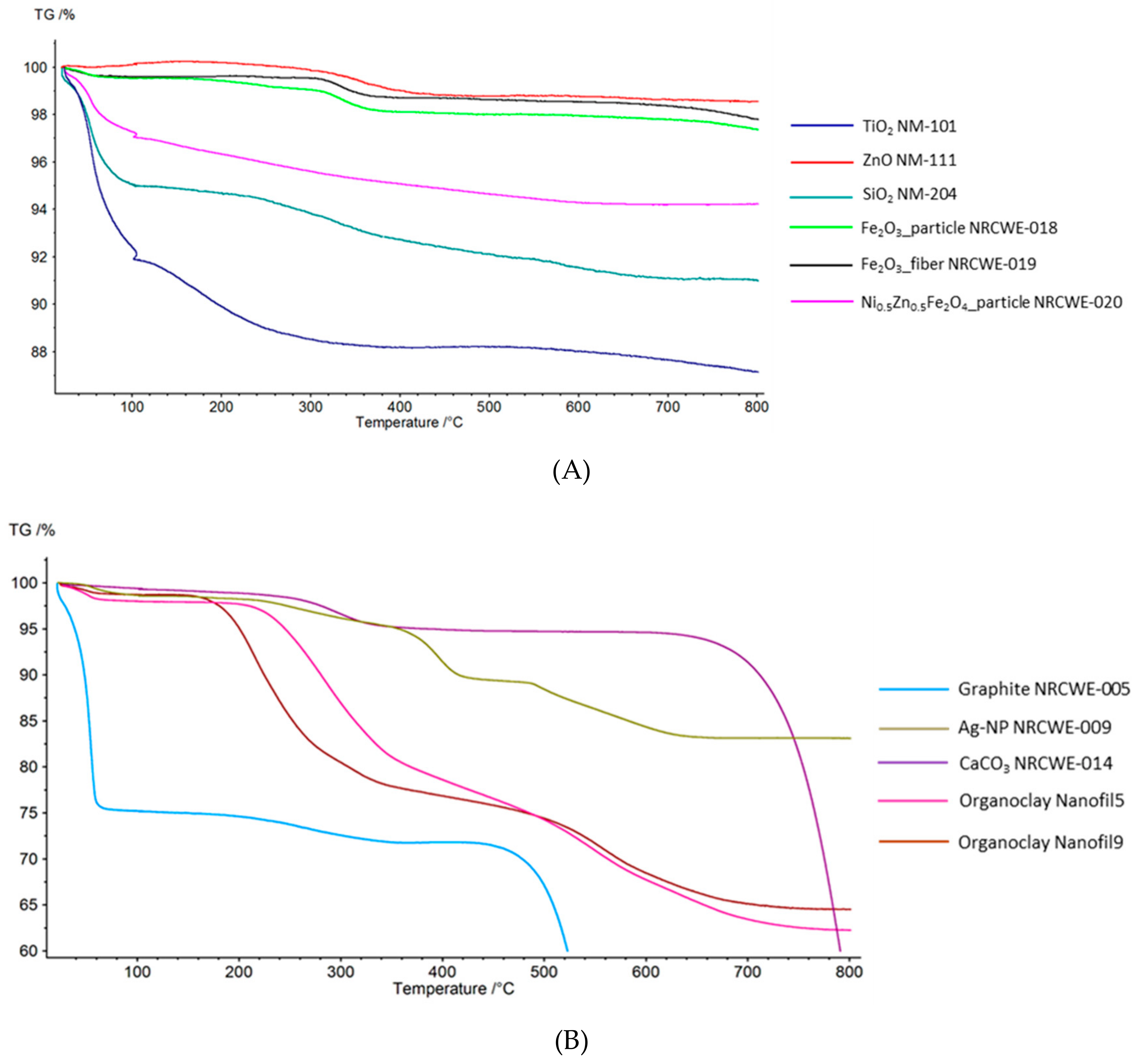
3.1. Thermogravimetric Analysis (TGA)
3.2. Comparison of TGA and the Laboratory Furnace Method for Water loss and Loss on Ignition (LOI)
3.3. Extraction, Separation, and Mass Spectrometric Analysis
3.4. Summary Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baraton, M.-I. Synthesis, Functionalization and Surface Treatment of Nanoparticles; American Scientific Publishers: Stevenson Ranche, CA, USA, 2003. [Google Scholar]
- Gref, R.; Couvreur, P.; Barratt, G.; Mysiakine, E. Surface-engineered nanoparticles for multiple ligand coupling. Biomater. Synth. Biomim. Polym. 2003, 24, 4529–4537. [Google Scholar] [CrossRef]
- Kinge, S.; Crego-Calama, M.; Reinhoudt, D.N. Self-Assembling Nanoparticles at Surfaces and Interfaces. ChemPhysChem 2008, 9, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Basiruddin, S.K.; Saha, A.; Pradhan, N.; Jana, N.R. Advances in Coating Chemistry in Deriving Soluble Functional Nanoparticle. J. Phys. Chem. C 2010, 114, 11009–11017. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. R. Soc. A 2010, 368, 1333–1383. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.; Byard, S.; Bloxham, M.J.; Botterill, J.; Dawson, N.J.; Dennis, A.; Diart, V.; North, N.C.; Sherwood, J.D. A review of the terms agglomerate and aggregate with a recommendation for nomenclature used in powder and particle characterization. J. Pharm. Sci. 2002, 91, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Glogowski, E.; Tangirala, R.; Russell, T.P.; Emrick, T. Functionalization of nanoparticles for dispersion in polymers and assembly in fluids. J. Polym. Sci. Part. A. 2006, 44, 5076–5086. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Green, L.A.W. Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Matsui, I. Nanoparticles for electronic device applications: A brief review. J. Chem. Eng. Jpn. 2005, 38, 535–546. [Google Scholar] [CrossRef]
- Braun, J.H.; Baidins, A.; Marganski, R.E. TiO2 pigment technology: A review. Prog. Org. Coat. 1992, 20, 105–138. [Google Scholar] [CrossRef]
- Atluri, R.; Jensen, K.A. Engineered Nanomaterials: Their Physicochemical Characteristics and How to Measure Them. In Modelling the Toxicity of Nanoparticles; Tran, L., Banares, M.A., Rallo, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–23. [Google Scholar]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. In Chemical Reviews; American Chemical Society: Washington, DC, USA, 2012; pp. 2373–2433. [Google Scholar]
- Bouwmeester, H.; Lynch, I.; Marvin, H.J.P.; Dawson, K.A.; Berges, M.; Braguer, D.; Byrne, H.J.; Casey, A.; Chambers, G.; Clift, M.J.D.; et al. Minimal analytical characterization of engineered nanomaterials needed for hazard assessment in biological matrices. Nanotoxicology 2011, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stone, V.; Nowack, B.; Baun, A.; van den Brink, N.; von der Kammer, F.; Dusinska, M.; Handy, R.; Hankin, S.; Hassellöv, M.; Joner, E.; et al. Nanomaterials for environmental studies: Classification, reference material issues, and strategies for physico-chemical characterisation. Sci. Total Environ. 2010, 408, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Hassellöv, M.; Readman, J.; Ranville, J.; Tiede, K. Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles. Ecotoxicology 2008, 17, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, D.H. Control Banding Approaches for Nanomaterials. Ann. Occup. Hyg. 2012, 56, 506–514. [Google Scholar] [PubMed]
- Arts, J.H.E.; Hadi, M.; Irfan, M.A.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Petry, T.; Sauer, U.G.; et al. A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regul. Toxicol. Pharmacol. 2015, 71 (Suppl. 2), 1–27. [Google Scholar] [CrossRef]
- Oomen, G.A.; Bleeker, A.E.; Bos, M.P.; van Broekhuizen, F.; Gottardo, S.; Groenewold, M.; Hristozov, D.; Hund-Rinke, K.; Irfan, M.A.; Marcomini, A.; et al. Grouping and Read-Across Approaches for Risk Assessment of Nanomaterials. Int. J. Environ. Res. Public Health 2015, 12, 13415–13434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Chemicals Agency. Guidance on Information Requirements and Chemical Safety Assessment—Appendix R.6-1 for Nanomaterials Applicable to the Guidance on QSARs and Grouping of Chemicals; ECHA: Helsinki, Finland, 2017; pp. 1–28. [Google Scholar]
- European Commission. EC decrete on REACH [18]-COMMISSION REGULATION (EU) 2018/1881 of 3 December 2018. OJ L 308. Off. J. Eur. Union 2018, 61, 1–20. [Google Scholar]
- Suleiman, A.M.; Svendsen, K.V.H. Are safety data sheets for cleaning products used in Norway a factor contributing to the risk of workers exposure to chemicals? Int. J. Occup. Med. Environ. Health 2014, 27, 840–853. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kuk, W.K.; Kwon, M.; Lee, J.H.; Lee, K.S.; Yu, I.J. Evaluation of information in nanomaterial safety data sheets. In Nanotoxicology; Taylor & Francis: Abingdon, UK, 2012; pp. 338–345. [Google Scholar]
- Du, F.; Zhang, B.; Zhou, H.; Yan, B.; Chen, L. Structure elucidation of nanoparticle-bound organic molecules by 1H NMR. TrAC Trends Anal. Chem. 2009, 28, 88–95. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, B. Analytical strategies for characterizing the surface chemistry of nanoparticles. Anal. Bioanal. Chem. 2010, 396, 973–982. [Google Scholar] [CrossRef]
- Baer, D.; Gaspar, D.; Nachimuthu, P.; Techane, S.; Castner, D. Application of surface chemical analysis tools for characterization of nanoparticles. Anal. Bioanal. Chem. 2010, 396, 983–1002. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yan, B. Characterizing the surface chemistry of nanoparticles: An analogy to solid-phase synthesis samples. Comb. Chem. High Throughput Screen 2011, 14, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.R.; Engelhard, M.H.; Johnson, G.E.; Laskin, J.; Lai, J.; Mueller, K.; Munusamy, P.; Thevuthasan, S.; Wang, H.; Washton, N.; et al. Surface characterization of nanomaterials and nanoparticles: Important needs and challenging opportunities. J. Vac. Sci. Technol. A 2013, 31, 050820. [Google Scholar] [CrossRef] [PubMed]
- Belsey, N.A.; Shard, A.G.; Minelli, C. Surface Chemistry. In Nanomaterial Characterization; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 153–178. [Google Scholar]
- Colangelo, E.; Comenge, J.; Paramelle, D.; Volk, M.; Chen, Q.; Lévy, R. Characterizing Self-Assembled Monolayers on Gold Nanoparticles. Bioconjugate Chem. 2017, 28, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, X.; Lemoff, A.; Zhang, B.; Yan, B. Structural confirmation and quantification of individual ligands from the surface of multi-functionalized gold nanoparticles. Analyst 2010, 135, 1210–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Templeton, A.C.; Hostetler, M.J.; Kraft, C.T.; Murray, R.W. Reactivity of Monolayer-Protected Gold Cluster Molecules: Steric Effects. J. Am. Chem. Soc. 1998, 120, 1906–1911. [Google Scholar] [CrossRef]
- Yan, B.; Zhu, Z.J.; Miranda, O.; Chompoosor, A.; Rotello, V.; Vachet, R. Laser desorption/ionization mass spectrometry analysis of monolayer-protected gold nanoparticles. Anal. Bioanal. Chem. 2010, 396, 1025–1035. [Google Scholar] [CrossRef]
- Harkness, K.M.; Hixson, B.C.; Fenn, L.S.; Turner, B.N.; Rape, A.C.; Simpson, C.A.; Huffman, B.J.; Okoli, T.C.; McLean, J.A.; Cliffel, D.E. A Structural Mass Spectrometry Strategy for the Relative Quantitation of Ligands on Mixed Monolayer-Protected Gold Nanoparticles. In Analytical Chemistry; American Chemical Society: Washington, DC, USA, 2010; pp. 9268–9274. [Google Scholar]
- Dyke, C.A.; Stewart, M.P.; Maya, F.; Tour, J.M. Diazonium-Based Functionalization of Carbon Nanotubes: XPS and GC-MS Analysis and Mechanistic Implications. Synlett 2004, 2004, 155–160. [Google Scholar] [CrossRef]
- Kingston, C.; Martínez-Rubí, Y.; Guan, J.; Barnes, M.; Scriver, C.; Sturgeon, R.; Simard, B. Coupled thermogravimetry, mass spectrometry, and infrared spectroscopy for quantification of surface functionality on single-walled carbon nanotubes. Anal. Bioanal. Chem. 2010, 396, 1037–1044. [Google Scholar] [CrossRef]
- Roonasi, P.; Holmgren, A. A Fourier transform infrared (FTIR) and thermogravimetric analysis (TGA) study of oleate adsorbed on magnetite nano-particle surface. Appl. Surf. Sci. 2009, 255, 5891–5895. [Google Scholar] [CrossRef]
- Yan, B.; Jeong, Y.; Mercante, L.A.; Tonga, G.Y.; Kim, C.; Zhu, Z.J.; Vachet, R.W.; Rotello, V.M. Characterization of surface ligands on functionalized magnetic nanoparticles using laser desorption/ionization mass spectrometry (LDI-MS). Nanoscale 2013, 5, 5063–5066. [Google Scholar] [CrossRef]
- Hougaard, K.; Jackson, P.; Jensen, K.; Sloth, J.; Loschner, K.; Larsen, E.; Birkedal, R.; Vibenholt, A.; Boisen, A.M.; Wallin, H.; et al. Effects of prenatal exposure to surface-coated nanosized titanium dioxide (UV-Titan). A study in mice. Part. Fibre Toxicol. 2010, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; He, P. Surface modifications of nanoparticles and nanotubes by plasma polymerization. Rev. Adv. Mater. Sci. 2004, 7, 97–107. [Google Scholar]
- Polizu, S.; Maugey, M.; Poulin, S.; Poulin, P.; Yahia, L.H. Nanoscale surface of carbon nanotube fibers for medical applications: Structure and chemistry revealed by TOF-SIMS analysis. Appl. Surf. Sci. 2006, 252, 6750–6753. [Google Scholar] [CrossRef]
- Nilsson, P.; Eriksson, A.C.; Ludvigsson, L.; Messing, M.E.; Nordin, E.Z.; Gudmundsson, A.; Meuller, B.O.; Deppert, K.; Fortner, E.C.; Onasch, T.B.; et al. In situ characterization of metal nanoparticles and their their organic coatings using laser vaporization aerosol mass spectrometry. Nano Res. 2015, 8, 3780–3795. [Google Scholar] [CrossRef]
- Baer, D.R.; Munusamy, P.; Thrall, B.D. Provenance information as a tool for addressing engineered nanoparticle reproducibility challenges. Biointerphases 2016, 11, 04B401. [Google Scholar] [CrossRef] [Green Version]
- Mülhopt, S.; Diabaté, S.; Dilger, M.; Adelhelm, C.; Anderlohr, C.; Bergfeldt, T.; de la Torre, J.G.; Jiang, Y.; Valsami-Jones, E.; Langevin, D. Characterization of Nanoparticle Batch-To-Batch Variability. Nanomaterials 2018, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Clausen, P.A.; Francis, L.; Ponti, A.; Guidotti, M.; Kling, K.I.; Kofoed-Sørensen, V.; Nielsen, S.H.; Jensen, K.A. Protocol for Quantitative Analysis of Inorganic and Organic MNM Surface Coatings—Deliverable 2.4; D2.04; NanoReg Project, EU; European Commission: Bruxelles, Belgium, 2015; pp. 1–106. [Google Scholar]
- SpecialChem Homepage UV-Titan M111. Available online: https://cosmetics.specialchem.com/product/i-sachtleben-uv-titan-m111 (accessed on 5 November 2019).
- Rasmussen, K.; Mast, J.; De Temmerman, P.-J.; Verleysen, E.; Waegeneers, N.; Van Steen, F.; Pizzolon, J.C.; De Temmerman, L.; Van Doren, E.; Jensen, K.A.; et al. Titanium Dioxide, NM-100, NM-101, NM-102, NM-103, NM-104, NM-105: Characterisation and Physico-Chemical Properties; EUR 26637 EN; European Commission: Bruxelles, Belgium, 2014; pp. 1–208. [Google Scholar]
- Kühnel, D.; Nickel, C.; Hellack, B.; van der Zalm, E.; Kussatz, C.; Herrchen, M.; Meisterjahn, B.; Hund-Rinke, K. Closing gaps for environmental risk screening of engineered nanomaterials. NanoImpact 2019, 15, 100173. [Google Scholar] [CrossRef]
- Rasmussen, K.; Mech, A.; Mast, J.; De Temmerman, P.-J.; Van Doren, E.; Waegeneers, N.; Van Steen, F.; Pizzolon, J.C.; De Temmerman, L.; Jensen, K.A.; et al. Synthetic Amorphous Silicon Dioxide (NM-200, NM-201, NM-202, NM-203, NM-204). Characterisation and Physico-Chemical Properties; EUR 26046 EN; European Commission: Bruxelles, Belgium, 2013; pp. 1–208. [Google Scholar]
- Nanostructured & Amorphous Materials (NanoAmor) Homepage. Available online: https://www.nanoamor.com/home (accessed on 5 November 2019).
- Skyspring Nanomaterials Catalog -Nanomaterials from Skyspring. Available online: https://ssnano.com/i/u/10035073/h/CAT/Catalog_SSNano-2015.pdf (accessed on 5 November 2019).
- Singh, C.; Friederics, S.; Levin, M.; Birkedal, R.; Jensen, K.A.; Pojana, G.; Wohlleben, W.; Schulz, S.; Wiench, K.; Turney, T.; et al. NM-Series of Representative Manufactured Nanomaterials: Zinc Oxide NM-110, NM-111, NM-112, NM-113—Characterization and Test Item Preparation; EUR 25066 EN-201; European Commission: Bruxelles, Belgium, 2011. [Google Scholar]
- Nymark, P.; Catalán, J.; Suhonen, S.; Järventaus, H.; Birkedal, R.; Clausen, P.A.; Jensen, K.A.; Vippola, M.; Savolainen, K.; Norppa, H. Genotoxicity of polyvinylpyrrolidone-coated silver nanoparticles in BEAS 2B cells. Toxicology 2013, 313, 38–48. [Google Scholar] [CrossRef]
- Klein, C.L.; Comero, S.; Stahlmecke, B.; Romazanov, J.; Kuhlbusch, T.; Van Doren, E.; De Temmerman, P.J.; Mast, J.; Wick, P.; Krug, H. NM-Series of Representative Manufactured Nanomaterials: NM-300 Silver Characterisation, Stability, Homogeneity; EUR 24693 EN-2011; European Commission: Bruxelles, Belgium, 2011. [Google Scholar]
- Nair, B. Final report on the safety assessment of stearoxy dimethicone, dimethicone, methicone, amino bispropyl dimethicone, aminopropyl dimethicone, amodimethicone, amodimethicone hydroxystearate, behenoxy dimethicone, C24-28 alkyl methicone, C30-45 alkyl methicone, C30-45 alkyl dimethicone, cetearyl methicone, cetyl dimethicone, dimethoxysilyl ethylenediaminopropyl dimethicone, hexyl methicone, hydroxypropyldimethicone, stearamidopropyl dimethicone, stearyl dimethicone, stearyl methicone, and vinyldimethicone. Int. J. Toxicol. 2003, 22 (Suppl. 2), 11–35. [Google Scholar]
- Nanogenotox. NANOGENOTOX Final Report; ANSES: Buenos Aires, Argentina, 2013; pp. 1–60. [Google Scholar]
- Hensel, A.; Rischer, M.; Di Stefano, D.; Behr, I.; Wolf-Heuss, E. Full chromatographic characterization of nonionic surfactant polyoxyethylene glycerol trioleate. Pharm. Acta Helv. 1997, 72, 185–189. [Google Scholar] [CrossRef]
- Hadrup, N.; Saber, A.T.; Kyjovska, Z.O.; Jacobsen, N.R.; Vippola, M.; Sarlin, E.; Ding, Y.; Schmid, O.; Wallin, H.; Jensen, K.A.; et al. Pulmonary toxicity of Fe2O3, ZnFe2O4, NiFe2O4 and NiZnFe4O8 nanomaterials: Inflammation and DNA strand breaks. Environ. Toxicol. Pharmacol. 2019. (submitted). [Google Scholar]
- Nogueira, N.; Utuni, V.; Silva, Y.; Kiyohara, P.; Vasconcelos, I.; Miranda, M.; Sasaki, J. X-ray diffraction and Mossbauer studies on superparamagnetic nickel ferrite (NiFe2O4) obtained by the proteic sol–gel method. Mater. Chem. Phys. 2015, 163, 402–406. [Google Scholar] [CrossRef]
- Singh, R.K.; Upadhyay, C.; Layek, S.; Yadav, A. Cation distribution of Ni 0.5 Zn 0.5 Fe 2 O 4 nanoparticles. Int. J. Eng. Sci. Technol. 2010, 2, 104–109. [Google Scholar]
- Sanchez, J.; McCormick, A. Kinetic and thermodynamic study of the hydrolysis of silicon alkoxides in acidic alcohol solutions. J. Phys. Chem. 1992, 96, 8973–8979. [Google Scholar] [CrossRef]
- Kallos, G.J.; Tou, J.C.; Malczewski, R.M.; Boley, W.F. Stability Studies of Alkoxysilanes in Aqueous-Media. Am. Ind. Hyg. Assoc. J. 1991, 52, 259–262. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Adhesion Through Silane Coupling Agents. J. Adhes. 1970, 2, 184–201. [Google Scholar] [CrossRef]
- Antic, V.V.; Antic, M.P.; Kronimus, A.; Oing, K.; Schwarzbauer, J. Quantitative determination of poly(vinylpyrrolidone) by continuous-flow off-line pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2011, 90, 93–99. [Google Scholar] [CrossRef]
- Raith, K.; Schmelzer, C.E.H.; Neubert, R.H.H. Towards a molecular characterization of pharmaceutical excipients: Mass spectrometric studies of ethoxylated surfactants. Int. J. Pharm. 2006, 319, 1–12. [Google Scholar] [CrossRef]
- Arnim Cumme, G.; Blume, E.; Bublitz, R.; Hoppe, H.; Horn, A. Composition analysis of detergents of the polyoxyethylene type: Comparison of thin-layer chromatography, reversed-phase chromatography and matrix-assisted laser desorption/ionization mass spectrometry. J. Chromatogr. A 1997, 791, 245–253. [Google Scholar] [CrossRef]
- Birkedal, R.K.; Clausen, P.A.; Kofoed-Sørensen, V.; Burdett, G.; Jensen, K.A. Data Report on Complete Physico-Chemical Benchmark (Reference) Values. NANODEVICE Deliverable Report D3.2a; European Commission: Bruxelles, Belgium, 2011; pp. 1–31. [Google Scholar]
- Cao, Z.; Daly, M.; Clémence, L.; Geever, L.M.; Major, I.; Higginbotham, C.L.; Devine, D.M. Chemical surface modification of calcium carbonate particles with stearic acid using different treating methods. Appl. Surf. Sci. 2016, 378, 320–329. [Google Scholar] [CrossRef]
- Khaliullin, T.O.; Kisin, E.R.; Yanamala, N.; Guppi, S.; Harper, M.; Lee, T.; Shvedova, A.A. Comparative cytotoxicity of respirable surface-treated/untreated calcium carbonate rock dust particles in vitro. Toxicol. Appl. Pharmacol. 2019, 362, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Bumajdad, A.; Ali, S.; Mathew, A. Characterization of iron hydroxide/oxide nanoparticles prepared in microemulsions stabilized with cationic/non-ionic surfactant mixtures. J. Colloid Interface Sci. 2011, 355, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Testing Programme of Manufactured Nanomaterials—Overview. Available online: https://www.oecd.org/chemicalsafety/nanosafety/overview-testing-programme-manufactured-nanomaterials.htm (accessed on 5 November 2019).


| MNM | Technique* | Comments | Ref. |
|---|---|---|---|
| Multi-functionalized Au | HPLC-MS-UV-CLND | Identified and quantified individual ligands | [30,31] |
| Mixed-monolayer coated Au | MALDI-TOF-MS | Semi-quantitative measure of ligand composition | [32] |
| Mixed thiolate coated Au | IMS-MS | Relative quantity of ligands | [33] |
| f-CNT | GC-MS | Chemical decoupling of ligands | [34] |
| f-CNT | TGA-FTIR-MS | Identification by FTIR-MS, quantification by TGA | [35] |
| Oleate coated magnetite (Fe2+Fe3+2O4) | TGA-MS | [36] | |
| Functionalized FePt and Fe3O4 | MALDI-TOF-MS and LC-MS | Chemical decoupling followed by LC-MS | [37] |
| Organic coated TiO2 | ESI-MS and MALDI-TOF-MS | Extraction with PLE followed by MS | [38] |
| Organic coated ZnO, NiFe2O4, YYbErO2S, CNT | TOF-SIMS | Several other techniques were also applied | [39] |
| Organic coated CNT | TOF-SIMS | Several other techniques were also applied | [40] |
| Organic coated Ag, Au, Pd, PdAg, Fe, Ni, Cu | LV-AMS | Cannot be used in case of several unknown organic compounds | [41] |
| Substance | Code | Phase |
Supplier [Reference] | Batch Number | Suppliers’ Information on Purity/Coating | Water Loss by TGA @25–110 °C (wt.%) | σ |
Coating TGA >110 °C (wt.%) | σ | Water Loss Furnace @110 °C (wt.%) | σ |
Coating Furnace >110
°C (wt.%) | σ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TiO2 | M111 | Rutile | Kemira [45] | - | - | 3.8 | 0.19 | 4.08 | 0.2 | 4.86 | 0.01 | 5.72 | 0.04 |
| NM-101 | Anatase | JRC [46] | - | - | 8.21 | 0.69 | 4.41 | 0.35 | - | - | - | - | |
| NM-103 | Rutile | ==||== | - | 6wt.%Al2O3; 2wt.% Dimethicone | 1.62 | 0.16 | 2.54 | 0.24 | - | - | - | - | |
| NM-104 | Rutile | [47] Suppl. Mat. | - | 6wt.%Al2O3; 1wt.% glycerin | 1.49 | 0.1 | 3.17 | 0.07 | - | - | - | - | |
| SiO2 | NM-204 | £SAS | JRC [48] | - | - | 4.44 | 0.38 | 3.14 | 0.34 | ||||
| NRCWE-008 | £SAS | NanoAmor [49] | 4850MR | - | 3.13 | 0.98 | 4.49 | 0.49 | 5.86 | 0.02 | 4.19 | 0.1 | |
| Graphite | NRCWE-005 | $Graphite | SS Nano [50] | 0520BX | - | 25.38 | 1.47 | 2.68 | 0.28 | 3.13 | 0.48 | - | - |
| ZnO | NM-111 | Zincite | JRC [51] | - | Triethoxyoctyl silane | 0 | - | 2.1 | 0.31 | - | - | - | - |
| Ag | NRCWE-009 | Ag(m) | NANOGAP [52] | NP Ag-2103 | 85wt.% Ag* | 1.6 | 0.2 | 14.66 | 0.55 | 1.99 | 0.17 | 13.5 | 0.26 |
| NM-300K | Ag(m) | JRC [53] | - | Dispersion (see text) | 67.62 | 1.32 | 15.95 | 0.43 | - | - | - | - | |
| CaCO3 | NRCWE-012 | €Calcite | SS Nano [50] | 1952RH | Yes; < 0.5 wt.% H2O | 0.43 | 0.11 | 5.33 | 0.11 | 0.46 | 0.03 | 4.7 | 0.03 |
| NRCWE-013 | €Calcite | ==||== | 1953RH | Yes; < 0.5 wt.% H2O | 0.54 | 0.22 | 5.72 | 0.08 | 0.5 | 0.12 | 4.8 | 0.12 | |
| NRCWE-014 | €Calcite | ==||== | 1954RH | Yes; < 0.5 wt.% H2O | 0.24 | 0.08 | 5.09 | 0.3 | 0.43 | 0.01 | 4.66 | 0.02 | |
| NRCWE-015 | €Calcite | ==||== | 1955RH | Yes; < 1.2 wt.% H2O | 0.64 | 0.15 | 5.5 | 0.12 | 0.87 | 0.05 | 5.96 | 0.05 | |
| NRCWE-016 | €Calcite | ==||== | 1956RH | Yes; < 0.5 wt.% H2O | 0.15 | 0.03 | 4.75 | 0.24 | 0.53 | 0.02 | 5.28 | 0.02 | |
| NRCWE-017 | €Calcite | ==||== | 1957RH | < 0.5 wt.% H2O | 0.34 | 0.02 | 5.52 | 0.18 | 0.61 | 0.03) | 5.33 | 0.08 | |
| Fe2O3 | NRCWE-018 | α-Fe2O3 particle | NanoAmor [49] | 2520ZH | 99 wt.% purity | 0.69 | 0.15 | 1.97 | 0.11 | 1.02 | 0.07 | 3.1 | 0.06 |
| NRCWE-019 | α-Fe2O3 rod | ==||== | 8004NJ | None | 0.76 | 0.26 | 2.19 | 0.37 | 2.28 | 0.06 | 3.12 | 0.06 | |
| Ni/ZnFe2O4 | NRCWE-020 | Ni0.5Zn0.5Fe2O4 | NanoAmor [49] | 4115FY | 98.5 wt.% purity | 2.63 | 0.17 | 3.03 | 0.15 | 2.74 | 0.02 | 3.28 | 0.03 |
| NRCWE-022 | NiFe2O4 | ==||== | 4110FY | 98 wt.% purity | 2.81 | 0.17 | 3.04 | 0.33 | 2.61 | 0.03 | 3.21 | 0.04 | |
| Organoclay | Nanofil®5 | Organoclay | Südchemie | - | ca. 35 wt.% QAC | 2.24 | 0.18 | 35.12 | 0.24 | 1.22 | 0.03 | 35.47 | 0.04 |
| Nanofil®8 | Organoclay | Südchemie | - | ca. 45 wt.% QAC | 0.99 | 0.51 | 44.09 | 0.35 | 1.46 | 0.03 | 43.74 | 0.03 | |
| Nanofil®9 | Organoclay | Südchemie | - | ca. 35 wt.% QAC | 1.47 | 0.3 | 34.89 | 0.4 | 1.1 | 0.01 | 37.04 | 0.01 | |
| Nanofil®SE3000 | Organoclay | Südchemie | - | unknown QAC | 1.13 | 0.2 | 53.75 | 0.41 | 0.69 | 0.004 | 55.04 | 0.11 |
| Code | Chromatography | Mass Spectrometry | Trimethoxymethylsilane | Tetramethoxy silane, TMOS (Artifact not Added to the Sum of Compounds) | Hexamethyl cyclotrisiloxane | Silane? | Silane? | 2-pyrrolidoneB | Trimethoxyoctylsilane | 1,4-Benzenedicarboxylic acid dimethylester* | Aromatic Compound? Base Peak m/z = 269 | ΣFatty acid methyl esters (C8 – C18) | ΣFatty acids (C8 – C18) | Large Peak Cluster (probably Reduced (hydrated) PAH Mixture) | Sum of GC-MS un-identified Compounds (%) | Sum of GC-MS Identified and Tentatively Identified Compounds (%) | ΣDilkyldimethylammonium Compounds (C7 - C27, Mainly C18, C16, C14) by LC-MS | ΣAlkyldimethylbenzylammonium Compounds (C14 - C21, Mainly C18, C16, C14) by LC-MS | MALDI-TOF-MS results | Fraction of TGA Coating Explained by Extraction and GC- or LC-MS (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GC-MS retention (min) | 3.2 | 4.9 | 6.4 | 7.5 | 14.6 | 15.3 | 24.4 | 27.3 | 31.6 | 17–36 | 17–36 | 32–42 | |||||||||
| TiO2 | UV-Titan M111 | GC | MS$ | 0 | 0 | 0 | |||||||||||||||
| NM-101 | GC | MS, MALDI | 0 | 0 | A0 | 0 | |||||||||||||||
| NM-103 | GC, LC | MS, QTOF, MALDI | 9 × 10–4 | 4 × 10–2 | 9 × 10–4 | 3 × 10–2 | 1 × 10–2 | 4 × 10–2 | 2 × 10–3 | B3 × 10–2 | 3 | ||||||||||
| NM-104 | GC, LC | MS, QTOF, MALDI | 6 × 10–3 | 0 | 0 | 0 | 0 | ||||||||||||||
| SiO | NM-204 | GC | MS, MALDI | 2 × 10–1 | 0 | 0 | 0 | 0 | |||||||||||||
| NRCWE-008 | GC | MS, MALDI | E13 | 0 | 0 | 0 | 0 | ||||||||||||||
| Graphite | NRCWE-005 | GC | MS, MALDI | 2 × 10–2 | 4 × 10–4 | 6 × 10–6 | 2 × 10–2 | 1 × 10–1 | 6 × 10–4 | 2 × 10–1 | 0 | 6 | |||||||||
| ZnO | MN-111 | GC | MS | 2 × 10–1 | 0 | 2 × 10–1 | 10 | ||||||||||||||
| Ag | NRCWE-009 | GC | MS, MALDI | 2 | 7 × 10–2 | 2 | C | 12 | |||||||||||||
| NM-300K (liquid) | GC | MS, MALDI | D | ||||||||||||||||||
| CaCO3 | NRCWE-012 | GC | MS | 2 × 10–4 | 5 × 10–1 | 2 | 2 × 10–4 | 3 | 52 | ||||||||||||
| NRCWE-013 | GC | MS | 1 × 10–1 | 3–1 | 4–1 | 0 | 7–1 | 12 | |||||||||||||
| NRCWE-014 | GC | MS | 5 × 10–2 | 2 × 10–1 | 1 | 0 | 1 | 27 | |||||||||||||
| NRCWE-015 | GC | MS | 1 × 10–1 | 3 × 10–2 | 4 | 0 | 4 | 68 | |||||||||||||
| NRCWE-016 | GC | MS | 8 × 10–3 | 3 × 10–2 | 1 | 6 × 101 | 6 × 10–1 | 1 | 36 | ||||||||||||
| NRCWE-017 | GC | MS | 1 × 10–4 | 5 × 10–1 | 3 | 1 × 10–4 | 3 | 60 | |||||||||||||
| Fe2O4 | NRCWE-018 | GC, LC | MS, QTOF, MALDI | 8 × 10–2 | 0 | 0 | 0 | 0 | |||||||||||||
| NRCWE-019 | GC, LC | MS, QTOF, MALDI | 4 × 10–2 | 0 | 0 | 0 | 0 | ||||||||||||||
| Ni/ZnFe2O4 | NRCWE-020 | GC, LC | MS, QTOF, MALDI | 1 × 10–2 | 00A0 | 0 | 0 | 0 | 0 | ||||||||||||
| NRCWE-022 | GC, LC | MS, QTOF, MALDI | 2 × 10–2 | 0 | 0 | 0 | 0 | ||||||||||||||
| Organoclay | Nanofil 5® | LC | QTOF£ | 3.3 | 9 | ||||||||||||||||
| Nanofil 8® | LC | QTOF | 6.1 | 14 | |||||||||||||||||
| Nanofil 9® | LC | QTOF | 2.3 | 7 | |||||||||||||||||
| Nanofil SE3000® | LC | QTOF | 2.6 | 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clausen, P.A.; Kofoed-Sørensen, V.; Nørgaard, A.W.; Sahlgren, N.M.; Jensen, K.A. Thermogravimetry and Mass Spectrometry of Extractable Organics from Manufactured Nanomaterials for Identification of Potential Coating Components. Materials 2019, 12, 3657. https://doi.org/10.3390/ma12223657
Clausen PA, Kofoed-Sørensen V, Nørgaard AW, Sahlgren NM, Jensen KA. Thermogravimetry and Mass Spectrometry of Extractable Organics from Manufactured Nanomaterials for Identification of Potential Coating Components. Materials. 2019; 12(22):3657. https://doi.org/10.3390/ma12223657
Chicago/Turabian StyleClausen, Per Axel, Vivi Kofoed-Sørensen, Asger W. Nørgaard, Nicklas Mønster Sahlgren, and Keld Alstrup Jensen. 2019. "Thermogravimetry and Mass Spectrometry of Extractable Organics from Manufactured Nanomaterials for Identification of Potential Coating Components" Materials 12, no. 22: 3657. https://doi.org/10.3390/ma12223657