Rare Earth-Based Compounds as Inhibitors of Hot-Corrosion Induced by Vanadium Salts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Corrosive Medium
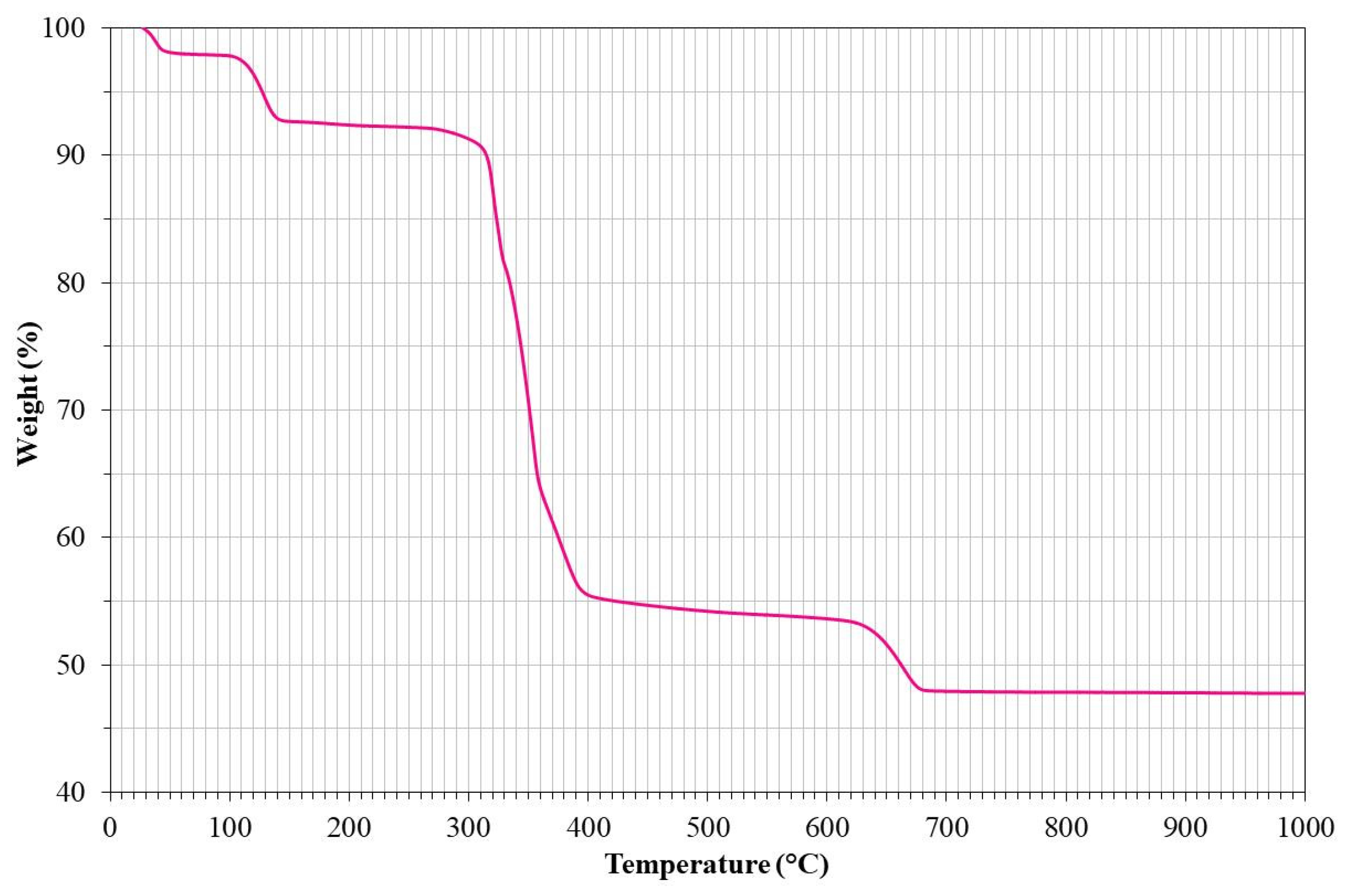
2.3. Corrosion Inhibitors
2.4. Corrosion Assays
3. Results and Discussion
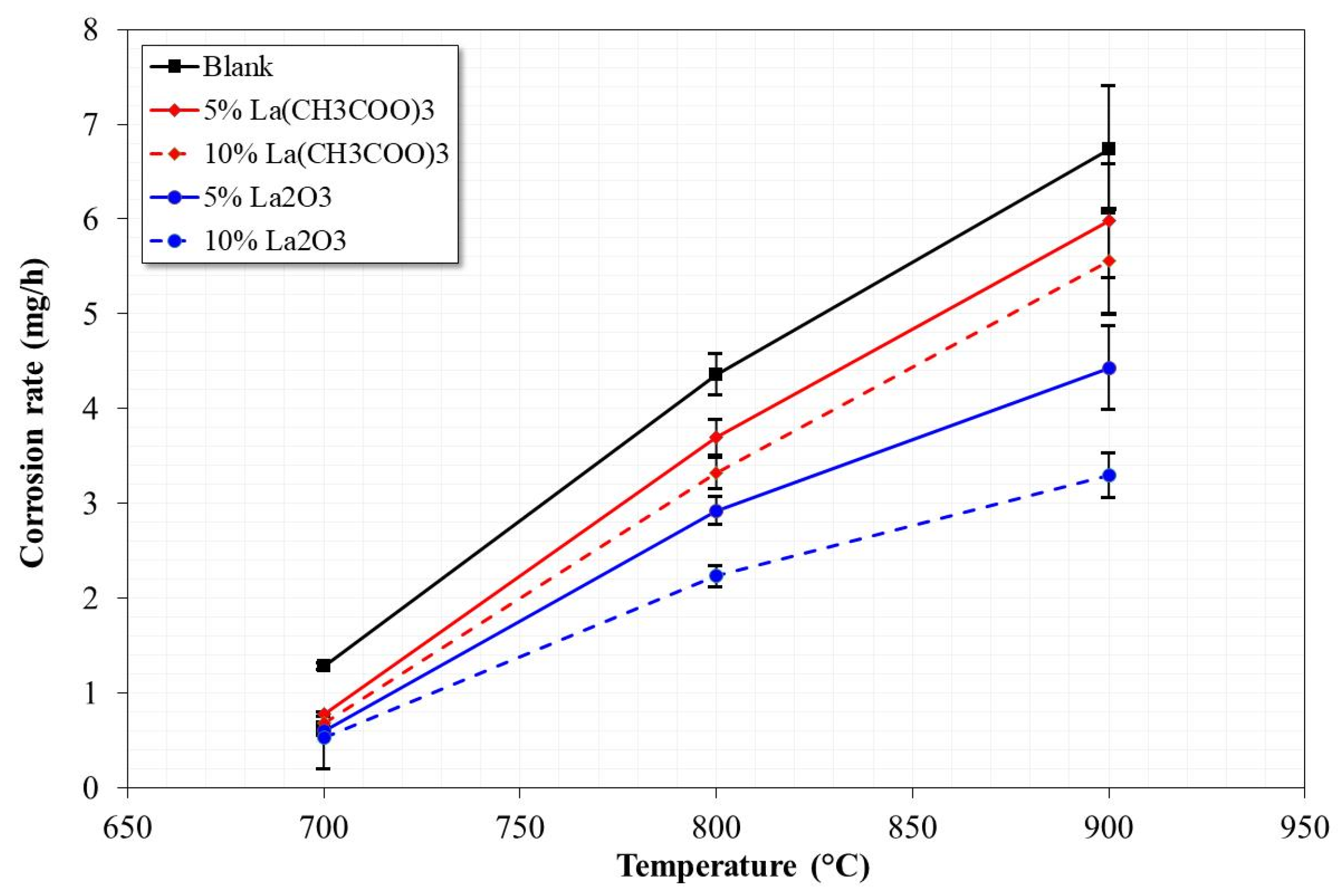
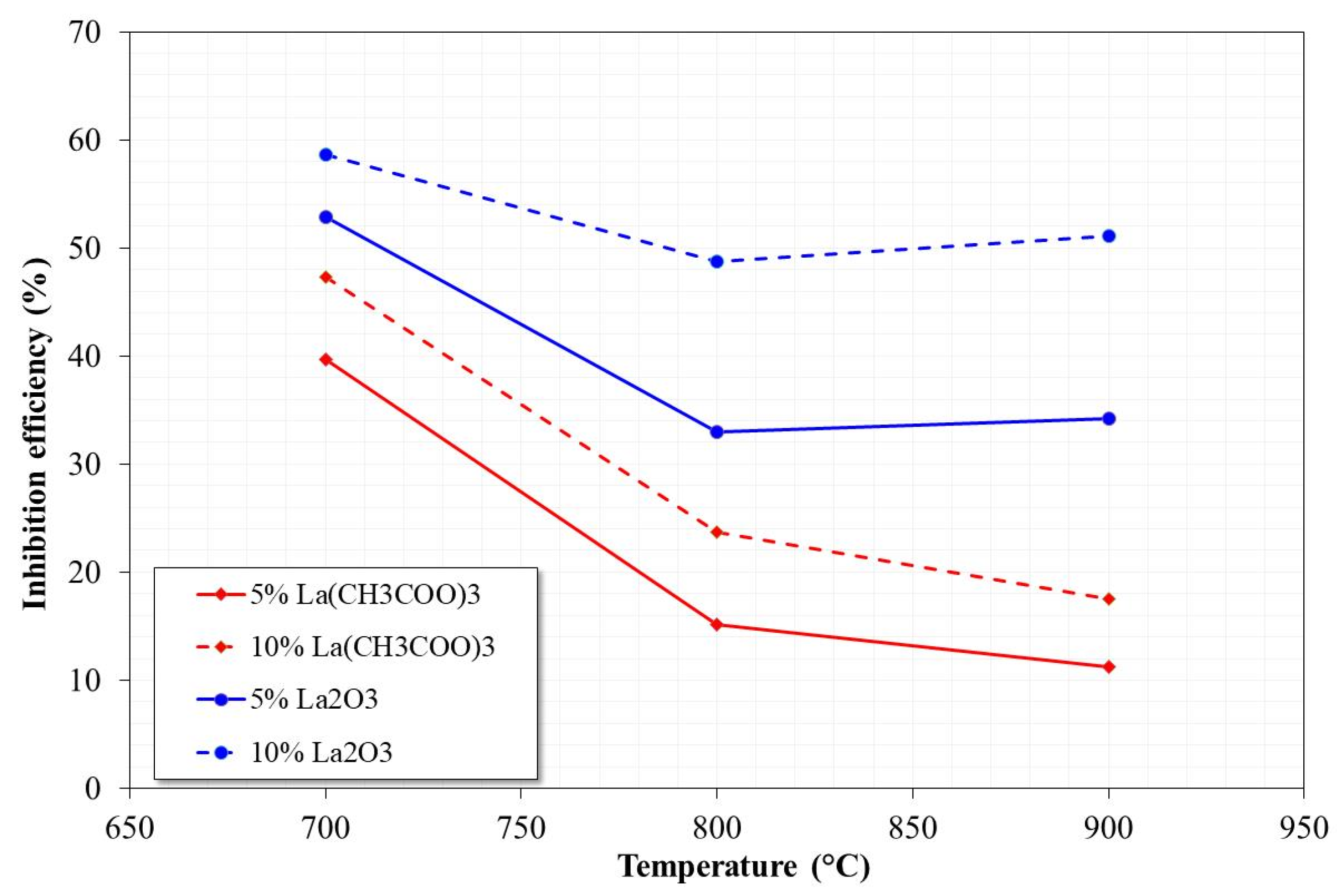
3.1. Mass Loss Tests
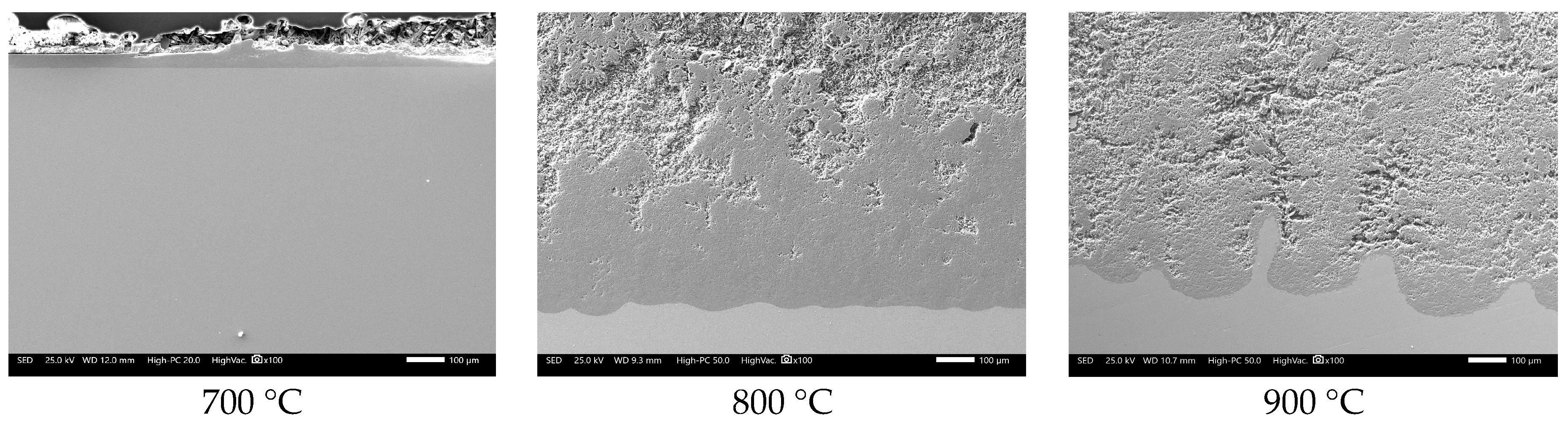
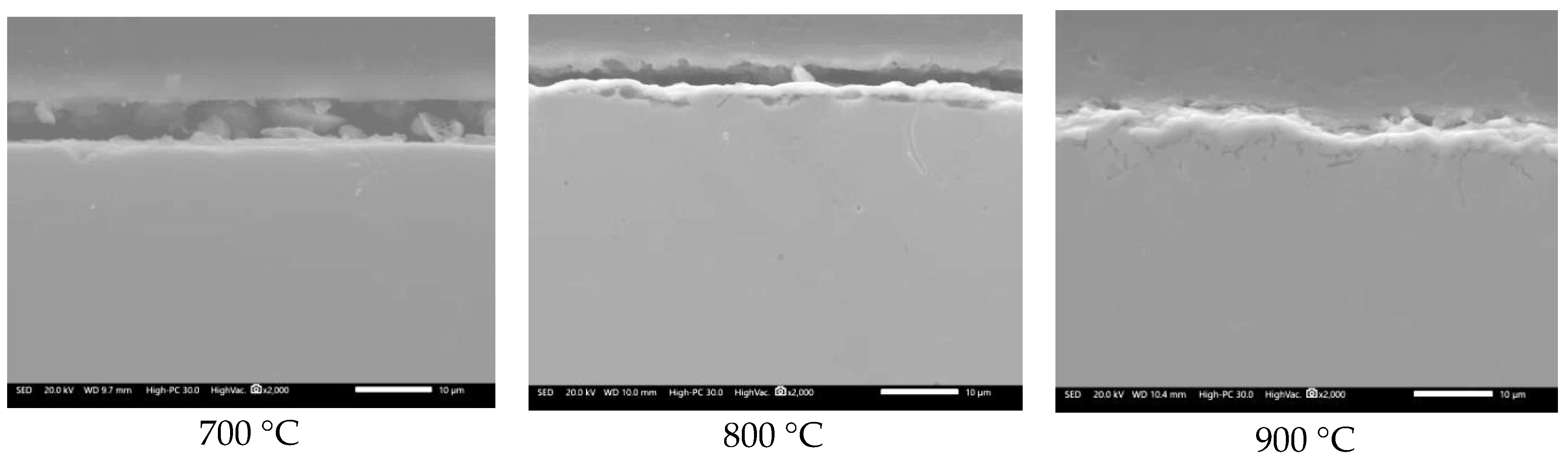
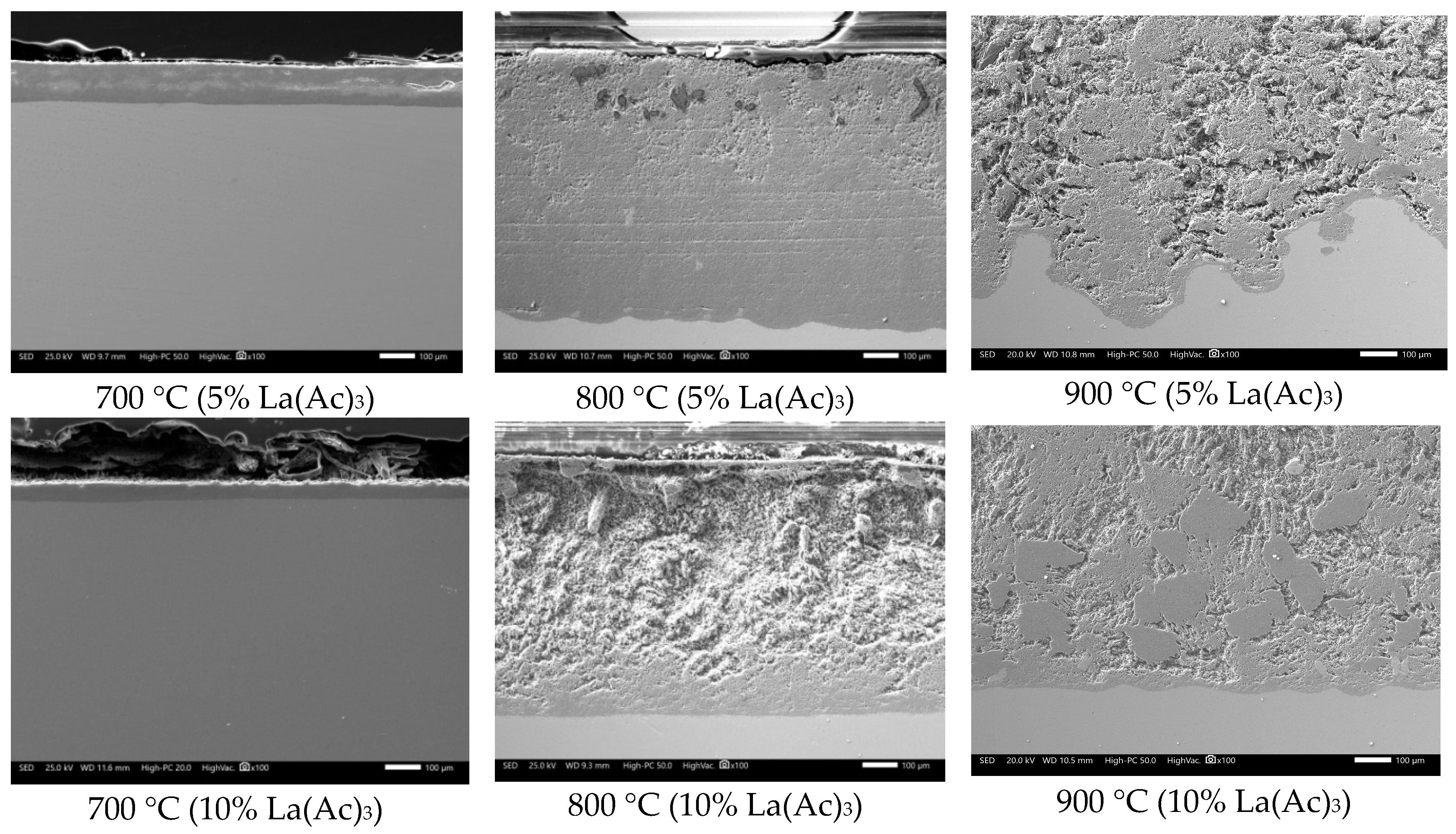
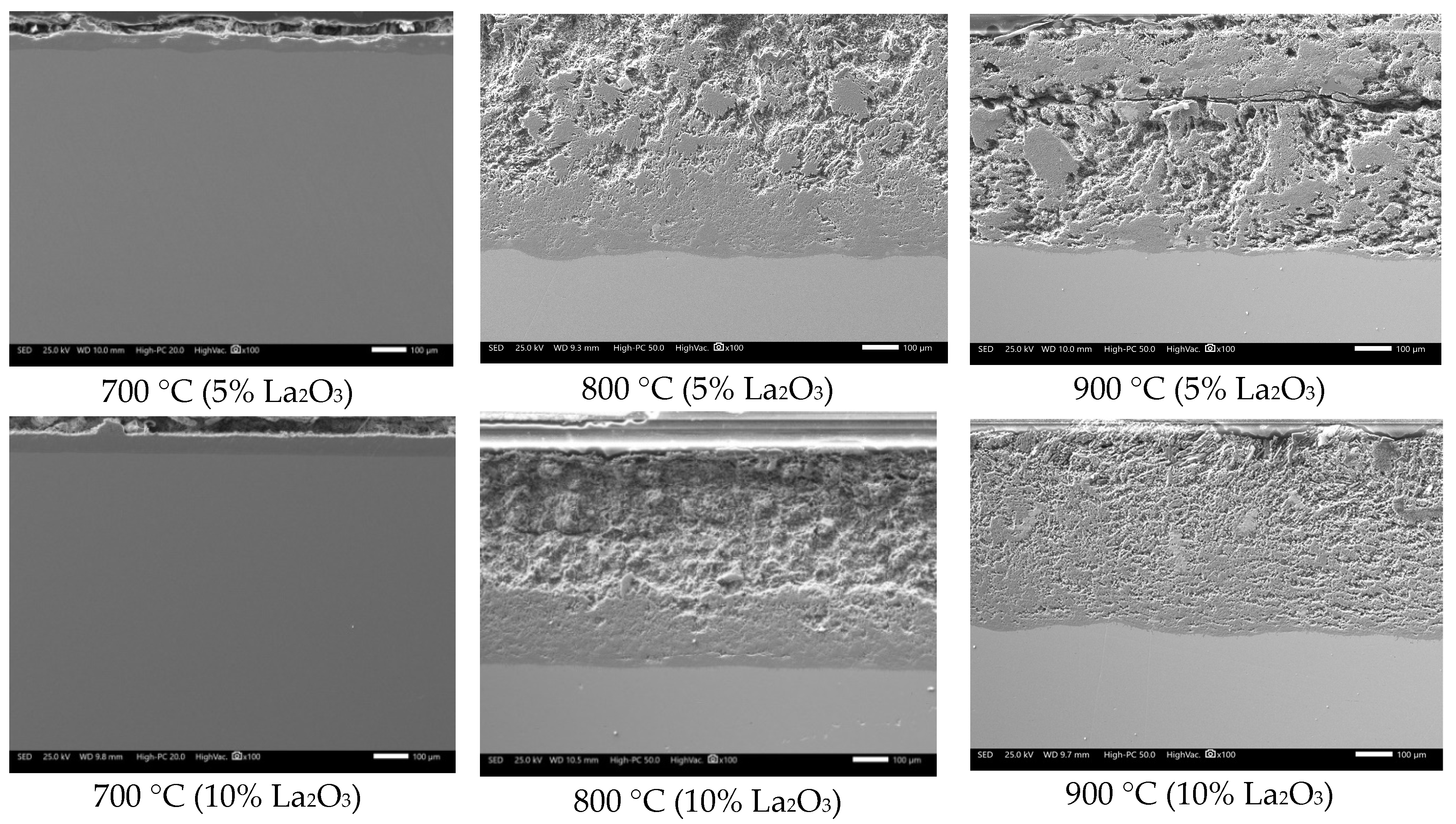
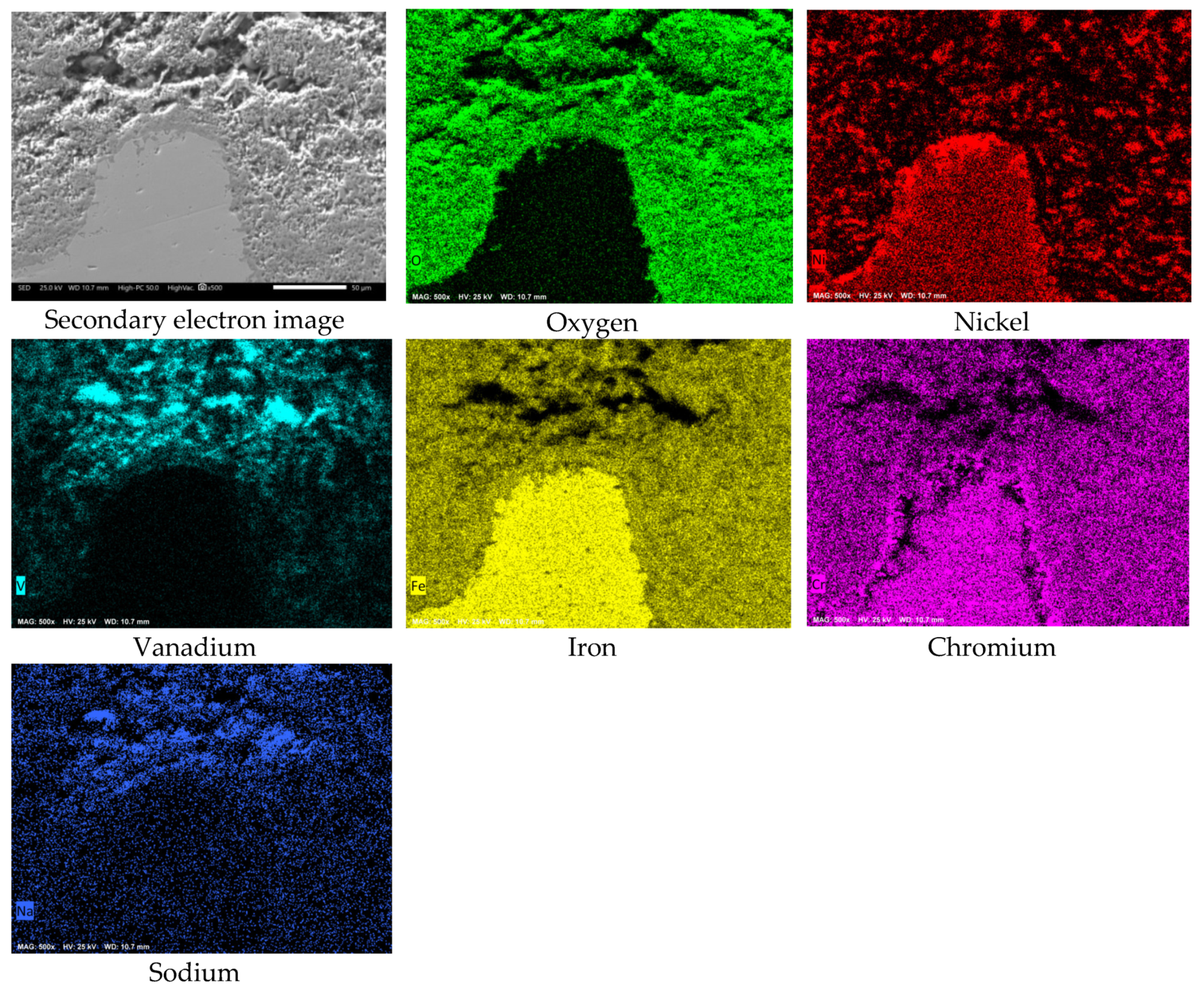
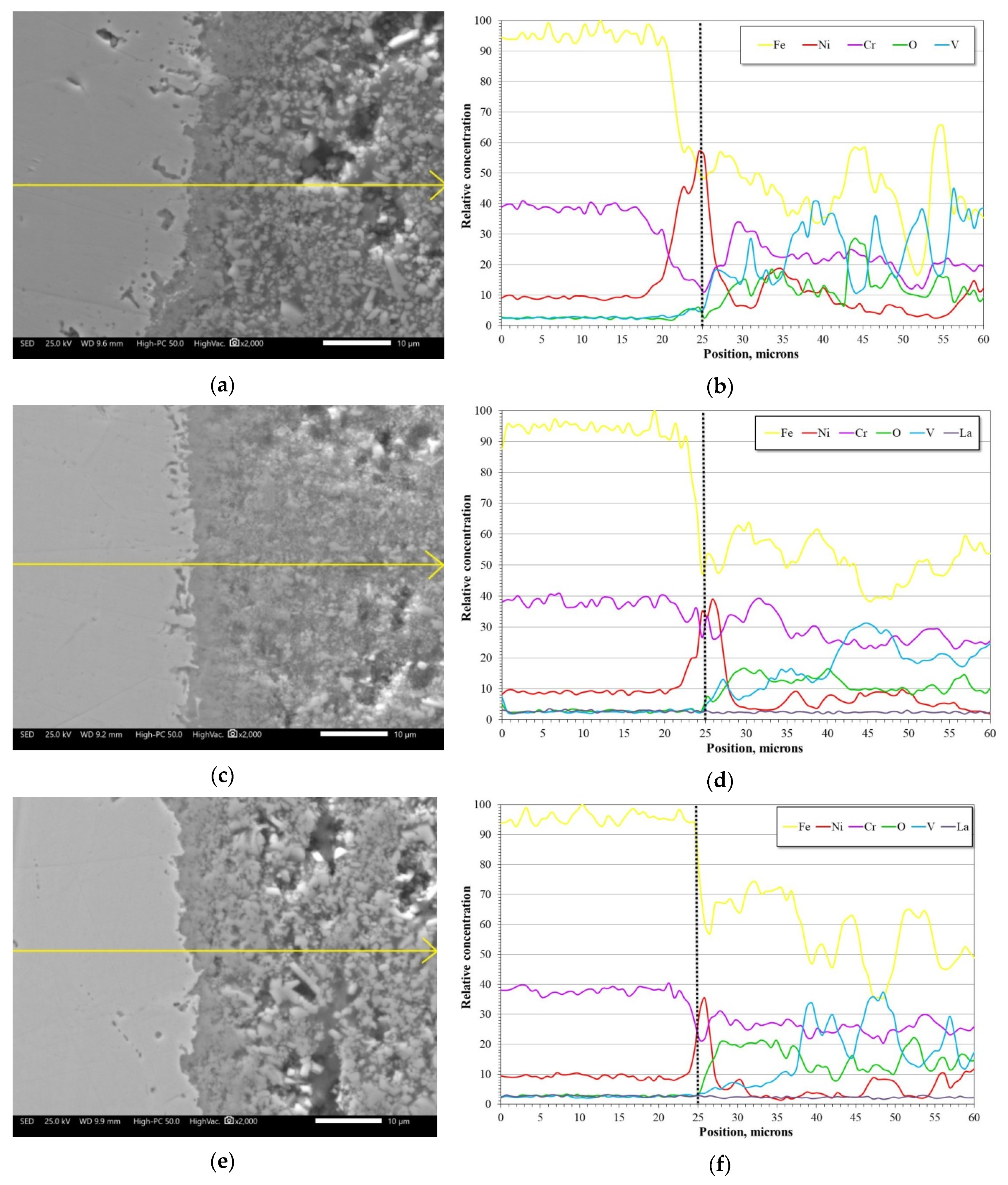
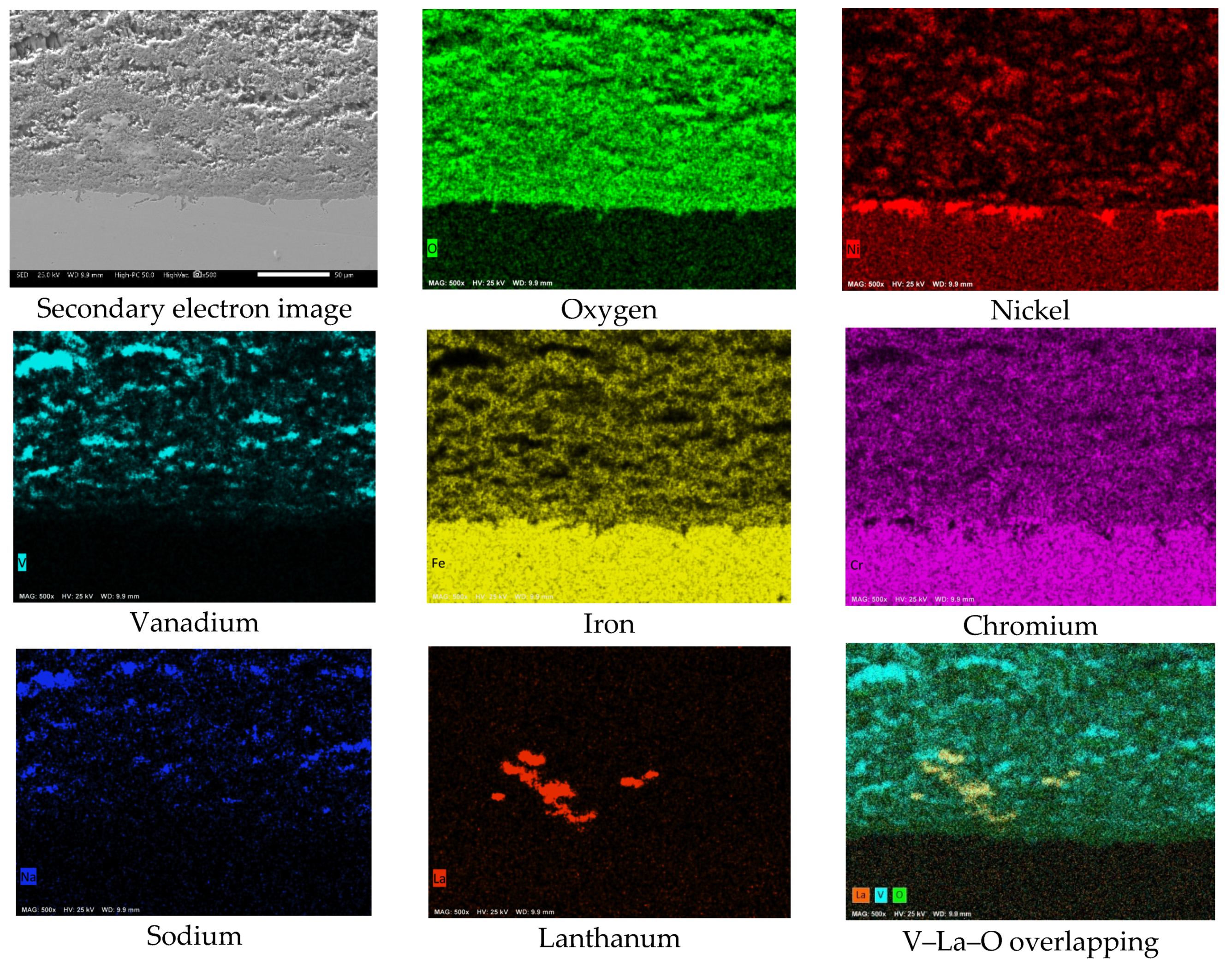
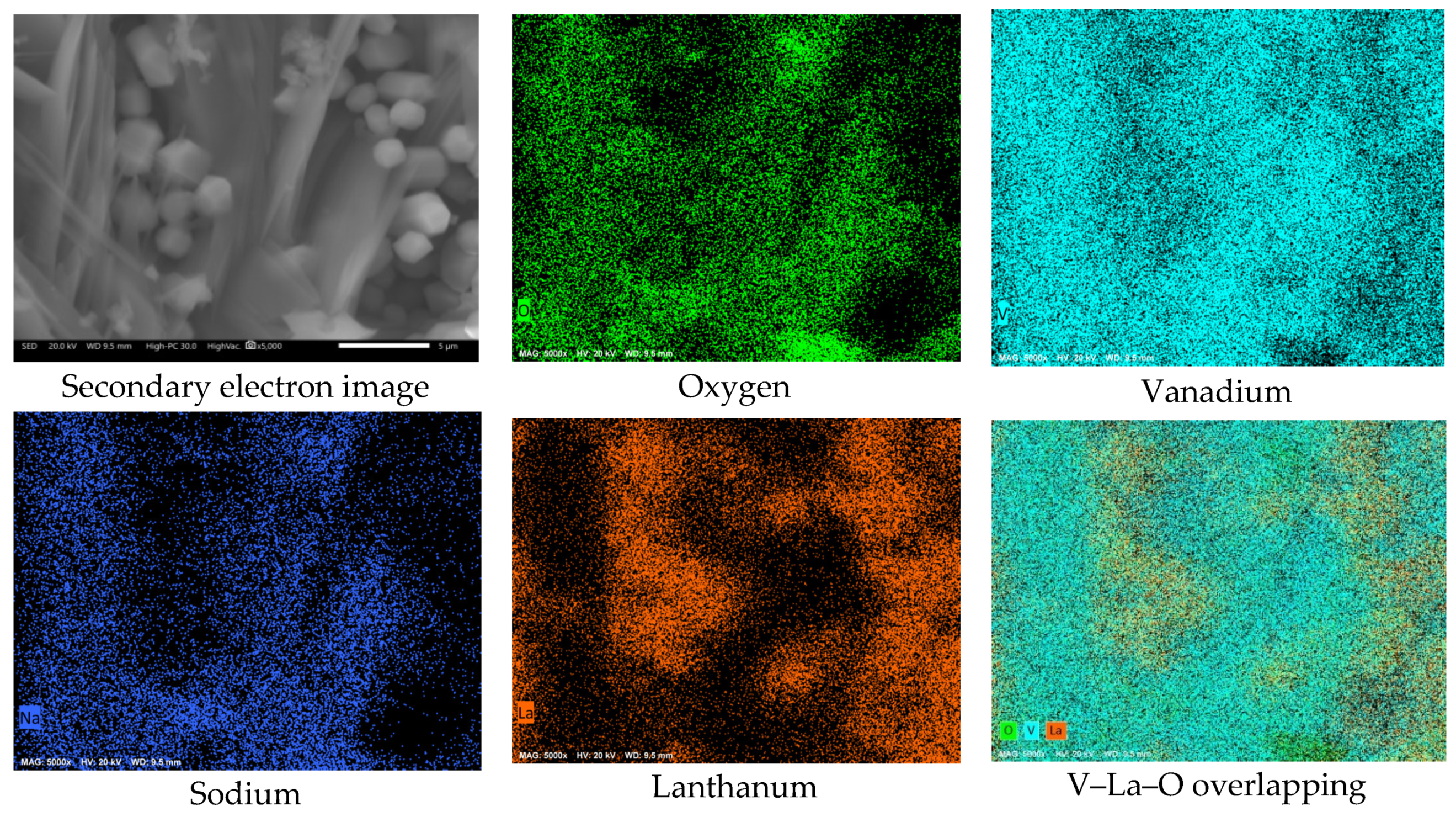
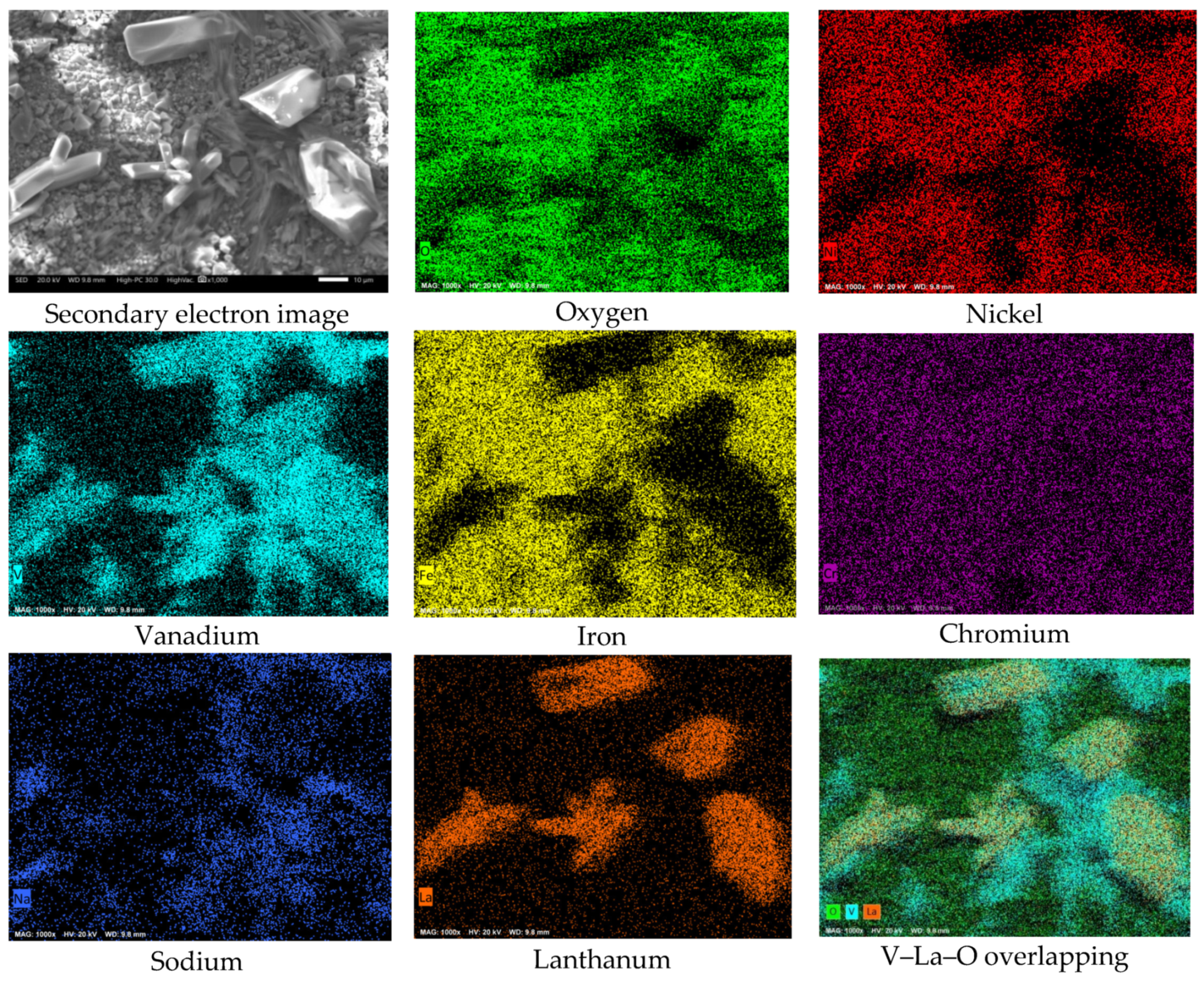
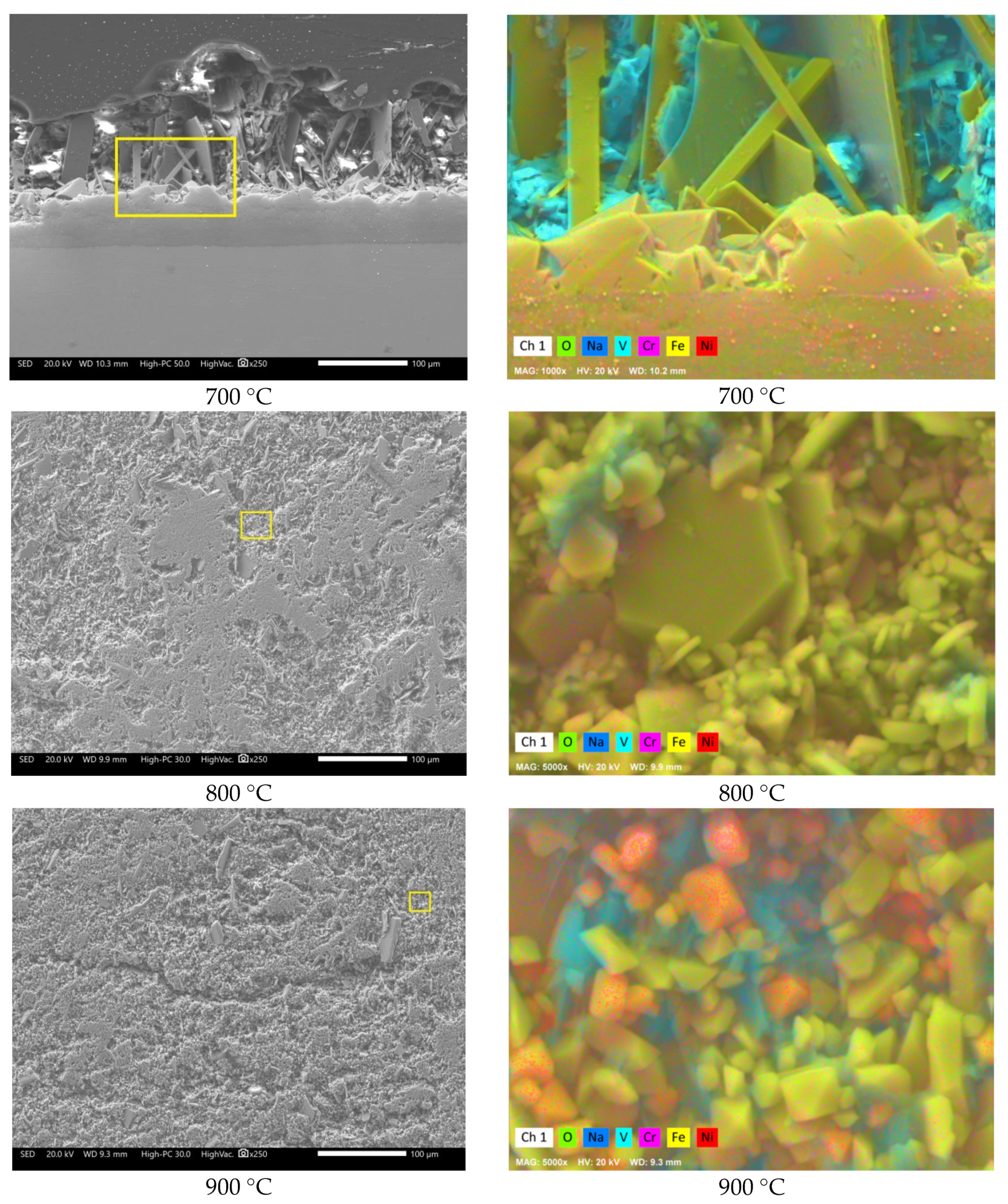
3.2. SEM Analysis
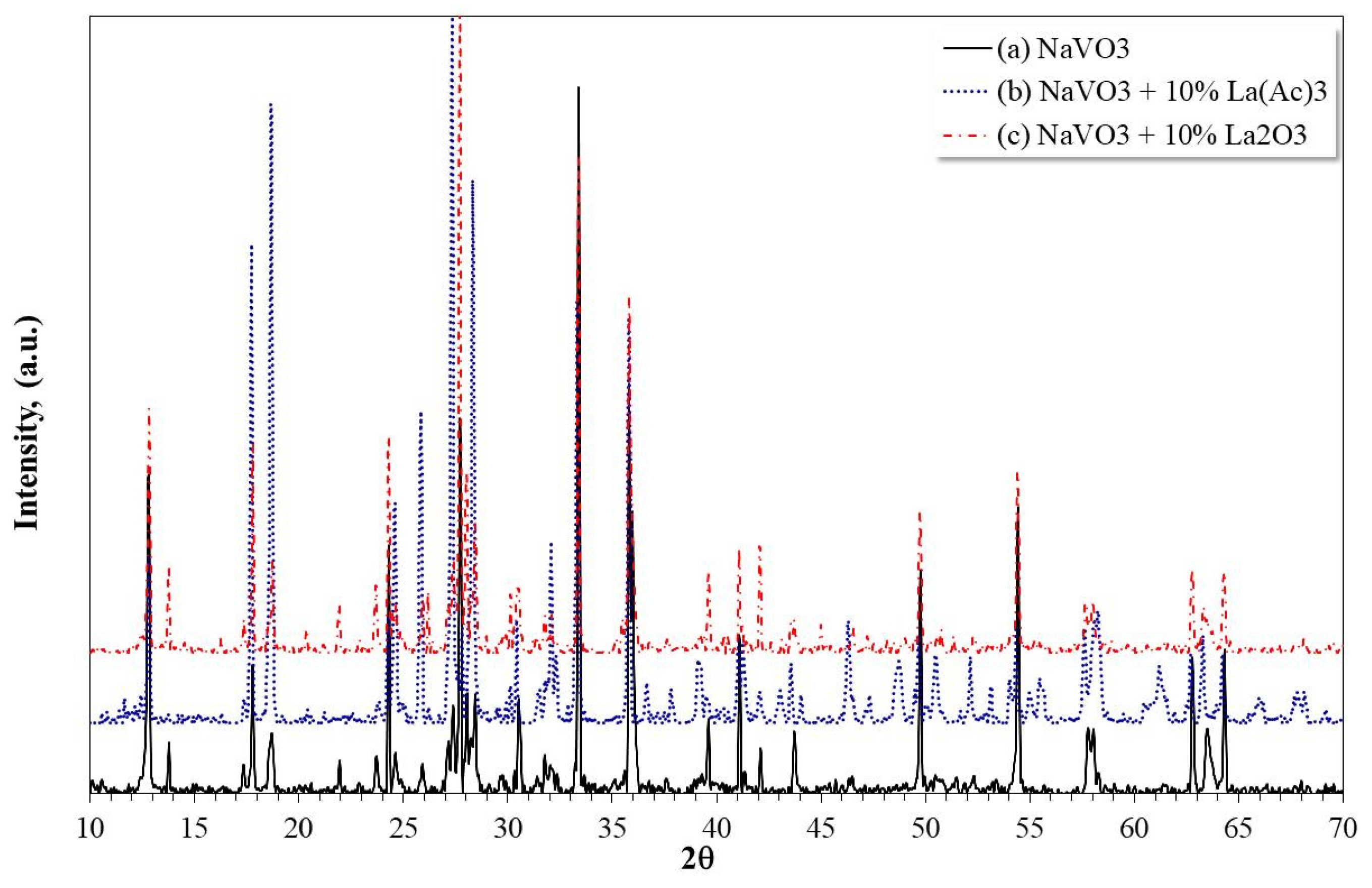
3.3. XRD Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wong-Moreno, A.; Mujica Martinez, Y.; Martinez, L. High temperature corrosion enhanced by residual fuel oil ash deposits. In CORROSION/94; NACE International: Houston, TX, USA, 1994; No. CONF-940222. [Google Scholar]
- Gonzalez-Rodriguez, J.G.; Haro, S.; Martinez-Villafañe, A.; Salinas-Bravo, V.M.; Porcayo-Calderon, J. Corrosion performance of heat resistant alloys in Na2SO4-V2O5 molten salts. Mater. Sci. Eng. A 2006, 435, 258–265. [Google Scholar] [CrossRef]
- Zheng, X.; Rapp, R.A. Electrochemical impedance study of platinum electrode in fused Na2SO4-10 mole percent NaVO3 melts. J. Electrochem. Soc. 1995, 142, 142–148. [Google Scholar] [CrossRef]
- Otsuka, N.; Rapp, R.A. Effects of chromate and vanadate anions on the hot corrosion of preoxidized Ni by a thin fused Na2SO4 film at 900 °C. J. Electrochem. Soc. 1990, 137, 53–60. [Google Scholar] [CrossRef]
- Hwang, Y.-S.; Rapp, R.A. Termochemistry and solubilities of oxides in sodiun sulfate-vanadate solutions. Corrosion 1989, 45, 933–937. [Google Scholar] [CrossRef]
- Porcayo-Calderon, J.; Salinas-Bravo, V.M.; Rodriguez-Diaz, R.A.; Martinez-Gomez, L. Effect of the NaVO3-V2O5 ratio on the high temperature corrosion of chromium. Int. J. Electrochem. Sci. 2015, 10, 4928–4945. [Google Scholar]
- Montero, X.; Galetz, M.C. Inhibitors and coatings against vanadate-containing oil ash corrosion of boilers. Surf. Coat. Technol. 2016, 304, 211–221. [Google Scholar] [CrossRef]
- Kerahroodi, M.S.A.; Rahmani, K.; Yousefi, M. The inhibitory effect of magnesium sulfonate as a fuel additive on hot corrosion of generating tubes of power plant boiler. Oxid. Met. 2018, 89, 565–588. [Google Scholar] [CrossRef]
- Rocca, E.; Aranda, L.; Moliere, M.; Steinmetz, P. Nickel oxide as a new inhibitor of vanadium-induced hot corrosion of Superalloys - comparison to MgO-based inhibitor. J. Mater. Chem. 2002, 12, 3766–3772. [Google Scholar] [CrossRef]
- Di Salvia, N.; Malavasi, M.P.; Di Salvia, G.; Vaccari, A. formation of Ni and Mg vanadates during the flameless oxy-combustion of heavy fuels. Fuel Process. Technol. 2015, 138, 534–539. [Google Scholar] [CrossRef]
- Rocca, E.; Aranda, L.; Molière, M. Chemistry of ash-deposits on gas turbines hot parts: Reactivity of nickel, zinc and iron oxides in (Na, V, S) molten salts. Mater. Sci. Forum 2008, 595–598, 169–176. [Google Scholar] [CrossRef]
- Rhys-Jones, T.N.; Nicholls, J.R.; Hancock, P. Effects of SO2/SO2 on the efficiency with which MgO inhibits vanadic corrosion in residual fuel fired gas turbines. Corrosion Sci. 1983, 23, 139–149. [Google Scholar] [CrossRef]
- Hancock, P. Vanadic and chloride attack of superalloys. Mater. Sci. Technol. 1987, 3, 536–544. [Google Scholar] [CrossRef]
- Porcayo-Calderon, J.; Ramos-Hernandez, J.J.; Mayén, J.; Porcayo-Palafox, E.; Pedraza-Basulto, G.K.; Gonzalez-Rodriguez, J.G.; Martinez-Gomez, L. High temperature corrosion of nickel in NaVO3-V2O5 Melts. Adv. Mater. Sci. Eng. 2017. [Google Scholar] [CrossRef]
- Sotelo-Mazón, O.; Porcayo-Calderon, J.; Cuevas-Arteaga, C.; Ramos-Hernandez, J.J.; Ascencio-Gutierrez, J.A.; Martinez-Gomez, L. EIS Evaluation of Fe, Cr, and Ni in NaVO3 at 700 °C. J. Spectrosc. 2014. [Google Scholar] [CrossRef]
- Rizzo da Rocha, S.M.; da Silva Queiroz, C.A.; Abrão, A. Synthesis and characterization of lanthanum acetate for application as a catalyst. J. Alloy. Compd. 2002, 344, 389–393. [Google Scholar] [CrossRef]
- Hussein, G.A.M. Spectrothermal investigation of the decomposition course of lanthanum acetate hydrate. J. Therm. Anal. 1994, 42, 1091–1102. [Google Scholar] [CrossRef]
- Salinas, G.; Gonzalez-Rodriguez, J.G.; Porcayo-Calderon, J.; Salinas-Bravo, V.M.; Espinosa-Medina, M.A. Effect of minor alloying elements on the corrosion behavior of Fe-40Al in NaCl-KCl molten salts. Int. J. Corrosion 2012. [Google Scholar] [CrossRef]
- Salinas-Solano, G.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.G.; Salinas-Bravo, V.M.; Ascencio-Gutierrez, J.A.; Martinez-Gomez, L. High temperature corrosion of inconel 600 in NaCl-KCl molten salts. Adv. Mater. Sci. Eng. 2014. [Google Scholar] [CrossRef]
- Rapp, R.A. Hot corrosion of materials: A fluxing mechanism? Corrosion Sci. 2002, 44, 209–221. [Google Scholar] [CrossRef]
- Hwang, Y.-S.; Rapp, R.A. Synergistic dissolution of oxides in molten sodium sulfate. J. Electrochem. Soc. 1990, 137, 1276–1280. [Google Scholar] [CrossRef]
- Espinosa-Medina, M.A.; Carbajal-De la Torre, G.; Liu, H.B.; Martínez-Villafañe, A.; González-Rodriguez, J.G. Hot corrosion behaviour of Fe-Al based intermetallic in molten NaVO3 salt. Corrosion Sci. 2009, 51, 1420–1427. [Google Scholar] [CrossRef]
- Longa-Nava, Y.; Zhang, Y.S.; Takemoto, M.; Rapp, R.A. Hot corrosion of nickel-chromium and nickel-chromium-aluminum thermal-spray coatings by sodium sulfate-sodium metavanadate salt. Corrosion 1996, 52, 680–689. [Google Scholar] [CrossRef]
- Walczak, J.; Filipek, E. Phase equilibria in the V2O5-Cr2O3 System. J. Therm. Anal. 1989, 35, 69–76. [Google Scholar] [CrossRef]
- Kerby, R.C.; Wilson, J.R. Solid-liquid phase equilibria for the ternary systems V2O5-Na2O-Fe2O3, V2O5-Na2O-Cr2O3, and V2O5-Na2O-MgO. Can. J. Chem. 1973, 51, 1032–1040. [Google Scholar] [CrossRef]
- Rost, E. Reduction of Fused Sodium Series A. Physical and inorganic chemistry. Vanadates Acta chem. 1985, 39, 405–410. [Google Scholar] [CrossRef]
- Huang, W.; Chen, M.; Shen, X.; Shan, Y.; He, M.; Wang, N. Viscosity property and Raman spectroscopy of FeO-SiO2-V2O3-TiO2-Cr2O3 slags. in advances in molten slags. In Proceedings of the 10th International Conference on Molten Slags, Fluxes and Salts 2016; Springer: Cham, Switzerland, 2016; pp. 455–462. [Google Scholar] [CrossRef]
- Tonkov, E.I.; Tonkov, E.Y. High Pressure Phase Transformations: A Handbook; CRC Press: Boca Raton, FL, USA, 1992; Volume 2. [Google Scholar]
- Kjellqvist, L.; Selleby, M.; Sundman, B. Thermodynamic modelling of the Cr–Fe–Ni–O system. Calphad 2008, 32, 577–592. [Google Scholar] [CrossRef]
- Jones, R.L. Oxide acid-base reactions relating to the inhibition of vanadium attack on REY zeolite catalysts. J. Catal. 1991, 129, 269–274. [Google Scholar] [CrossRef]
- Reidy, R.F.; Jones, R.L. Thermogravimetric analysis of the reaction of CeO2 with the NaVO3-SO3 system. J. Electrochem. Soc. 1995, 142, 1353–1357. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Rapp, R.A. Solubilities of CeO2, HfO2 and Y2O3 in fused Na2SO4-30 mol% NaVO3 and CeO2 in pure Na2SO4 at 900 °C. Corrosion 1987, 43, 348–352. [Google Scholar] [CrossRef]
- Jones, R.L. Some aspects of the hot corrosion of thermal barrier coatings. J. Therm. Spray Technol. 1997, 6, 77–84. [Google Scholar] [CrossRef]
- Kamada, T.; Kaneko, T.; Daimon, K.; Hikichi, Y.; Ota, T. Synthesis and properties of monazite type LaVO4. In Proceedings of the Preprints of Annual Meeting of the Ceramic Society of Japan, Tokyo, Japan, 22–23 March 2003. Abstract Number 1E28. [Google Scholar] [CrossRef]

| x/y Ratio | Reaction Products | Vanadium Salt | Melting Point |
|---|---|---|---|
| 3 (x = 3; y = 1) | Na3VO4 (Sodium Orthovanadate) | 850 °C | |
| 2 (x = 2; y = 1) | Na4V2O7 (Sodium Pyrovanadate) | 640 °C | |
| 1 (x = 1; y = 1) | NaVO3 (Sodium Metavanadate) | 630 °C | |
| 0.167 (x = 1; y = 6) | Na2O·V2O4·5V2O5 (β-sodium vanadyl vanadate) | 625 °C | |
| 0.417 (x = 5; y = 2) | 5Na2O·V2O4·11V2O5 (γ-sodium vanadyl vanadate) | 535 °C |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Garcia, N.S.; Arrieta-Gonzalez, C.D.; Ramos-Hernandez, J.J.; Pedraza-Basulto, G.K.; Gonzalez-Rodriguez, J.G.; Porcayo-Calderon, J.; Martinez-Gomez, L. Rare Earth-Based Compounds as Inhibitors of Hot-Corrosion Induced by Vanadium Salts. Materials 2019, 12, 3796. https://doi.org/10.3390/ma12223796
Flores-Garcia NS, Arrieta-Gonzalez CD, Ramos-Hernandez JJ, Pedraza-Basulto GK, Gonzalez-Rodriguez JG, Porcayo-Calderon J, Martinez-Gomez L. Rare Earth-Based Compounds as Inhibitors of Hot-Corrosion Induced by Vanadium Salts. Materials. 2019; 12(22):3796. https://doi.org/10.3390/ma12223796
Chicago/Turabian StyleFlores-Garcia, N.S., C.D. Arrieta-Gonzalez, J.J. Ramos-Hernandez, G.K. Pedraza-Basulto, J.G. Gonzalez-Rodriguez, J. Porcayo-Calderon, and L. Martinez-Gomez. 2019. "Rare Earth-Based Compounds as Inhibitors of Hot-Corrosion Induced by Vanadium Salts" Materials 12, no. 22: 3796. https://doi.org/10.3390/ma12223796






