Advances in the Stability of Halide Perovskite Nanocrystals
Abstract
:1. Introduction
2. Synthesis and Post-Synthesis Treatments
2.1. Key Synthesis Methods
2.1.1. Hot-Injection Method
2.1.2. Ligand Assisted Reprecipitation (LARP) Method
2.2. Post-Synthesis Modification
2.2.1. Surface Passivation
2.2.2. Phase Transition
2.2.3. Ions (Anion and Cation) Exchange
3. Perovskite Nanocrystals Structure
3.1. A-Cation
3.2. B-Cation: Lead-Free PNCs
3.3. Halides
3.4. Role of Dopants in Degradation
3.5. Morphological Dimensionality of PNCs
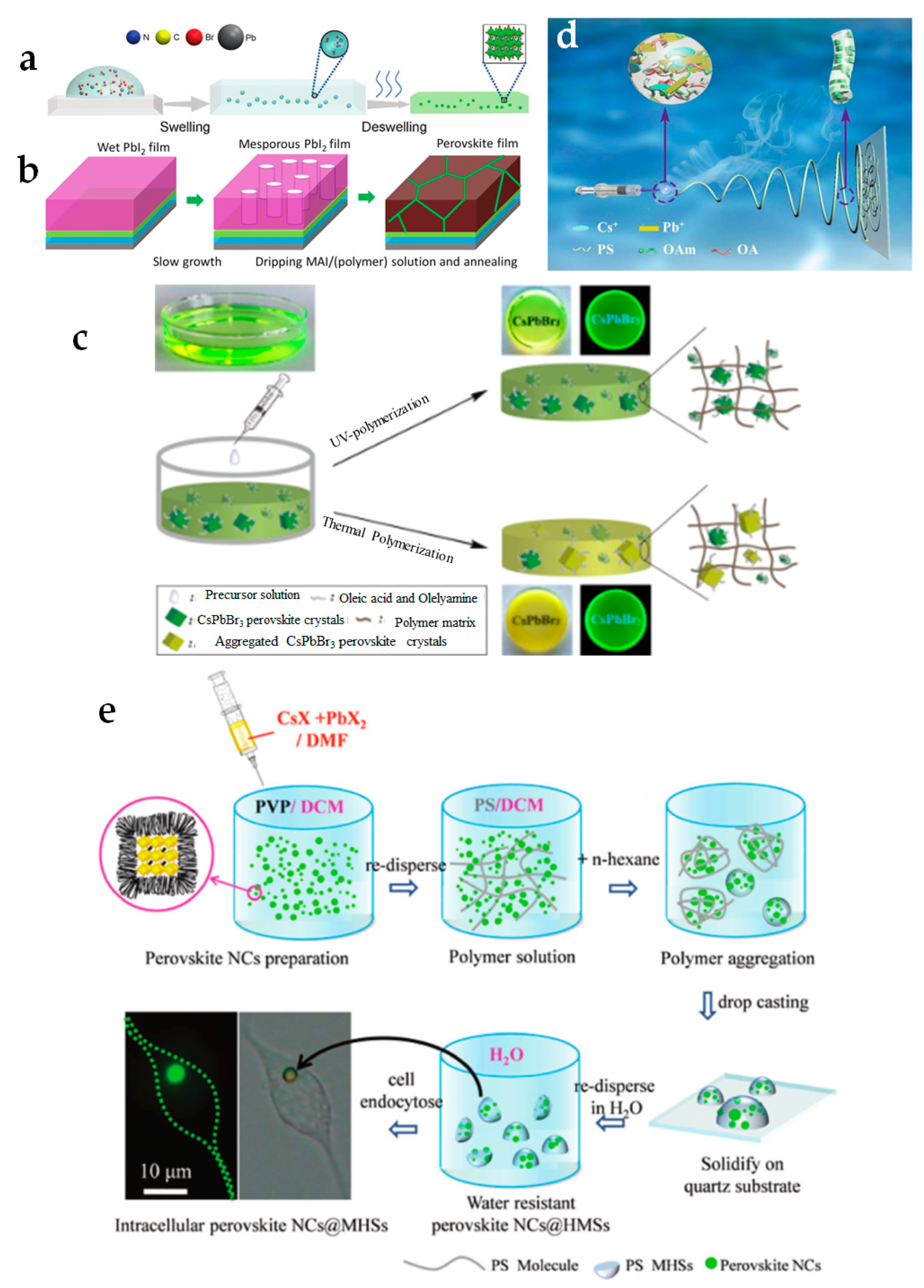
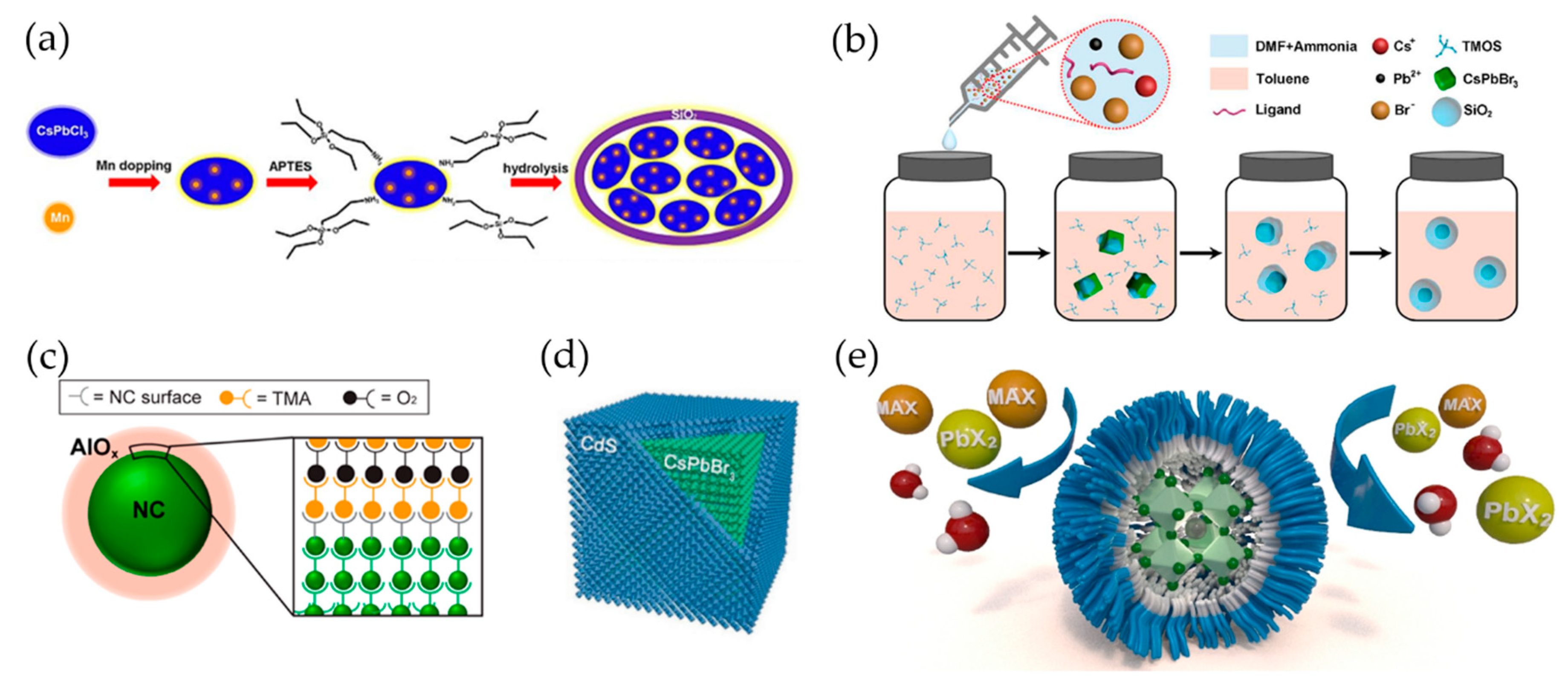
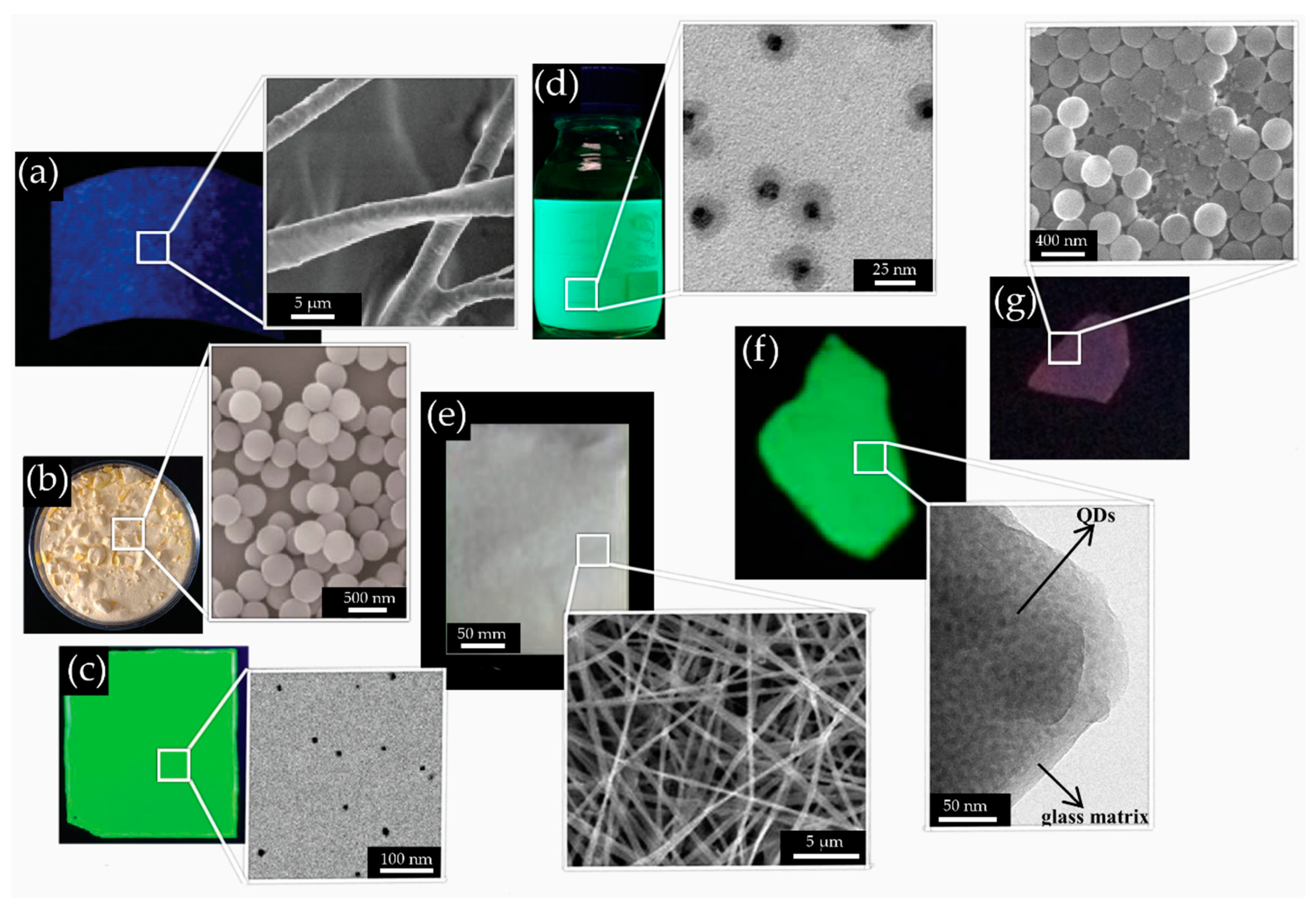
4. Environmental Stability (Heat, Moisture, Oxygen, UV Light) of PNCs
4.1. Encapsulation
4.2. Polymer Matrix
4.3. Core/Shell Structure
5. Conclusions and Future Outlook
Funding
Conflicts of Interest
References
- Kumar Jena, A.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, H.; Chen, J.; Hautzinger, M.P.; Zhu, X.-Y.; Jin, S. Metal halide perovskite nanostructures for optoelectronic applications and the study of physical properties. Nat. Rev. Mater. 2019, 4, 169–188. [Google Scholar] [CrossRef]
- NREL. Best Research-Cell Efficiencies. 2019. Available online: https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20190923.pdf (accessed on 25 September 2019).
- Talapin, D.V.; Lee, J.-S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of Colloidal Nanocrystals for Electronic and Optoelectronic Applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Huang, H.; Kershaw, S.V.; Rogach, A.L. Advances in metal halide perovskite nanocrystals: Synthetic strategies, growth mechanisms, and optoelectronic applications. Mater. Today 2019. In press. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, H.; Gedamu, D.; Fourmont, P.; Rekola, H.; Hiltunen, A.; Cloutier, S.G.; Nechache, R.; Priimagi, A.; Vivo, P. Halide Perovskite Nanocrystals for Next-Generation Optoelectronics. Small 2019, 15, 1900801. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, L.; Zhang, Q.; Krahne, R. Nanoscale & Nanoscale Advances joint themed collection on halide perovskite nanocrystals. Nanoscale 2019, 11, 8648–8650. [Google Scholar] [PubMed]
- Brittman, S.; Luo, J. A Promising Beginning for Perovskite Nanocrystals: A Nano Letters Virtual Issue. Nano Lett. 2018, 18, 2747–2750. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Phung, N.; Di Girolamo, D.; Vivo, P.; Abate, A. Enhancement in lifespan of halide perovskite solar cells. Energy Environ. Sci. 2019, 12, 865–886. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Protesescu, L.; Bodnarchuk, M.I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 2017, 358, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Aristidou, N.; Eames, C.; Sanchez-Molina, I.; Bu, X.; Kosco, J.; Islam, M.S.; Haque, S.A. Fast oxygen diffusion and iodide defects mediate oxygen-induced degradation of perovskite solar cells. Nat. Commun. 2017, 8, 15218. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Pradhan, B. Lead-Free Metal Halide Perovskite Nanocrystals: Challenges, Applications, and Future Aspects. ChemNanoMat 2019, 5, 300–312. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; de Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Qiu, Y.; Yang, S. Fully-Inorganic Trihalide Perovskite Nanocrystals: A New Research Frontier of Optoelectronic Materials. Adv. Mater. 2017, 29, 1700775. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhao, Y.; Zhang, S.; Dai, Y.; Liu, L.; Li, Y.; Chen, Q. Recent advances toward practical use of halide perovskite nanocrystals. J. Mater. Chem. A 2018, 6, 21729–21746. [Google Scholar] [CrossRef]
- Chan, S.; Liu, M.; Latham, K.; Haruta, M.; Kurata, H.; Teranishi, T.; Tachibana, Y. Monodisperse and size-tunable PbS colloidal quantum dots via heterogeneous precursors. J. Mater. Chem. C 2017, 5, 2182–2187. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3 ). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; He, B.; Fan, X.; Liu, Z.; Urban, J.J.; Alivisatos, A.P.; He, L.; Liu, Y. Insight into the Ligand-Mediated Synthesis of Colloidal CsPbBr 3 Perovskite Nanocrystals: The Role of Organic Acid, Base, and Cesium Precursors. ACS Nano 2016, 10, 7943–7954. [Google Scholar] [CrossRef] [PubMed]
- De Roo, J.; Ibáñez, M.; Geiregat, P.; Nedelcu, G.; Walravens, W.; Maes, J.; Martins, J.C.; Van Driessche, I.; Kovalenko, M.V.; Hens, Z. Highly Dynamic Ligand Binding and Light Absorption Coefficient of Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2016, 10, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xu, L.; Li, J.; Xue, J.; Dong, Y.; Li, X.; Zeng, H. Monolayer and Few-Layer All-Inorganic Perovskites as a New Family of Two-Dimensional Semiconductors for Printable Optoelectronic Devices. Adv. Mater. 2016, 28, 4861–4869. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, H.; Zhang, T.; Mi, L.; Zhang, Y.; Zhang, Z.; Zhang, W.; Jiang, Y. Solvent-Polarity-Engineered Controllable Synthesis of Highly Fluorescent Cesium Lead Halide Perovskite Quantum Dots and Their Use in White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 8478–8486. [Google Scholar] [CrossRef]
- Yang, B.; Chen, J.; Hong, F.; Mao, X.; Zheng, K.; Yang, S.; Li, Y.; Pullerits, T.; Deng, W.; Han, K. Lead-Free, Air-Stable All-Inorganic Cesium Bismuth Halide Perovskite Nanocrystals. Angew. Chem. Int. Ed. 2017, 56, 12471–12475. [Google Scholar] [CrossRef] [PubMed]
- Jellicoe, T.C.; Richter, J.M.; Glass, H.F.J.; Tabachnyk, M.; Brady, R.; Dutton, S.E.; Rao, A.; Friend, R.H.; Credgington, D.; Greenham, N.C.; et al. Synthesis and Optical Properties of Lead-Free Cesium Tin Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2016, 138, 2941–2944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Yan, X.; Zhang, M.; Sun, S.; Yang, M.; Shen, W.; Pan, X.; Wang, P.; Deng, Z. Controlled Synthesis of Lead-Free and Stable Perovskite Derivative Cs2SnI6 Nanocrystals via a Facile Hot-Injection Process. Chem. Mater. 2016, 28, 8132–8140. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, J.; Parida, M.R.; Ahmed, G.H.; Pan, J.; Bakr, O.M.; Brédas, J.-L.; Mohammed, O.F. Direct-Indirect Nature of the Bandgap in Lead-Free Perovskite Nanocrystals. J. Phys. Chem. Lett. 2017, 8, 3173–3177. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Kumar, G.S.; Sain, S.; Dalui, A.; Ghorai, U.K.; Pradhan, S.K.; Acharya, S. Size Tunable Cesium Antimony Chloride Perovskite Nanowires and Nanorods. Chem. Mater. 2018, 30, 2135–2142. [Google Scholar] [CrossRef]
- De Mello Donegá, C.; Liljeroth, P.; Vanmaekelbergh, D. Physicochemical Evaluation of the Hot-Injection Method, a Synthesis Route for Monodisperse Nanocrystals. Small 2005, 1, 1152–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot-induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Embden, J.; Chesman, A.S.R.; Jasieniak, J.J. The Heat-Up Synthesis of Colloidal Nanocrystals. Chem. Mater. 2015, 27, 2246–2285. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 Quantum Dots for Lighting and Displays: Room- Temperature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 2435–2445. [Google Scholar] [CrossRef]
- Wei, S.; Yang, Y.; Kang, X.; Wang, L.; Huang, L.; Pan, D. Room-temperature and gram-scale synthesis of CsPbX3 (X = Cl, Br, I) perovskite nanocrystals with 50–85% photoluminescence quantum yields. Chem. Commun. 2016, 52, 7265–7268. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.C.; Pertegás, A.; González-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Mínguez Espallargas, G.; Bolink, H.J.; Galian, R.E.; Pérez-Prieto, J. Nontemplate Synthesis of CH3NH3PbBr3 Perovskite Nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; Motti, S.G.; Srimath Kandada, A.R.; Mosconi, E.; D’Innocenzo, V.; Bertoni, G.; Marras, S.; Kamino, B.A.; Miranda, L.; De Angelis, F. Solution Synthesis Approach to Colloidal Cesium Lead Halide Perovskite Nanoplatelets with Monolayer-Level Thickness Control. J. Am. Chem. Soc. 2016, 138, 1010–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Zhao, F.; Liu, L.; Zhang, F.; Wu, X.; Shi, L.; Zou, B.; Pei, Q.; Zhong, H. Emulsion Synthesis of Size-Tunable CH3NH3PbBr3 Quantum Dots: An Alternative Route toward Efficient Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2015, 7, 28128–28133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhong, H.; Chen, C.; Wu, X.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly Luminescent and Color-Tunable Colloidal CH3NH3PbX 3 (X = Br, I, Cl) Quantum Dots: Potential Alternatives for Display Technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yuan, D.; Xu, Y.; Wang, A.; Deng, Z. Ligand-Mediated Synthesis of Shape-Controlled Cesium Lead Halide Perovskite Nanocrystals via Reprecipitation Process at Room Temperature. ACS Nano 2016, 10, 3648–3657. [Google Scholar] [CrossRef] [PubMed]
- Leng, M.; Yang, Y.; Zeng, K.; Chen, Z.; Tan, Z.; Li, S.; Li, J.; Xu, B.; Li, D.; Hautzinger, M.P. All-Inorganic Bismuth-Based Perovskite Quantum Dots with Bright Blue Photoluminescence and Excellent Stability. Adv. Funct. Mater. 2018, 28, 1704446. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Deng, H.; Farooq, U.; Yang, X.; Khan, J.; Tang, J.; Song, H. High Quantum Yield Blue Emission from Lead-Free Inorganic Antimony Halide Perovskite Colloidal Quantum Dots. ACS Nano 2017, 11, 9294–9302. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.K.; Wang, C.; Jasieniak, J.J. Synthetic Evolution of Colloidal Metal Halide Perovskite Nanocrystals. Langmuir 2019, 35, 11609–11628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, M.; Di Stasio, F.; Dang, Z.; Canale, C.; Khan, A.H.; Shamsi, J.; Brescia, R.; Prato, M.; Manna, L. Colloidal Synthesis of Strongly Fluorescent CsPbBr 3 Nanowires with Width Tunable down to the Quantum Confinement Regime. Chem. Mater. 2016, 28, 6450–6454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boles, M.A.; Ling, D.; Hyeon, T.; Talapin, D.V. The surface science of nanocrystals. Nat. Mater. 2016, 15, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, L.; Wang, T.; Song, J.; Chen, J.; Xue, J.; Dong, Y.; Cai, B.; Shan, Q.; Han, B. 50-Fold EQE Improvement up to 6.27% of Solution-Processed All-Inorganic Perovskite CsPbBr3 QLEDs via Surface Ligand Density Control. Adv. Mater. 2017, 29, 1603885. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Yun, S.; Yoon, S.-G.; Choi, J. Surface engineering for improved stability of CH3NH3PbBr3 perovskite nanocrystals. Nanoscale 2018, 10, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Ripka, E.G.; Deschene, C.R.; Franck, J.M.; Bae, I.-T.; Maye, M.M. Understanding the Surface Properties of Halide Exchanged Cesium Lead Halide Nanoparticles. Langmuir 2018, 34, 11139–11146. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.K.; Santra, P.K.; Joshi, N.; Chugh, J.; Singh, S.K.; Rensmo, H.; Ghosh, P.; Nag, A. Origin of the Substitution Mechanism for the Binding of Organic Ligands on the Surface of CsPbBr3 Perovskite Nanocubes. J. Phys. Chem. Lett. 2017, 8, 4988–4994. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Pu, Y.-C.; Lindley, S.A.; Yang, Y.; Lu, L.; Li, Y.; Li, X.; Zhang, J.Z. Organolead Halide Perovskite Nanocrystals: Branched Capping Ligands Control Crystal Size and Stability. Angew. Chem. Int. Ed. 2016, 55, 8864–8868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Zhang, Y.; Ding, C.; Kobayashi, S.; Izuishi, T.; Nakazawa, N.; Toyoda, T.; Ohta, T.; Hayase, S.; Minemoto, T.; et al. Highly Luminescent Phase-Stable CsPbI 3 Perovskite Quantum Dots Achieving Near 100% Absolute Photoluminescence Quantum Yield. ACS Nano 2017, 11, 10373–10383. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Guo, Y.; Muhammad, F.; Deng, Z. Controlled Synthesis of Lead-Free Cesium Tin Halide Perovskite Cubic Nanocages with High Stability. Chem. Mater. 2017, 29, 6493–6501. [Google Scholar] [CrossRef]
- Suh, Y.-H.; Kim, T.; Choi, J.W.; Lee, C.-L.; Park, J. High-Performance CsPbX3 Perovskite Quantum-Dot Light-Emitting Devices via Solid-State Ligand Exchange. ACS Appl. Nano Mater. 2018, 1, 488–496. [Google Scholar] [CrossRef]
- Pan, J.; Shang, Y.; Yin, J.; De Bastiani, M.; Peng, W.; Dursun, I.; Sinatra, L.; El-Zohry, A.M.; Hedhili, M.N.; Emwas, A.-H. Bidentate Ligand-Passivated CsPbI 3 Perovskite Nanocrystals for Stable Near-Unity Photoluminescence Quantum Yield and Efficient Red Light-Emitting Diodes. J. Am. Chem. Soc. 2018, 140, 562–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravi, V.K.; Scheidt, R.A.; Nag, A.; Kuno, M.; Kamat, P.V. To Exchange or Not to Exchange. Suppressing Anion Exchange in Cesium Lead Halide Perovskites with PbSO4 –Oleate Capping. ACS Energy Lett. 2018, 3, 1049–1055. [Google Scholar] [CrossRef]
- Huang, S.; Wang, B.; Zhang, Q.; Li, Z.; Shan, A.; Li, L. Postsynthesis Potassium-Modification Method to Improve Stability of CsPbBr3 Perovskite Nanocrystals. Adv. Opt. Mater. 2018, 6, 1701106. [Google Scholar] [CrossRef]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, H.; Zhu, Z.; Chang, Y.; Zhang, T.; Song, Z.; Jiang, Y. Shape and phase evolution from CsPbBr 3 perovskite nanocubes to tetragonal CsPb2 Br5 nanosheets with an indirect bandgap. Chem. Commun. 2016, 52, 11296–11299. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Wang, S.; Long, D.; Li, M.; Wang, D.; Zhang, T. Inter-Conversion between Different Compounds of Ternary Cs-Pb-Br System. Materials 2018, 11, 717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balakrishnan, S.K.; Kamat, P.V. Ligand Assisted Transformation of Cubic CsPbBr 3 Nanocrystals into Two-Dimensional CsPb2Br5 Nanosheets. Chem. Mater. 2018, 30, 74–78. [Google Scholar] [CrossRef]
- Palazon, F.; Almeida, G.; Akkerman, Q.A.; De Trizio, L.; Dang, Z.; Prato, M.; Manna, L. Changing the Dimensionality of Cesium Lead Bromide Nanocrystals by Reversible Postsynthesis Transformations with Amines. Chem. Mater. 2017, 29, 4167–4171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udayabhaskararao, T.; Houben, L.; Cohen, H.; Menahem, M.; Pinkas, I.; Avram, L.; Wolf, T.; Teitelboim, A.; Leskes, M.; Yaffe, O. A Mechanistic Study of Phase Transformation in Perovskite Nanocrystals Driven by Ligand Passivation. Chem. Mater. 2018, 30, 84–93. [Google Scholar] [CrossRef]
- Chen, X.; Chen, D.; Li, J.; Fang, G.; Sheng, H.; Zhong, J. Tunable CsPbBr3/Cs4PbBr6 phase transformation and their optical spectroscopic properties. Dalton Trans. 2018, 47, 5670–5678. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bekenstein, Y.; Ye, X.; Nguyen, S.C.; Swabeck, J.; Zhang, D.; Lee, S.-T.; Yang, P.; Ma, W.; Alivisatos, A.P. Ligand Mediated Transformation of Cesium Lead Bromide Perovskite Nanocrystals to Lead Depleted Cs4PbBr6 Nanocrystals. J. Am. Chem. Soc. 2017, 139, 5309–5312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palazon, F.; Urso, C.; De Trizio, L.; Akkerman, Q.; Marras, S.; Locardi, F.; Nelli, I.; Ferretti, M.; Prato, M.; Manna, L. Postsynthesis Transformation of Insulating Cs4PbBr6 Nanocrystals into Bright Perovskite CsPbBr3 through Physical and Chemical Extraction of CsBr. ACS Energy Lett. 2017, 2, 2445–2448. [Google Scholar] [CrossRef] [PubMed]
- Palazon, F.; Dogan, S.; Marras, S.; Locardi, F.; Nelli, I.; Rastogi, P.; Ferretti, M.; Prato, M.; Krahne, R.; Manna, L. From CsPbBr3 Nano-Inks to Sintered CsPbBr3–CsPb2Br5 Films via Thermal Annealing: Implications on Optoelectronic Properties. J. Phys. Chem. C 2017, 121, 11956–11961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.; Ruan, L.; Shen, Z.; Deng, Z. Reversible light-mediated compositional and structural transitions between CsPbBr3 and CsPb2Br5 nanosheets. Chem. Commun. 2018, 54, 2804–2807. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Hu, H.; Xu, Y.; Jiang, S.; Chen, M.; Zhong, Q.; Yang, D.; Liu, Q.; Zhao, Y.; Sun, B. From Nonluminescent Cs4 PbX6 (X = Cl, Br, I) Nanocrystals to Highly Luminescent CsPbX3 Nanocrystals: Water-Triggered Transformation through a CsX-Stripping Mechanism. Nano Lett. 2017, 17, 5799–5804. [Google Scholar] [CrossRef] [PubMed]
- Turedi, B.; Lee, K.J.; Dursun, I.; Alamer, B.; Wu, Z.; Alarousu, E.; Mohammed, O.F.; Cho, N.; Bakr, O.M. Water-Induced Dimensionality Reduction in Metal-Halide Perovskites. J. Phys. Chem. C 2018, 122, 14128–14134. [Google Scholar] [CrossRef] [Green Version]
- Long, Z.; Ren, H.; Sun, J.; Ouyang, J.; Na, N. High-throughput and tunable synthesis of colloidal CsPbX3 perovskite nanocrystals in a heterogeneous system by microwave irradiation. Chem. Commun. 2017, 53, 9914–9917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Yang, Y.; Bekenstein, Y.; Yu, Y.; Gibson, N.A.; Wong, A.B.; Eaton, S.W.; Kornienko, N.; Kong, Q.; Lai, M. Synthesis of Composition Tunable and Highly Luminescent Cesium Lead Halide Nanowires through Anion-Exchange Reactions. J. Am. Chem. Soc. 2016, 138, 7236–7239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Zhang, X.; Lu, S.; Yang, P. Phase transformation, morphology control, and luminescence evolution of cesium lead halide nanocrystals in the anion exchange process. RSC Adv. 2016, 6, 103382–103389. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the optical properties of cesium lead halide perovskite nanocrystals by anion exchange reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protesescu, L.; Yakunin, S.; Kumar, S.; Bär, J.; Bertolotti, F.; Masciocchi, N.; Guagliardi, A.; Grotevent, M.; Shorubalko, I.; Bodnarchuk, M.I. Dismantling the “Red Wall” of Colloidal Perovskites: Highly Luminescent Formamidinium and Formamidinium–Cesium Lead Iodide Nanocrystals. ACS Nano 2017, 11, 3119–3134. [Google Scholar] [CrossRef] [PubMed]
- Van der Stam, W.; Geuchies, J.J.; Altantzis, T.; van den Bos, K.H.W.; Meeldijk, J.D.; Van Aert, S.; Bals, S.; Vanmaekelbergh, D.; de Mello Donega, C. Highly Emissive Divalent-Ion-Doped Colloidal CsPb1–xMxBr3 Perovskite Nanocrystals through Cation Exchange. J. Am. Chem. Soc. 2017, 139, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Mondal, N.; De, A.; Samanta, A. Achieving Near-Unity Photoluminescence Efficiency for Blue-Violet-Emitting Perovskite Nanocrystals. ACS Energy Lett. 2019, 4, 32–39. [Google Scholar] [CrossRef]
- Huang, G.; Wang, C.; Xu, S.; Zong, S.; Lu, J.; Wang, Z.; Lu, C.; Cui, Y. Postsynthetic Doping of MnCl2 Molecules into Preformed CsPbBr3 Perovskite Nanocrystals via a Halide Exchange-Driven Cation Exchange. Adv. Mater. 2017, 29, 1700095. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, F.; Lin, F.; Chen, Y.; Cai, Z.; Wang, Y.; Chen, X. Synthesis of CsPbCl3-Mn Nanocrystals via Cation Exchange. Adv. Opt. Mater. 2017, 1700520. [Google Scholar] [CrossRef]
- Saliba, M. Polyelemental, Multicomponent Perovskite Semiconductor Libraries through Combinatorial Screening. Adv. Energy Mater. 2019, 9, 1803754. [Google Scholar] [CrossRef]
- Linaburg, M.R.; McClure, E.T.; Majher, J.D.; Woodward, P.M. Cs1–xRbxPbCl3 and Cs1–xRbxPbBr3 Solid Solutions: Understanding Octahedral Tilting in Lead Halide Perovskites. Chem. Mater. 2017, 29, 3507–3514. [Google Scholar] [CrossRef]
- Lim, D.-H.; Ramasamy, P.; Kwak, D.-H.; Lee, J.-S. Solution-phase synthesis of rubidium lead iodide orthorhombic perovskite nanowires. Nanotechnology 2017, 28, 255601. [Google Scholar] [CrossRef] [PubMed]
- Amgar, D.; Binyamin, T.; Uvarov, V.; Etgar, L. Near ultra-violet to mid-visible band gap tuning of mixed cation Rbx Cs1−xPbX3 (X = Cl or Br) perovskite nanoparticles. Nanoscale 2018, 10, 6060–6068. [Google Scholar] [CrossRef] [PubMed]
- Saidaminov, M.I.; Mohammed, O.F.; Bakr, O.M. Low-Dimensional-Networked Metal Halide Perovskites: The Next Big Thing. ACS Energy Lett. 2017, 2, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Fan, Q.; Biesold-McGee, G.V.; Xu, Q.; Pan, S.; Peng, J.; Ma, J.; Lin, Z. Lead-Free Halide Perovskite Nanocrystals: Crystal Structures, Synthesis, Stabilities, and Optical Properties. Angew. Chem. 2019, 1904862. [Google Scholar]
- Qiu, L.; Ono, L.K.; Qi, Y. Advances and challenges to the commercialization of organic–inorganic halide perovskite solar cell technology. Mater. Today Energy 2018, 7, 169–189. [Google Scholar] [CrossRef]
- Giustino, F.; Snaith, H.J. Toward Lead-Free Perovskite Solar Cells. ACS Energy Lett. 2016, 1, 1233–1240. [Google Scholar] [CrossRef] [Green Version]
- Lyu, M.; Yun, J.-H.; Chen, P.; Hao, M.; Wang, L. Addressing Toxicity of Lead: Progress and Applications of Low-Toxic Metal Halide Perovskites and Their Derivatives. Adv. Energy Mater. 2017, 7, 1602512. [Google Scholar] [CrossRef]
- Shi, Z.; Guo, J.; Chen, Y.; Li, Q.; Pan, Y.; Zhang, H.; Xia, Y.; Huang, W. Lead-Free Organic-Inorganic Hybrid Perovskites for Photovoltaic Applications: Recent Advances and Perspectives. Adv. Mater. 2017, 29, 1605005. [Google Scholar] [CrossRef] [PubMed]
- Hoefler, S.F.; Trimmel, G.; Rath, T. Progress on lead-free metal halide perovskites for photovoltaic applications: A review. Monatshefte Für Chem. Chem. Mon. 2017, 148, 795–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Yang, J.; Lee, J.I.; Cho, J.H.; Kang, M.S. Lead-Free Perovskite Nanocrystals for Light-Emitting Devices. J. Phys. Chem. Lett. 2018, 9, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Khalfin, S.; Bekenstein, Y. Advances in lead-free double perovskite nanocrystals, engineering band-gaps and enhancing stability through composition tunability. Nanoscale 2019, 11, 8665–8679. [Google Scholar] [CrossRef] [PubMed]
- Bekenstein, Y.; Dahl, J.C.; Huang, J.; Osowiecki, W.T.; Swabeck, J.K.; Chan, E.M.; Yang, P.; Alivisatos, A.P. The Making and Breaking of Lead-Free Double Perovskite Nanocrystals of Cesium Silver–Bismuth Halide Compositions. Nano Lett. 2018, 18, 3502–3508. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, A.; Ravi, V.K.; Nag, A. Beyond Colloidal Cesium Lead Halide Perovskite Nanocrystals: Analogous Metal Halides and Doping. ACS Energy Lett. 2017, 2, 1089–1098. [Google Scholar] [CrossRef]
- Scaife, D.E.; Weller, P.F.; Fisher, W.G. Crystal preparation and properties of cesium tin(II) trihalides. J. Solid State Chem. 1974, 9, 308–314. [Google Scholar] [CrossRef]
- Yamada, K.; Kawaguchi, H.; Matsui, T.; Okuda, T.; Ichiba, S. Structural Phase Transition and Electrical Conductivity of the Perovskite CH3NH3Sn1-xPbxBr3 and CsSnBr3. Bull. Chem. Soc. Jpn. 1990, 63, 2521–2525. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Cao, B.; Yuan, S.; Chen, X.; Qiu, Z.; Jiang, Y.; Ye, Q.; Wang, H.; Zeng, H.; Liu, J. From unstable CsSnI3 to air-stable Cs2SnI6: A lead-free perovskite solar cell light absorber with bandgap of 1.48 eV and high absorption coefficient. Sol. Energy Mater. Sol. Cells 2017, 159, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Leng, M.; Chen, Z.; Yang, Y.; Li, Z.; Zeng, K.; Li, K.; Niu, G.; He, Y.; Zhou, Q.; Tang, J. Lead-Free, Blue Emitting Bismuth Halide Perovskite Quantum Dots. Angew. Chem. Int. Ed. 2016, 55, 15012–15016. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.D.; Santra, K.; Wang, Y.; Hadi, A.; Petrich, J.W.; Panthani, M.G. Synthesis and optical properties of ordered-vacancy perovskite cesium bismuth halide nanocrystals. Chem. Commun. 2018, 54, 3640–3643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, Y.; Fang, M.; Chen, J.; Zhao, Y. Formation of highly luminescent cesium bismuth halide perovskite quantum dots tuned by anion exchange. Chem. Commun. 2018, 54, 3779–3782. [Google Scholar] [CrossRef] [PubMed]
- Pal, J.; Manna, S.; Mondal, A.; Das, S.; Adarsh, K.V.; Nag, A. Colloidal Synthesis and Photophysics of M3Sb2I9 (M = Cs and Rb) Nanocrystals: Lead-Free Perovskites. Angew. Chem. Int. Ed. 2017, 56, 14187–14191. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-G.; Yang, J.-H.; Fu, Y.; Yang, D.; Xu, Q.; Yu, L.; Wei, S.-H.; Zhang, L. Design of Lead-Free Inorganic Halide Perovskites for Solar Cells via Cation-Transmutation. J. Am. Chem. Soc. 2017, 139, 2630–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Mao, X.; Hong, F.; Meng, W.; Tang, Y.; Xia, X.; Yang, S.; Deng, W.; Han, K. Lead-Free Direct Bandgap Double Perovskite Nanocrystals with Bright Dual-Color Emission. J. Am. Chem. Soc. 2018, 140, 17001–17006. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Stoumpos, C.C.; Kanatzidis, M.G. “Unleaded” Perovskites: Status Quo and Future Prospects of Tin-Based Perovskite Solar Cells. Adv. Mater. 2018, 1803230. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Liu, J.; Portale, G.; Fang, H.-H.; Blake, G.R.; ten Brink, G.H.; Koster, L.J.A.; Loi, M.A. Highly Reproducible Sn-Based Hybrid Perovskite Solar Cells with 9% Efficiency. Adv. Energy Mater. 2018, 8, 1702019. [Google Scholar] [CrossRef]
- Babayigit, A.; Duy Thanh, D.; Ethirajan, A.; Manca, J.; Muller, M.; Boyen, H.-G.; Conings, B. Assessing the toxicity of Pb- and Sn-based perovskite solar cells in model organism Danio rerio. Sci. Rep. 2016, 6, 18721. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ju, M.-G.; Garces, H.F.; Carl, A.D.; Ono, L.K.; Hawash, Z.; Zhang, Y.; Shen, T.; Qi, Y.; Grimm, R.L. Highly stable and efficient all-inorganic lead-free perovskite solar cells with native-oxide passivation. Nat. Commun. 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Pasanen, H.; Ali-Löytty, H.; Lahtonen, K.; Qudsia, S.; Smått, J.H.; Valden, M.; Tkachenko, N.V.; Vivo, P. All-inorganic Tin–Germanium Perovskite Nanocubes: Towards Highly Efficient Lead-free Perovskite Nanocrystals-based Solar Cells. J. Am. Chem. Soc. 2019. under peer review. [Google Scholar]
- Zhou, Y.; Zhao, Y. Chemical stability and instability of inorganic halide perovskites. Energy Environ. Sci. 2019, 12, 1495–1511. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, Y.; Hosono, H.; Kamiya, T.; Padture, N.P. Bandgap Optimization of Perovskite Semiconductors for Photovoltaic Applications. Chem. A Eur. J. 2018, 24, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.K.; Singhal, N.; Nag, A. Initiation and future prospects of colloidal metal halide double-perovskite nanocrystals: Cs2AgBiX6 (X = Cl, Br, I). J. Mater. Chem. A 2018, 6, 21666–21675. [Google Scholar] [CrossRef]
- McClure, E.T.; Ball, M.R.; Windl, W.; Woodward, P.M. Cs2 AgBiX6 (X = Br, Cl): New Visible Light Absorbing, Lead-Free Halide Perovskite Semiconductors. Chem. Mater. 2016, 28, 1348–1354. [Google Scholar] [CrossRef]
- Slavney, A.H.; Hu, T.; Lindenberg, A.M.; Karunadasa, H.I. A Bismuth-Halide Double Perovskite with Long Carrier Recombination Lifetime for Photovoltaic Applications. J. Am. Chem. Soc. 2016, 138, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, J.; Yang, S.; Hong, F.; Sun, L.; Han, P.; Pullerits, T.; Deng, W.; Han, K. Lead-Free Silver-Bismuth Halide Double Perovskite Nanocrystals. Angew. Chem. Int. Ed. 2018, 57, 5359–5363. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.C.; Osowiecki, W.T.; Cai, Y.; Swabeck, J.K.; Bekenstein, Y.; Asta, M.; Chan, E.M.; Alivisatos, A.P. Probing the Stability and Band Gaps of Cs2AgInCl6 and Cs2AgSbCl6 Lead-Free Double Perovskite Nanocrystals. Chem. Mater. 2019, 31, 3134–3143. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, B.; Chang, J.; Ding, B.; Zheng, S.; Wu, Y.; Yang, J.; Yang, G.; Zhong, X.; Wang, J. (C6H5CH2NH3)2CuBr4: A Lead-Free, Highly Stable Two-Dimensional Perovskite for Solar Cell Applications. ACS Appl. Energy Mater. 2018, 1, 2709–2716. [Google Scholar] [CrossRef]
- Vargas, B.; Ramos, E.; Pérez-Gutiérrez, E.; Alonso, J.C.; Solis-Ibarra, D. A Direct Bandgap Copper–Antimony Halide Perovskite. J. Am. Chem. Soc. 2017, 139, 9116–9119. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, W.; Wang, W.; Xu, B.; Wu, D.; Hao, J.; Cao, W.; Fang, F.; Li, Y.; Zeng, Y. Halide-Rich Synthesized Cesium Lead Bromide Perovskite Nanocrystals for Light-Emitting Diodes with Improved Performance. Chem. Mater. 2017, 29, 5168–5173. [Google Scholar] [CrossRef]
- Woo, J.Y.; Kim, Y.; Bae, J.; Kim, T.G.; Kim, J.W.; Lee, D.C.; Jeong, S. Highly Stable Cesium Lead Halide Perovskite Nanocrystals through in Situ Lead Halide Inorganic Passivation. Chem. Mater. 2017, 29, 7088–7092. [Google Scholar] [CrossRef]
- Imran, M.; Caligiuri, V.; Wang, M.; Goldoni, L.; Prato, M.; Krahne, R.; De Trizio, L.; Manna, L. Benzoyl Halides as Alternative Precursors for the Colloidal Synthesis of Lead-Based Halide Perovskite Nanocrystals. J. Am. Chem. Soc. 2018, 140, 2656–2664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creutz, S.E.; Crites, E.N.; De Siena, M.C.; Gamelin, D.R. Colloidal Nanocrystals of Lead-Free Double-Perovskite (Elpasolite) Semiconductors: Synthesis and Anion Exchange To Access New Materials. Nano Lett. 2018, 18, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S. Il Chemical Management for Colorful, Efficient, and Stable Inorganic–Organic Hybrid Nanostructured Solar Cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; Meggiolaro, D.; Dang, Z.; De Angelis, F.; Manna, L. Fluorescent Alloy CsPbxMn1–xI3 Perovskite Nanocrystals with High Structural and Optical Stability. ACS Energy Lett. 2017, 2, 2183–2186. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Liu, Y.; Li, J.; Liu, C.; Feng, R.; Jiang, F.; Li, Y.; Song, J.; Zeng, H.; Hong, M.; et al. Stabilizing Cesium Lead Halide Perovskite Lattice through Mn(II) Substitution for Air-Stable Light-Emitting Diodes. J. Am. Chem. Soc. 2017, 139, 11443–11450. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, Z.; Shao, J.; Yao, D.; Gao, H.; Liu, Y.; Yu, W.; Zhang, H.; Yang, B. CsPbxMn1– xCl3 Perovskite Quantum Dots with High Mn Substitution Ratio. ACS Nano 2017, 11, 2239–2247. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lin, Q.; Li, H.; Wu, K.; Robel, I.; Pietryga, J.M.; Klimov, V.I. Mn2+-Doped Lead Halide Perovskite Nanocrystals with Dual-Color Emission Controlled by Halide Content. J. Am. Chem. Soc. 2016, 138, 14954–14961. [Google Scholar] [CrossRef] [PubMed]
- Mir, W.J.; Jagadeeswararao, M.; Das, S.; Nag, A. Colloidal Mn-Doped Cesium Lead Halide Perovskite Nanoplatelets. ACS Energy Lett. 2017, 2, 537–543. [Google Scholar] [CrossRef]
- Chen, D.; Fang, G.; Chen, X. Silica-Coated Mn-Doped CsPb(Cl/Br)3 Inorganic Perovskite Quantum Dots: Exciton-to-Mn Energy Transfer and Blue-Excitable Solid-State Lighting. ACS Appl. Mater. Interfaces 2017, 9, 40477–40487. [Google Scholar] [CrossRef] [PubMed]
- Begum, R.; Parida, M.R.; Abdelhady, A.L.; Murali, B.; Alyami, N.M.; Ahmed, G.H.; Hedhili, M.N.; Bakr, O.M.; Mohammed, O.F. Engineering Interfacial Charge Transfer in CsPbBr3 Perovskite Nanocrystals by Heterovalent Doping. J. Am. Chem. Soc. 2017, 139, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Fang, G.; Chen, X.; Lei, L.; Zhong, J.; Mao, Q.; Zhou, S.; Li, J. Mn-Doped CsPbCl3 perovskite nanocrystals: Solvothermal synthesis, dual-color luminescence and improved stability. J. Mater. Chem. C 2018, 6, 8990–8998. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Wang, A.; Wang, Q.; Tang, H.; Shen, W.; Li, Z.; Deng, Z. Controlled Synthesis of Composition Tunable Formamidinium Cesium Double Cation Lead Halide Perovskite Nanowires and Nanosheets with Improved Stability. Chem. Mater. 2017, 29, 2157–2166. [Google Scholar] [CrossRef]
- Mosconi, E.; Azpiroz, J.M.; De Angelis, F. Ab Initio Molecular Dynamics Simulations of Methylammonium Lead Iodide Perovskite Degradation by Water. Chem. Mater. 2015, 27, 4885–4892. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, H.; Schrader, A.W.; Liang, D.; Ding, Q.; Joshi, P.; Hwang, L.; Zhu, X.-Y.; Jin, S. Nanowire Lasers of Formamidinium Lead Halide Perovskites and Their Stabilized Alloys with Improved Stability. Nano Lett. 2016, 16, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Asuo, I.M.; Gedamu, D.; Ka, I.; Gerlein, L.F.; Fortier, F.-X.; Pignolet, A.; Cloutier, S.G.; Nechache, R. High-performance pseudo-halide perovskite nanowire networks for stable and fast-response photodetector. Nano Energy 2018, 51, 324–332. [Google Scholar] [CrossRef]
- Yuan, H.; Debroye, E.; Janssen, K.; Naiki, H.; Steuwe, C.; Lu, G.; Moris, M.; Orgiu, E.; Uji-i, H.; De Schryver, F.; et al. Degradation of Methylammonium Lead Iodide Perovskite Structures through Light and Electron Beam Driven Ion Migration. J. Phys. Chem. Lett. 2016, 7, 561–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.C.; Lin, S.Y.; Tang, A.C.; Singh, B.P.; Tong, H.C.; Chen, C.Y.; Lee, Y.C.; Tsai, T.L.; Liu, R.S. Mesoporous Silica Particles Integrated with All-Inorganic CsPbBr3 Perovskite Quantum-Dot Nanocomposites (MP-PQDs) with High Stability and Wide Color Gamut Used for Backlight Display. Angew. Chem. Int. Ed. 2016, 55, 7924–7929. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, J.; Ye, S.; Zhang, Q. A stimuli responsive material of perovskite quantum dots composited nano-porous glass. J. Mater. Chem. C 2018, 6, 11184–11192. [Google Scholar] [CrossRef]
- Guhrenz, C.; Benad, A.; Ziegler, C.; Haubold, D.; Gaponik, N.; Eychmüller, A. Solid-State Anion Exchange Reactions for Color Tuning of CsPbX3 Perovskite Nanocrystals. Chem. Mater. 2016, 28, 9033–9040. [Google Scholar] [CrossRef]
- Liu, S.; Shao, G.; Ding, L.; Liu, J.; Xiang, W.; Liang, X. Sn-doped CsPbBr3 QDs glasses with excellent stability and optical properties for WLED. Chem. Eng. J. 2019, 361, 937–944. [Google Scholar] [CrossRef]
- Di, X.; Jiang, J.; Hu, Z.; Zhou, L.; Li, P.; Liu, S.; Xiang, W.; Liang, X. Stable and brightly luminescent all-inorganic cesium lead halide perovskite quantum dots coated with mesoporous silica for warm WLED. Dye. Pigment. 2017, 146, 361–367. [Google Scholar] [CrossRef]
- Di, X.; Shen, L.; Jiang, J.; He, M.; Cheng, Y.; Zhou, L.; Liang, X.; Xiang, W. Efficient white LEDs with bright green-emitting CsPbBr3 perovskite nanocrystal in mesoporous silica nanoparticles. J. Alloy. Compd. 2017, 729, 526–532. [Google Scholar] [CrossRef]
- He, M.; Liu, S.; Ding, L.; Zhang, Z.; Liu, J.; Xiang, W.; Liang, X. SiO2 -improved stability of Mn-doped CsPbBr0.5I2.5 NC and their application for white LED. J. Am. Ceram. Soc. 2019, 102, 930–935. [Google Scholar]
- Sun, C.; Shen, X.; Zhang, Y.; Wang, Y.; Chen, X.; Ji, C.; Shen, H.; Shi, H.; Wang, Y.; Yu, W.W. Highly luminescent, stable, transparent and flexible perovskite quantum dot gels towards light-emitting diodes. Nanotechnology 2017, 28, 36. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, Z.; Kong, L.; Zhu, N.; Shan, A.; Li, L. Enhancing the Stability of CH3NH3PbBr3 Quantum Dots by Embedding in Silica Spheres Derived from Tetramethyl Orthosilicate in “waterless” Toluene. J. Am. Chem. Soc. 2016, 138, 5749–5752. [Google Scholar] [CrossRef] [PubMed]
- Milstein, T.J.; Kluherz, K.T.; Kroupa, D.M.; Erickson, C.S.; Yoreo, J.J.D.; Gamelin, D.R. Anion Exchange and the Quantum-Cutting Energy Threshold in Ytterbium-Doped CsPb(Cl1–xBrx)3 Perovskite Nanocrystals. Nano Lett. 2019, 19, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Pandey, O.P.; Singh, K.; Homa, D.; Scott, B.; Pickrell, G. A review of bioactive glasses: Their structure, properties, fabrication and apatite formation. J. Biomed. Mater. Res. Part A 2014, 102, 254–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Luo, Y.; He, M.; Liang, X.; Xiang, W. Novel CsPbI3 QDs glass with chemical stability and optical properties. J. Eur. Ceram. Soc. 2017, 38, 1998–2004. [Google Scholar] [CrossRef]
- Diodes, M.L.; Br, C.X.; Dots, I.Q. Multicolour Light-emitting Diodes Based on CsPbX3 (X = Br, I) Quantum Dots Glasses Solid Materials. Mater. Lett. 2018, 229, 290–292. [Google Scholar]
- Li, P.; Cheng, Y.; Zhou, L.; Yu, X.; Jiang, J.; He, M.; Liang, X.; Xiang, W. Photoluminescence properties and device application of CsPb2Br5 quantum dots in glasses. Mater. Res. Bull. 2018, 105, 63–67. [Google Scholar] [CrossRef]
- He, M.; Ding, L.; Liu, S.; Shao, G.; Zhang, Z.; Liang, X.; Xiang, W. Superior fluorescence and high stability of B-Si-Zn glasses based on Mn-doped CsPbBrxI3-x nanocrystals. J. Alloy. Compd. 2018, 780, 318–325. [Google Scholar] [CrossRef]
- Jiang, J.; Shao, G.; Zhang, Z.; Ding, L.; Zhang, H.; Liu, J.; Chen, Z.; Xiang, W.; Liang, X. Ultrastability and color-tunability of CsPb(Br/I)3 nanocrystals in P-Si-Zn glass for white LEDs. Chem. Commun. 2018, 54, 12302–12305. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Hu, Z.; Jiang, J.; He, M.; Zhou, L.; Xiang, W.; Liang, X. Use of long-term stable CsPbBr3 perovskite quantum dots in phospho-silicate glass for highly efficient white LEDs. Chem. Commun. 2017, 53, 11068–11071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ye, Y.; Liu, C.; Zhao, Z.; Wang, J.; Han, J.; Zhao, X. Revealing the Effects of Defects on Ultrafast Carrier Dynamics of CsPbI3 Nanocrystals in Glass. J. Phys. Chem. C 2019, 123, 15851–15858. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, D.; Li, X.; Zhong, J.; Xu, X. Functional Inorganic Materials and Devices In Situ Crystallization Synthesis of CsPbBr3 Perovskite Quantum Dots Embedded Glasses with Improved Stability for Solid-State-Lighting and Random Upconverted Lasing. ACS Appl. Mater. Interfaces 2018, 10, 18918–18926. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, J.; Chen, H.; Chen, J.; Zhu, R.; Ma, P.; Towers, A.; Lin, Y.; Gesquiere, A.J.; Wu, S.T. Ultrastable, Highly Luminescent Organic–Inorganic Perovskite–Polymer Composite Films. Adv. Mater. 2016, 28, 10710–10717. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Guo, H.; DeQuilettes, D.W.; Jariwala, S.; De Marco, N.; Dong, S.; DeBlock, R.; Ginger, D.S.; Dunn, B.; Wang, M. Polymer-modified halide perovskite films for efficient and stable planar heterojunction solar cells. Sci. Adv. 2017, 3, 1700106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, Y.; Zhao, H.; Zhang, J. Highly Stable and Luminescent Perovskite-Polymer Composites from a Convenient and Universal Strategy. ACS Appl. Mater. Interfaces 2018, 10, 4971–4980. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Guo, S.; Cao, S.; Wang, L.; Gao, F.; Yang, Z.; Zheng, J.; Yang, W. A General Strategy for In Situ Growth of All-Inorganic CsPbX3 (X = Br, I, and Cl) Perovskite Nanocrystals in Polymer Fibers toward Significantly Enhanced Water/Thermal Stabilities. Adv. Opt. Mater. 2018, 6, 1800346. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Liao, Q.; Xu, Z.; Li, H.; Zheng, L.; Fu, H. Embedding Perovskite Nanocrystals into a Polymer Matrix for Tunable Luminescence Probes in Cell Imaging. Adv. Funct. Mater. 2017, 27, 1604382. [Google Scholar] [CrossRef]
- Raja, S.N.; Bekenstein, Y.; Koc, M.A.; Fischer, S.; Zhang, D.; Lin, L.; Ritchie, R.O.; Yang, P.; Alivisatos, A.P. Encapsulation of Perovskite Nanocrystals into Macroscale Polymer Matrices: Enhanced Stability and Polarization. ACS Appl. Mater. Interfaces 2016, 8, 35523–35533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.; Huang, Y.; Xu, W.; Yao, Q.; Liu, X.; Ding, C.; Chen, X. Cesium lead halide perovskite nanocrystals for ultraviolet and blue light blocking. Chin. Chem. Lett. 2018, 30, 1021–1023. [Google Scholar] [CrossRef]
- Fang, M.; Huang, S.; Li, D.; Jiang, C.; Tian, P.; Lin, H.; Luo, C.; Yu, W.; Peng, H. Stretchable and self-healable organometal halide perovskite nanocrystal-embedded polymer gels with enhanced luminescence stability. Nanophotonics 2018, 7, 1949–1958. [Google Scholar] [CrossRef]
- Wei, Y.; Deng, X.; Xie, Z.; Cai, X.; Liang, S.; Ma, P.; Hou, Z.; Cheng, Z.; Lin, J. Enhancing the Stability of Perovskite Quantum Dots by Encapsulation in Crosslinked Polystyrene Beads via a Swelling–Shrinking Strategy toward Superior Water Resistance. Adv. Funct. Mater. 2017, 27, 1703535. [Google Scholar] [CrossRef]
- Li, Y.; Lv, Y.; Guo, Z.; Dong, L.; Zheng, J.; Chai, C.; Chen, N.; Lu, Y.; Chen, C. One-Step Preparation of Long-Term Stable and Flexible CsPbBr3 Perovskite Quantum Dots/Ethylene Vinyl Acetate Copolymer Composite Films for White Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 15888–15894. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh-Khajehmarjan, E.; Nikniazi, A.; Olyaeefar, B.; Ahmadi-Kandjani, S.; Nunzi, J.-M. Bulk luminescent solar concentrators based on organic-inorganic CH3NH3PbBr3 perovskite fluorophores. Sol. Energy Mater. Sol. Cells 2019, 192, 44–51. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, Y.; Benetti, D.; Ma, D.; Rosei, F. Perovskite quantum dots integrated in large-area luminescent solar concentrators. Nano Energy 2017, 37, 214–223. [Google Scholar] [CrossRef]
- Lin, C.C.; Jiang, D.H.; Kuo, C.C.; Cho, C.J.; Tsai, Y.H.; Satoh, T.; Su, C. Water-Resistant Efficient Stretchable Perovskite-Embedded Fiber Membranes for Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Hai, J.; Li, H.; Zhao, Y.; Chen, F.; Peng, Y.; Wang, B. Designing of blue, green, and red CsPbX3 perovskite-codoped flexible films with water resistant property and elimination of anion-exchange for tunable white light emission. Chem. Commun. 2017, 53, 5400–5403. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shi, T.; Du, J.; Zang, Z.; Yao, Z.; Li, M.; Sun, K.; Hu, W.; Leng, Y.; Tang, X. Highly Stable Silica-Wrapped Mn-Doped CsPbCl3 Quantum Dots for Bright White Light-Emitting Devices. ACS Appl. Mater. Interfaces 2018, 10, 43978–43986. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Cao, M.; Hu, H.; Yang, D.; Chen, M.; Li, P.; Wu, L.; Zhang, Q. One-pot synthesis of highly stable CsPbBr3@SiO2 core-shell nanoparticles. ACS Nano 2018, 12, 8579–8587. [Google Scholar] [CrossRef] [PubMed]
- Loiudice, A.; Strach, M.; Saris, S.; Chernyshov, D.; Buonsanti, R. Universal Oxide Shell Growth Enables in Situ Structural Studies of Perovskite Nanocrystals during the Anion Exchange Reaction. J. Am. Chem. Soc. 2019, 141, 8254–8263. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yang, J.; Li, S.; Liu, Z.; Hu, Z.; Hao, J.; Du, J.; Leng, Y.; Qin, H.; Lin, X. Single Halide Perovskite/Semiconductor Core/Shell Quantum Dots with Ultrastability and Nonblinking Properties. Adv. Sci. 2019, 6, 1900412. [Google Scholar] [CrossRef] [PubMed]
- Hintermayr, V.; Lampe, C.; Löw, M.; Roemer, J.; Vanderlinden, W.; Gramlich, M.; Böhm, A.X.; Sattler, C.; Nickel, B.; Lohmueller, T. Polymer nanoreactors shield perovskite nanocrystals from degradation. Nano Lett. 2019, 19, 4928–4933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innocenzi, P. The Sol to Gel Transition; Briefs Mater; Springer: Berlin, Germany, 2016. [Google Scholar]
- Ding, N.; Zhou, D.; Sun, X.; Xu, W.; Xu, H.; Pan, G.; Li, D.; Zhang, S.; Dong, B.; Song, H. Highly stable and water-soluble monodisperse CsPbX3/SiO2 nanocomposites for white-LED and cells imaging. Nanotechnology 2018, 29, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shi, Z.; Li, S.; Ma, Z.; Li, Y.; Wang, L.; Wu, D.; Tian, Y.; Du, G.; Li, X. Synergetic Effect of the Surfactant and Silica Coating on the Enhanced Emission and Stability of Perovskite Quantum Dots for Anticounterfeiting. ACS Appl. Mater. Interfaces 2019, 11, 28013–28022. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Hofman, E.; Li, J.; Davis, A.H.; Tung, C.H.; Wu, L.Z.; Zheng, W. Photoelectrochemically active and environmentally stable CsPbBr3/TiO2 core/shell nanocrystals. Adv. Funct. Mater. 2018, 28, 1704288. [Google Scholar] [CrossRef]
- Tang, X.; Yang, J.; Li, S.; Chen, W.; Hu, Z.; Qiu, J. CsPbBr3/CdS Core/Shell Structure Quantum Dots for Inverted Light-Emitting Diodes Application. Front. Chem. 2019, 7, 499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Zhang, C.; Huang, S.; Li, Z.; Kong, L.; Jin, L.; Wang, J.; Wu, K.; Li, L. Postsynthesis Phase Transformation for CsPbBr3/Rb4PbBr6 Core/Shell Nanocrystals with Exceptional Photostability. ACS Appl. Mater. Interfaces 2018, 10, 23303–23310. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Song, P.; Cao, J.; Zhao, S.; Shen, Z.; Di, G.; Liang, Z.; Xu, Z.; Song, D.; Xu, X. Water-resistant, monodispersed and stably luminescent CsPbBr3/CsPb2Br5 core-shell-like structure lead halide perovskite nanocrystals. Nanotechnology 2017, 28, 44. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Li, H.; Meng, X.; Li, H. CsPbX3/Cs4PbX6 core/shell perovskite nanocrystals. Chem. Commun. 2018, 54, 6300–6303. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bi, C.; Yuan, J.; Zhang, L.; Tian, J. Original Core-Shell Structure of Cubic CsPbBr3@Amorphous CsPbBrx Perovskite Quantum Dots with a High Blue Photoluminescence Quantum Yield of over 80%. ACS Energy Lett. 2018, 3, 245–251. [Google Scholar] [CrossRef]
- Xu, K.; Lin, C.C.; Xie, X.; Meijerink, A. Efficient and Stable Luminescence from Mn2+ in Core and Core-Isocrystalline Shell CsPbCl3 Perovskite Nanocrystals. Chem. Mater. 2017, 29, 4265–4272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaumik, S.; Veldhuis, S.A.; Ng, Y.F.; Li, M.; Muduli, S.K.; Sum, T.C.; Damodaran, B.; Mhaisalkar, S.; Mathews, N. Highly stable, luminescent core-shell type methylammonium-octylammonium lead bromide layered perovskite nanoparticles. Chem. Commun. 2016, 52, 7118–7121. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, T.; Jiang, C.; Qi, R. Luminescent CH3NH3PbBr3/β-cyclodextrin Core/Shell Nanodots with Controlled Size and Ultrastability Through Host-guest Interaction. ChemNanoMat. 2019, 1900381. [Google Scholar]
- Mary Vijila, C.V.; Rajeev Kumar, K.; Jayaraj, M.K. Stokes shift engineered, stable core-shell perovskite nanoparticle – Poly(methyl methacrylate) composites with high photoluminescence quantum yield. Opt. Mater. 2019, 94, 241–248. [Google Scholar] [CrossRef]
- Ushakova, E.; Matuhina, A.; Sokolova, A.; Cherevkov, S.A.; Dubavik, A.; Medvedev, O.; Litvin, A.; Kurdyukov, D.; Golubev, V.; Baranov, A.V. Enhanced stability of the optical responses from the all-inorganic perovskite nanocrystals embedded in synthetic opal matrix. Nanotechnology 2019, 30, 40. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, B.; Wang, Z.; Hung, T.F.; Susha, A.S.; Zhong, H.; Rogach, A.L. Water resistant CsPbX3 nanocrystals coated with polyhedral oligomeric silsesquioxane and their use as solid state luminophores in all-perovskite white light-emitting devices. Chem. Sci. 2016, 7, 5699–5703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirershadi, S.; Ahmadi-Kandjani, S. Efficient thin luminescent solar concentrator based on organometal halide perovskite. Dye. Pigment. 2015, 120, 15–21. [Google Scholar] [CrossRef]
- Zhou, Q.; Bai, Z.; Lu, W.G.; Wang, Y.; Zou, B.; Zhong, H. In Situ Fabrication of Halide Perovskite Nanocrystal-Embedded Polymer Composite Films with Enhanced Photoluminescence for Display Backlights. Adv. Mater. 2016, 28, 9163–9168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wei, J.; Li, H.; Yan, Y.; Zhou, W.; Yu, D.; Zhao, Q. A polymer scaffold for self-healing perovskite solar cells. Nat. Commun. 2016, 7, 10228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, Y.; Zhou, Y.; Zhang, Y.; Li, Z.; Zhang, L.; Ju, M.G.; Chen, M.; Pang, S.; Zeng, X.C.; Padture, N.P. Continuous Grain-Boundary Functionalization for High-Efficiency Perovskite Solar Cells with Exceptional Stability. Chem 2018, 4, 1404–1415. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Song, Z.; Zhao, D.; Awni, R.A.; Li, C.; Shrestha, N.; Chen, C.; Yin, X.; Li, D.; Ellingson, R.J. Improving Performance and Stability of Planar Perovskite Solar Cells through Grain Boundary Passivation with Block Copolymers. Sol. RRL 2019, 3, 1900078. [Google Scholar] [CrossRef] [Green Version]
- Meyns, M.; Perálvarez, M.; Heuer-Jungemann, A.; Hertog, W.; Ibáñez, M.; Nafria, R.; Genç, A.; Arbiol, J.; Kovalenko, M.V.; Carreras, J. Polymer-Enhanced Stability of Inorganic Perovskite Nanocrystals and Their Application in Color Conversion LEDs. ACS Appl. Mater. Interfaces 2016, 8, 19579–19586. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Material | Crystal Structure | Optical Transition | Bandgap, eV | Ref. |
|---|---|---|---|---|
| CsSnX3 (X = Br, Cl, I) | Cubic (Cl), orthorhombic (Br, I) | Hot injection or anion exchange | High hole mobility; Tunable bandgap through Vis/NIR | [24,91,92,93] |
| Cs2SnI6 | Defect-variant of ABX3 perovskite structure | Phosphine-free hot injection | Stable films; direct bandgap (1.48 eV); high absorption coefficient; tunable morphology; tested in solar cells and FETs | [25,94] |
| MA3Bi2X9 (X = Cl, Br, I) | Distorted-layered structure | Collaborative solvent LARP (Co-LARP) | PLQY = 15% (X = Br); tunable PL peaks from 360 to 540 nm; good ethanolic stability; minor re-absorption effect | [95] |
| Cs3Bi2X9 (X = Br, Cl, I) | Vacancy-ordered ABX3 perovskite structure | Hot injection [96] or room T synthesis [97] | Widely-tunable absorbance; air stable | [96,97] |
| Cs3Sb2X9 (X = Cl, I) | Trigonal and orthorhombic phases | Ionic metathesis process (X = Cl) | Band-edge emission in the yellow-red (X = I); Sharp band-edge excitonic emission | [27,98] |
| Cs2InSbCl6 | - | - | Direct bandgap (1.0 eV); stable | [99] |
| Cs2AgBiX6 (X = Cl, Br, I) | Elpasolite (X = Cl, Br) | Hot injection (X = Cl, Br), anion exchange reaction (X = I) | Stable, strong absorption throughout the visible region. Indirect bandgap | - |
| Cs2InSbCl6 | - | - | Direct bandgap (1.0 eV); stable | [99] |
| Cs2AgInxBi1−xCl6 (x = 0.75 and 0.9) | Fm3m cubic space group | Anti-solvent recrystallization | Direct bandgap; PLQY = 36.6%; bright dual color (violet and orange) emission | [100] |
| Composition | Method | PLQY of Composite, % | UV Resistance, h | Thermal Stability, °C | Water Resistance, h | Stability under Ambient Condition, Days | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Dramatic Changes | Full Degradation | Dramatic Changes | Full Degradation | Dramatic Changes | Full Degradation | |||||
| CsPbBr3 | Mesoporous particles (SiO2) | 46.2 | >120 | >120 | 95 | >125 | >120 | >120 | >30 | [138] |
| CsPbBr3 | Mesoporous particles (SiO2) | - | >96 (6 W) | >96 (6 W) | >100 | >100 | - | - | - | [133] |
| Mn-doped-CsPbBr0.5I2.5 | Mesoporous particles (SiO2) | - | - | - | - | - | - | >240 | >10 | [139] |
| Mn-doped-CsPbBr0.5I2.5 | Mesoporous particles (Al2O3) | - | - | - | - | - | - | 72 | 5 | [139] |
| CsPbBr3 | polyhedral-oligomeric-silsesquioxane-(POSS) | 61 | - | - | - | - | - | >1680 | - | [185] |
| CsPb(Br/I)3 | 45 | - | - | - | - | - | >1680 | - | [185] | |
| CsPbI3 | Glass encapsulation (B2O3-SiO2-ZnO) | 4.2 | - | - | - | - | - | - | >8.3 * | [144] |
| CsPbBr3 | Glass encapsulation (B2O3-SiO2-ZnO) | 42.5 | - | - | - | - | - | - | >60- | [145] |
| CsPbBr1.5I1.5 | Glass encapsulation (B2O3-SiO2-ZnO) | 15.5 | - | - | - | - | - | - | >60 | [145] |
| CsPbI3 | Glass encapsulation (B2O3-SiO2-ZnO) | 17.6 | - | - | - | - | - | - | >60 | [145] |
| CsPb2Br5 | Glass encapsulation (ZnO-SiO2-B2O3) | 30.6 | - | - | 60 | >160 | - | - | - | [146] |
| CsPbBr1.2I1.8 | Glass encapsulation (P2O5-SiO2-ZnO) | - | >500 | >500 | 89 | 180 | >960 | >960 | >20.8 | [148] |
| CsPbBr3 | Glass encapsulation (P2O2-SiO2-ZnO)- | 42 | >144 | >144 | >125 | >125 | 24 | - | >30 | [149] |
| CsPbBr3 | Glass encapsulation (TeO2-Al2O3-H3BO3-ZnO-Na2CO3) | 72 | >20 (20 W) | >20 (20 W) | 80 | 180 | >1080 | >1080 | >45 | [151] |
| Mn-doped-CsPbBr1.0I2.0 | Glass encapsulation (B2O3-SiO2-ZnO) | - | - | - | 81 | >107 | - | - | >70 | [147] |
| CsPb0.64Sn0.36Br3 | Glass encapsulation (B2O3-SiO2-ZnO) | 43 | - | - | 140 | >220 | - | - | >100 * | [136] |
| CsPbBr3 | Porous glass encapsulation (SiO2-B2O3-Na2O-CaO) | 28 | >12 (65 mW) | >12 (65 mW) | 77 | 190 | >0.17 | - | - | [134] |
| CsPb(Cl0.5Br0.5)3 | Porous glass encapsulation (SiO2-B2O3-Na2O-CaO) | 2.6 | 8 (65 mW) | >12 (65 mW) | 158 | 280 | - | - | - | [134] |
| CsPb(Br0.4I0.6)3 | Porous glass encapsulation (SiO2-B2O3-Na2O-CaO) | 8.9 | 6 (65 mW) | >6 (65 mW) | 54 | 140 | - | - | - | [134] |
| CsPbX3 (X = Cl/Br, Br, I) | Porous glass encapsulation (opal matrix) | - | - | - | - | - | - | - | >30 | [184] |
| CsPb(Cl0.5Br0.5)3 | Polymer matrix (SBS) | 10.8 | - | - | - | - | 0.12 | 1 | - | [164] |
| CsPb(Br0.8I0.2)3 | Polymer matrix (SBS) | 23 | - | - | - | - | 0.17 | 1 | - | [164] |
| CsPb(Br0.6I0.4)3 | Polymer matrix (SBS) | 14.6 | - | - | - | - | 0.1 | 0.5 | - | [164] |
| CsPb(Br0.4I0.6)3 | Polymer matrix (SBS) | 12.2 | - | - | - | - | 0.12 | 0.67 | - | [164] |
| (CsPb(Cl/Br)3) | Polymer matrix (ethylcellulose) | - | - | - | - | - | - | - | 6 | [158] |
| MAPbBr3 | Polymer matrix (MMA:PMMA) | - | 12 (9 W) | >24 (9 W) | - | - | - | - | - | [162] |
| CsPbBr3 | Polymer matrix (PLMA EGDA) | - | 14 (0.1 W/cm2) | >14 (0.1 W/cm2) | - | - | - | - | - | [163] |
| CsPb(Br0.2I0.8)3 | Polymer matrix (PLMA EGDA) | - | >14 (0.1 W/cm2) | >14 (0.1 W/cm2) | - | - | - | - | >150 | [163] |
| MAPbBr3 | Polymer matrix (PS) | 34 | - | - | 70 | >100 | - | >1440 | >150 | [152] |
| MAPbBr3 | Polymer matrix (PC) | 31 | - | - | 60 | >180 | - | >1440 | >150 | [152] |
| MAPbBr3 | Polymer matrix (ABS) | 48 | - | - | 75 | >100 | - | >1440 | >150 | [152] |
| MAPbBr3 | Polymer matrix (CA) | 47 | - | - | - | - | - | >48 | >150 | [152] |
| MAPbBr3 | Polymer matrix (PVC) | 16 | - | - | - | - | - | >1440 | >150 | [152] |
| MAPbBr3 | Polymer matrix (PMMA) | - | - | - | - | - | - | 0 | 1 | [152] |
| MAPbBr3 | Polymer matrix (PDMS-urea) | 23.8 | - | - | - | - | >216 | - | - | [159] |
| MAPbI3 | Polymer matrix (PDMS-urea) | - | - | - | - | - | 24 | >48 | - | [159] |
| CsPbBr3 | Polymer matrix (EVA) | 40.5 | >54 | - | 65 | >75 | >240 | >720 | >8 | [161] |
| CsPbBr3 | Polymer matrix (PS) | 48 | - | - | - | - | - | >720 | - | [155] |
| CsPbBr3 | Polymer matrix (PVP/silicone resin) | 24 | >120 | - | >100 | - | - | >4 | >5 | [165] |
| CsPbBr3 | Polymer matrix (PVP/PS) | 27 | >10 | - | - | - | - | - | - | [156] |
| CsPbBr3 | Polymer matrix (SEBS) | - | - | >2.8 × 10−4 (50 kW/cm2) | - | - | - | >2928 | - | [157] |
| CsPbBr3 | Polymer matrix (PS) | 68 | 288 (16 W) | >384 (16 W) | - | - | 528 | >6480 | - | [160] |
| CsPbBr3 | Polymer matrix (PMMA) | 54.6 | - | - | - | >80 | >48 | - | >30 | [154] |
| CsPbBr3 | Polymer matrix (PBMA) | 62.2 | - | - | - | - | >48 | >720 | >30 | [154] |
| MAPbBr3 | Polymer matrix (PVA) | - | >2 (2 pW) | - | - | - | - | - | - | [186] |
| MAPbBr3 | Polymer matrix (PVDF) | 94.6 | - | >400 (6 W) | - | - | - | >400 | >9 * | [187] |
| MAPbI3 | Polymer matrix (PEG) | - | - | - | - | - | - | - | >12.5 * | [188] |
| MAPbI3 | Polymer matrix (P123) | - | - | - | - | - | - | - | >20 * | [189] |
| MA0.7FA0.3PbI3 | Polymer matrix (F127) | - | - | - | - | - | - | - | >10 | [190] |
| CsPbMnCl3 | Core/Shell (SiO2) | 55.4 | - | - | >100 | >100 | - | - | >15 | [166] |
| CsPbX3 | Core/Shell (SiO2) | 60 | 360 | >528 | 50 | 90 | >24 | - | - | [140] |
| CsPbCl3 | Core/Shell (SiO2) | 11.2 | - | - | - | - | - | - | >30 | [172] |
| CsPbBr3 | Core/Shell (SiO2) | 84 | - | - | - | - | - | - | >30 | [172] |
| CsPbI3 | Core/Shell (SiO2) | 45 | - | - | - | - | - | - | >60 | [172] |
| CsPb(Cl0.5/Br0.5)3 | Core/Shell (SiO2) | - | - | - | - | - | 2 | >240 | - | [172] |
| CsPbBr3 | Core/Shell (SiO2) | - | - | - | - | - | 2 | >240 | - | [172] |
| CsPb(Br0.3/I0.7)3 | Core/Shell (SiO2) | - | - | - | - | - | 1.3 | >240 | - | [172] |
| CsPbBr3 | Core/Shell (SiO2) | 90 | - | - | - | - | >0.7 ** | - | >28 * | [167] |
| CsPbBr3 | Core/Shell (LP/SiO2) | 90.5 | >168 (8 W) | - | 60 | 120 | - | >0.6 | >30 | [173] |
| CsPbBr3 | Core/Shell (TiO2) | - | >24 | - | - | - | >2160 | - | - | [174] |
| CsPbBr3 | Core/Shell (AlOx) | 75.3 | - | - | - | - | - | >168 | - | [168] |
| CsPbBr3 | Core/Shell (CsPb2Br5) | - | - | - | - | - | >72 | >120 | - | [177] |
| CsPbI3 | Core/Shell (Cs4PbI6) | - | - | - | - | - | - | - | >7 | [178] |
| CsPbBr3 | Core/Shell (amorphous CsPbBrx) | 84 | - | - | - | - | - | - | - | [179] |
| CsPbBr3 | Core/Shell (Rb4PbBr6) | 85 | >10 (175 mW/cm2) | - | - | - | - | - | - | [176] |
| Mn2+-doped CsPbCl3 | Core/Shell (CsPbCl3) | 40 | - | - | >110 | - | - | - | - | [180] |
| MAPbBr3 | Core/Shell ((C8H17NH3)2PbBr4) | 92 | - | - | - | - | - | - | >60 | [181] |
| CsPbBr3 | Core/Shell (PMA) | 53 | >12 | - | - | - | - | - | - | [191] |
| MAPbBr3 | Core/Shell (PMMA) | 88 | >7 | - | - | - | - | >18 | >365 | [183] |
| MAPbBr3 | Core/Shell (β-cyclodextrin) | 89.7 | >144 | - | - | - | >240 | - | - | [182] |
| MAPbI3 | Core/Shell (PS-b-P2VP)) | 55 | - | - | - | - | 312 | 1800 | >220 | [170] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Matuhina, A.; Zhang, H.; Vivo, P. Advances in the Stability of Halide Perovskite Nanocrystals. Materials 2019, 12, 3733. https://doi.org/10.3390/ma12223733
Liu M, Matuhina A, Zhang H, Vivo P. Advances in the Stability of Halide Perovskite Nanocrystals. Materials. 2019; 12(22):3733. https://doi.org/10.3390/ma12223733
Chicago/Turabian StyleLiu, Maning, Anastasia Matuhina, Haichang Zhang, and Paola Vivo. 2019. "Advances in the Stability of Halide Perovskite Nanocrystals" Materials 12, no. 22: 3733. https://doi.org/10.3390/ma12223733





