Lead-Free Perovskites for Lighting and Lasing Applications: A Minireview
Abstract
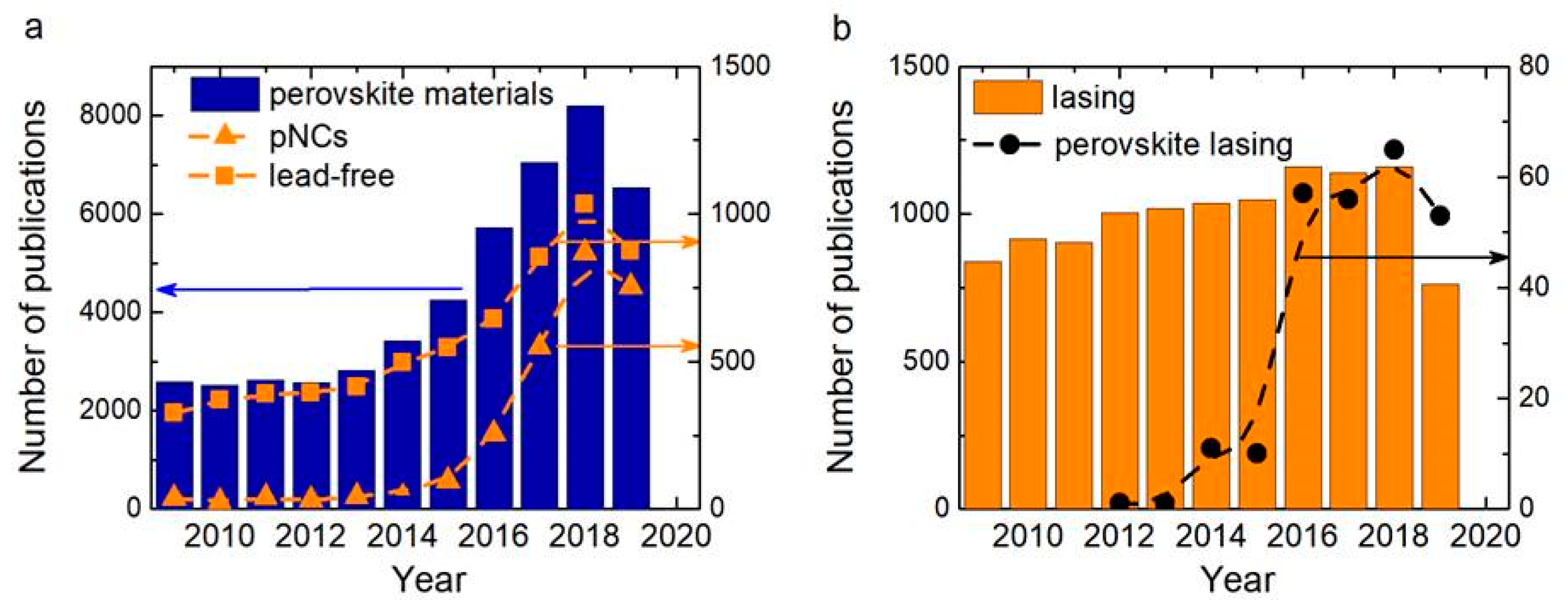
:1. Introduction
- Layered or 2D, including Ruddlesden–Popper (RP) perovskites with formula L2BX4 where L is organic molecule (ligand),
- Double perovskites where the 2 B is replaced by 1 atom with 1+ valence and 1 atom with 3+ valence resulting in A2B+B3+X6 structure;
- Perovskite-related crystal structures with formula A4BX6 or AB2X5.
2. Synthesis
2.1. Chemical Composition
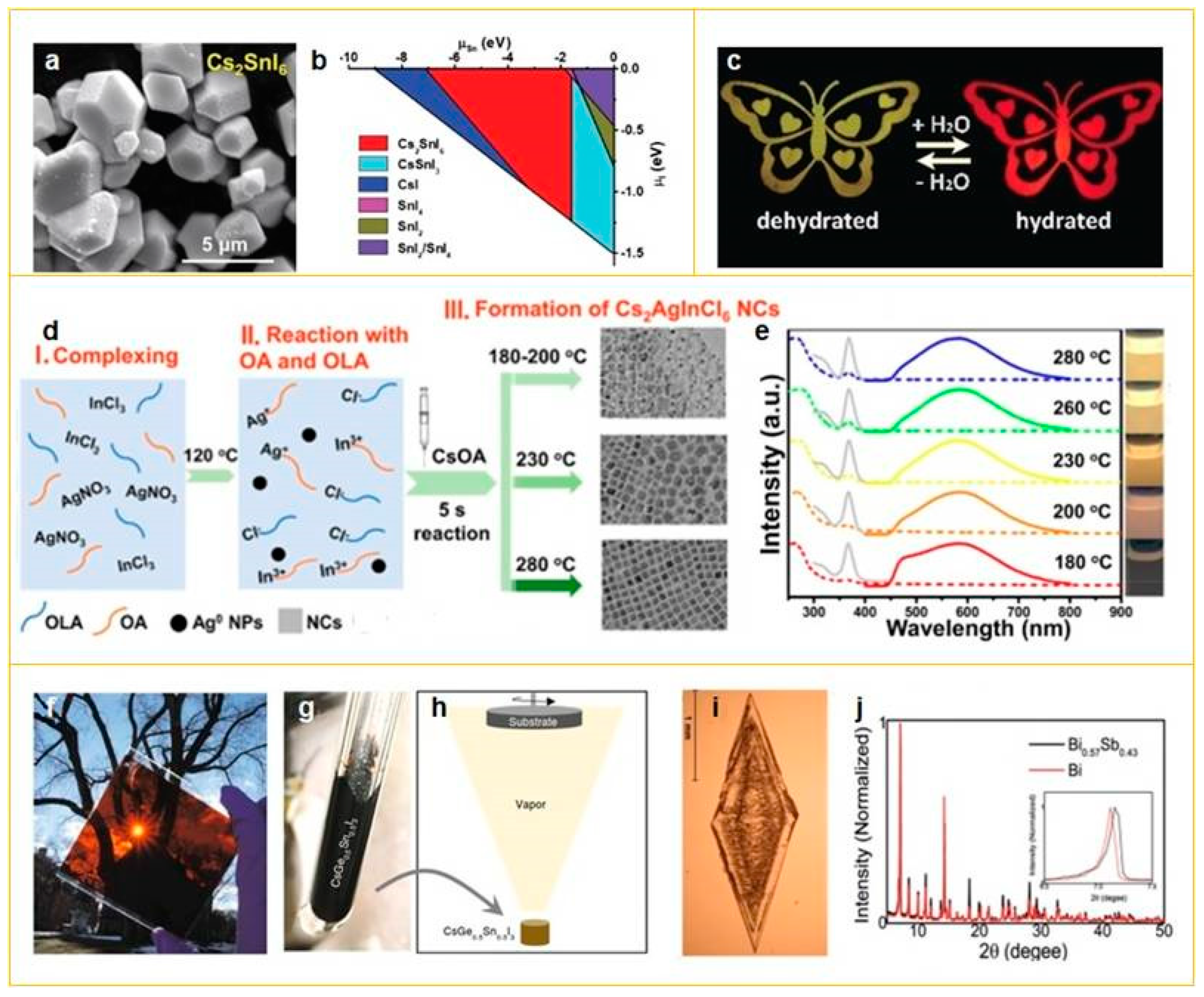
2.1.1. Pb-Substituted Perovskites
2.1.2. Double Perovskites
2.1.3. Alloyed Perovskites
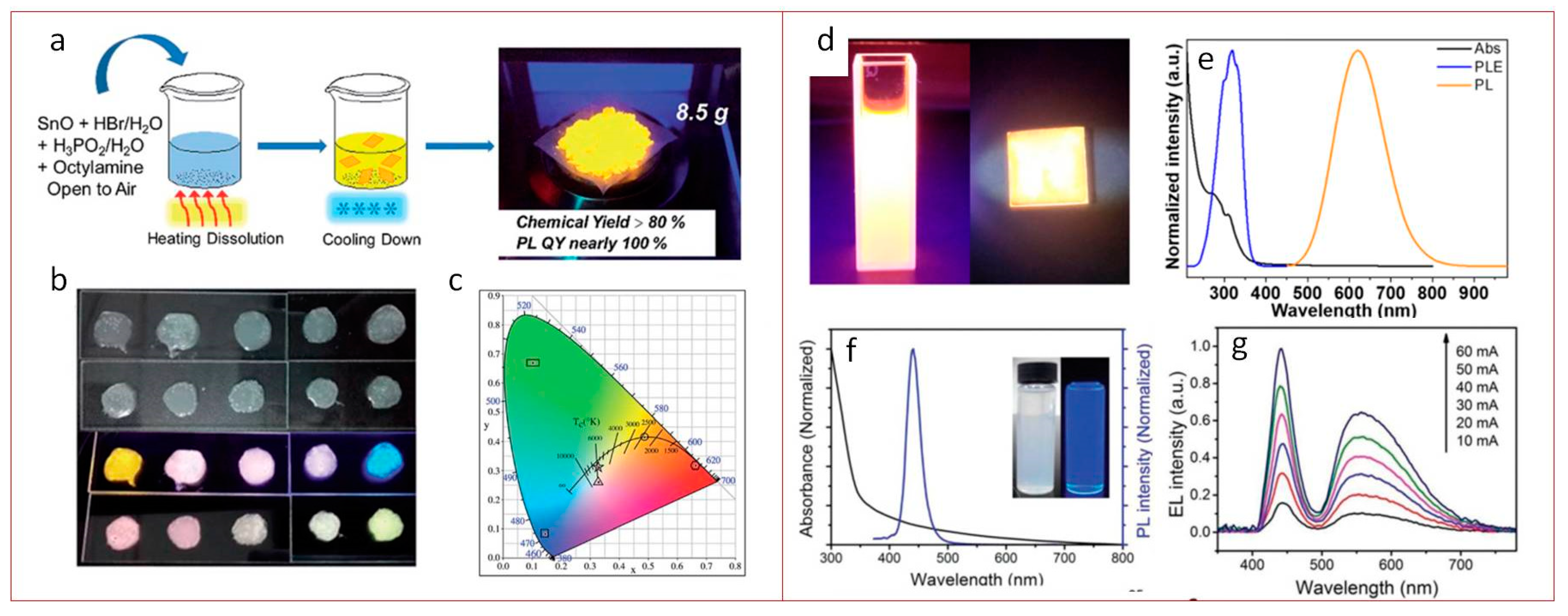
2.2. Lead-Free Perovskites Morphology
2.3. Stability
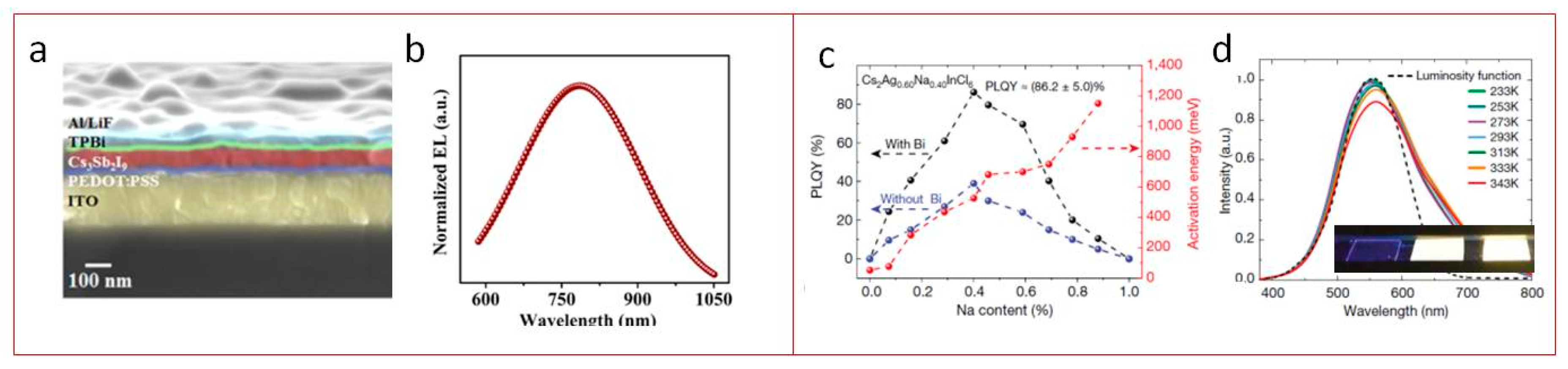
3. Lighting Applications
4. Lasing
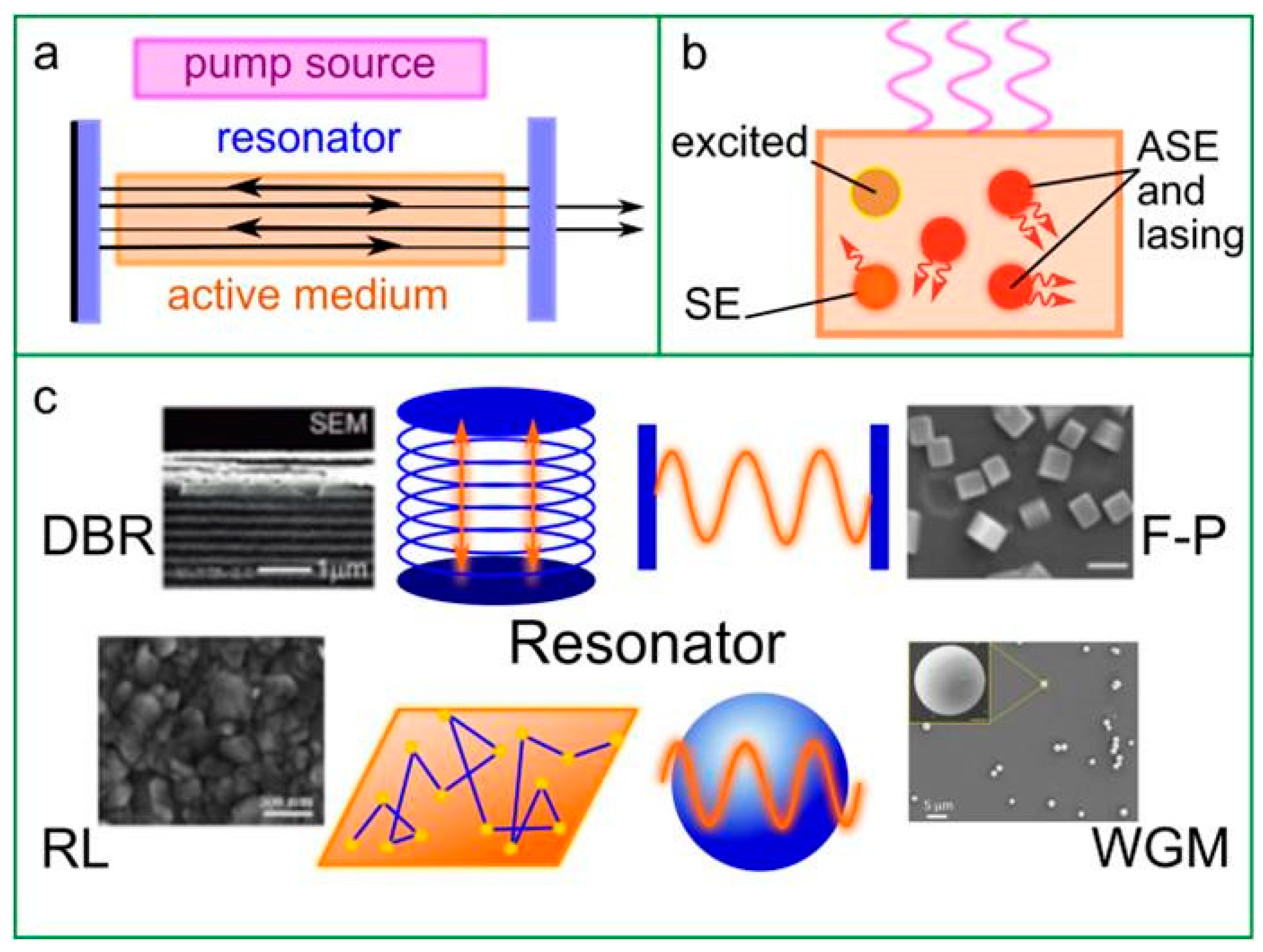
4.1. Basic Principles
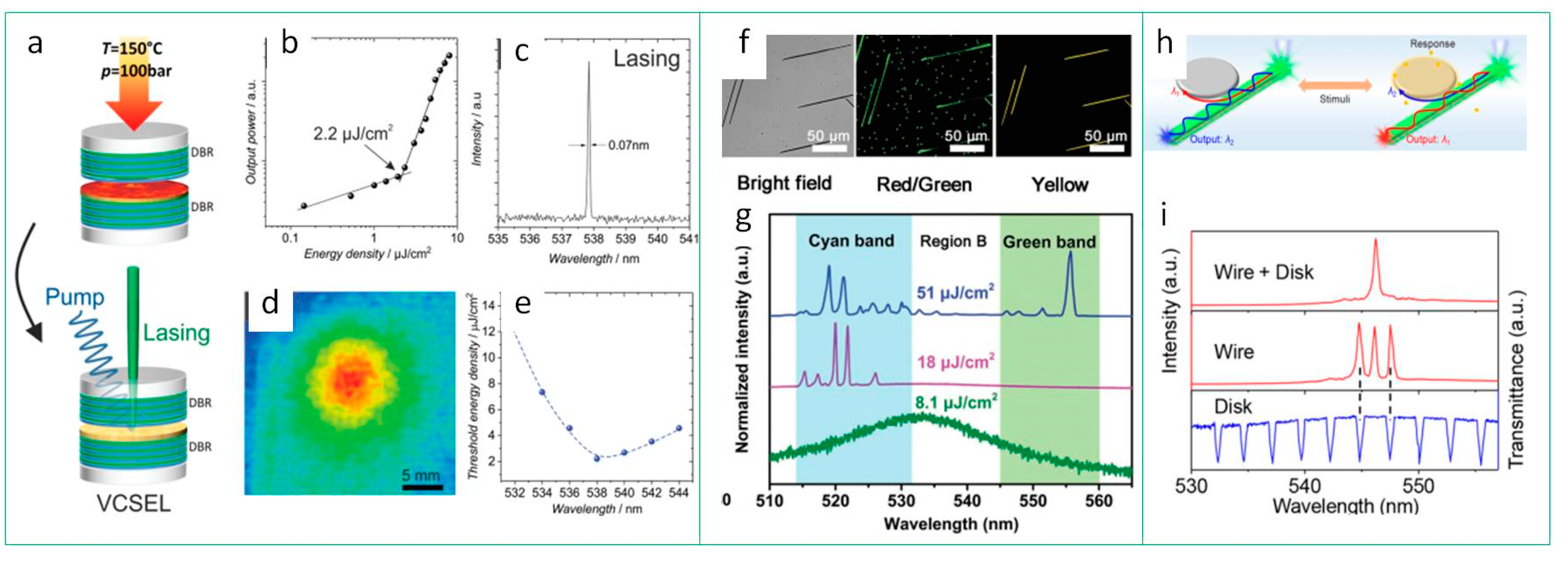
4.2. Perovskite Materials in Lasers
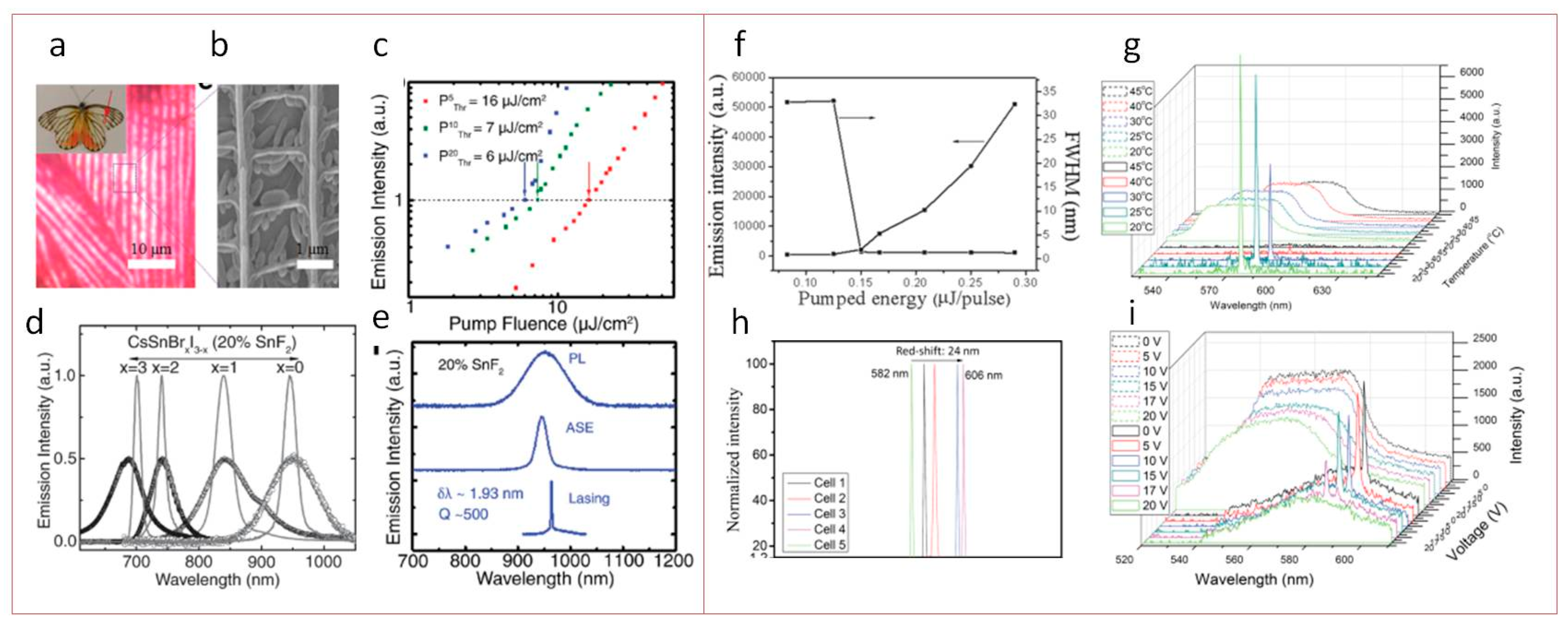
Lead-Free Perovskite for Lasing
5. Outline and Perspectives
- Chemical composition and morphology. To date, Bi-based perovskite materials provide a wide variety of morphology of materials with perovskite symmetry, including double perovskites, along with the excellent and stable performance. The other direction is the search of novel chemical compounds for substituting the lead atom in lanthanide series, such as Yb;
- Synthetic approaches. Inspired by the chemical routes implemented for lead-based perovskites, the vapor deposition approaches might be admitted as synthesis route for formation micrometer-sized lead-free perovskites, the shape of which can meet the resonance conditions for stimulated amplification of the emission;
- Active medium protection. The use of the matrices for in-situ synthesis or as host matrices for perovskite materials can expand the variety of chemical compounds used, i.e., unstable in ambient, together with simple control of the architecture of the active medium defined by matrix morphology.
Funding
Conflicts of Interest
References
- Radhakrishna, S. Polarised luminescence from lead centers in cesium halides. J. Lumin. 1976, 12–13, 409–411. [Google Scholar] [CrossRef]
- Mitzi, D.B. Synthesis, Structure, and Properties of Organic-Inorganic Perovskites and Related Materials; John Wiley & Sons, Inc.: New York, NY, USA, 1999; Volume 48, ISBN 9780470166499. [Google Scholar]
- Tan, Z.K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Arveson, S.M.; Tisdale, W.A. Colloidal Organohalide Perovskite Nanoplatelets Exhibiting Quantum Confinement. J. Phys. Chem. Lett. 2015, 6, 1911–1916. [Google Scholar] [CrossRef] [PubMed]
- Sichert, J.A.; Tong, Y.; Mutz, N.; Vollmer, M.; Fischer, S.; Milowska, K.Z.; García Cortadella, R.; Nickel, B.; Cardenas-Daw, C.; Stolarczyk, J.K.; et al. Quantum Size Effect in Organometal Halide Perovskite Nanoplatelets. Nano Lett. 2015, 15, 6521–6527. [Google Scholar] [CrossRef]
- Schmidt, L.C.; Pertegás, A.; González-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Mínguez Espallargas, G.; Bolink, H.J.; Galian, R.E.; Pérez-Prieto, J. Nontemplate synthesis of CH3NH3PbBr3 perovskite nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Ha, S.T.; Su, R.; Xing, J.; Zhang, Q.; Xiong, Q. Metal halide perovskite nanomaterials: Synthesis and applications. Chem. Sci. 2017, 8, 2522–2536. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 2018, 17, 394. [Google Scholar] [CrossRef]
- Weidman, M.C.; Goodman, A.J.; Tisdale, W.A. Colloidal Halide Perovskite Nanoplatelets: An Exciting New Class of Semiconductor Nanomaterials. Chem. Mater. 2017, 29, 5019–5030. [Google Scholar] [CrossRef]
- Shi, E.; Gao, Y.; Finkenauer, B.P.; Akriti, A.; Coffey, A.H.; Dou, L. Two-dimensional halide perovskite nanomaterials and heterostructures. Chem. Soc. Rev. 2018, 47, 6046–6072. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Connor, B.A.; Karunadasa, H.I. Tuning the Luminescence of Layered Halide Perovskites. Chem. Rev. 2019, 119, 3104–3139. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.K.; Kulkarni, A.; Miyasaka, T. Halide Perovskite Photovoltaics: Background, Status, and Future Prospects. Chem. Rev. 2019, 119, 3036–3103. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Cahen, D.; Kahn, A. Halide Perovskites: Is It All about the Interfaces? Chem. Rev. 2019, 119, 3349–3417. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef]
- Shi, Z.; Guo, J.; Chen, Y.; Li, Q.; Pan, Y.; Zhang, H.; Xia, Y.; Huang, W. Lead-Free Organic–Inorganic Hybrid Perovskites for Photovoltaic Applications: Recent Advances and Perspectives. Adv. Mater. 2017, 29, 1605005. [Google Scholar] [CrossRef]
- Sani, F.; Shafie, S.; Lim, H.N.; Musa, A.O. Advancement on lead-free organic-inorganic halide perovskite solar cells: A review. Materials 2018, 11, 1008. [Google Scholar] [CrossRef]
- Sun, J.; Yang, J.; Lee, J.I.; Cho, J.H.; Kang, M.S. Lead-Free Perovskite Nanocrystals for Light-Emitting Devices. J. Phys. Chem. Lett. 2018, 9, 1573–1583. [Google Scholar] [CrossRef]
- Luo, J.; Hu, M.; Niu, G.; Tang, J. Lead-Free Halide Perovskites and Perovskite Variants as Phosphors toward Light-Emitting Applications. ACS Appl. Mater. Interfaces 2019, 11, 31575–31584. [Google Scholar] [CrossRef]
- Wang, J.; Dong, J.; Lu, F.; Sun, C.; Zhang, Q.; Wang, N. Two-dimensional lead-free halide perovskite materials and devices. J. Mater. Chem. A 2019, 7, 23563–23576. [Google Scholar] [CrossRef]
- Stylianakis, M.M.; Maksudov, T.; Panagiotopoulos, A.; Kakavelakis, G.; Petridis, K. Inorganic and Hybrid Perovskite Based Laser Devices: A Review. Materials 2019, 12, 859. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, M.; Anni, M. Amplified Spontaneous Emission and Lasing in Lead Halide Perovskites: State of the Art and Perspectives. Appl. Sci. 2019, 9, 4591. [Google Scholar] [CrossRef]
- Ling, Y.; Tian, Y.; Wang, X.; Wang, J.C.; Knox, J.M.; Perez-Orive, F.; Du, Y.; Tan, L.; Hanson, K.; Ma, B.; et al. Enhanced Optical and Electrical Properties of Polymer-Assisted All-Inorganic Perovskites for Light-Emitting Diodes. Adv. Mater. 2016, 28, 8983–8989. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Peng, Y.; Fu, Y.; Shearer, M.J.; Zhang, J.; Zhai, J.; Zhang, Y.; Hamers, R.J.; Andrew, T.L.; Jin, S. Color-Pure Violet-Light-Emitting Diodes Based on Layered Lead Halide Perovskite Nanoplates. ACS Nano 2016, 10, 6897–6904. [Google Scholar] [CrossRef]
- Mondal, N.; De, A.; Samanta, A. Achieving Near-Unity Photoluminescence Efficiency for Blue-Violet-Emitting Perovskite Nanocrystals. ACS Energy Lett. 2019, 4, 32–39. [Google Scholar] [CrossRef]
- Gonzalez-Carrero, S.; Francés-Soriano, L.; González-Béjar, M.; Agouram, S.; Galian, R.E.; Pérez-Prieto, J. The Luminescence of CH3NH3PbBr3 Perovskite Nanoparticles Crests the Summit and Their Photostability under Wet Conditions is Enhanced. Small 2016, 12, 5245–5250. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, Y.; Ju, M.-G.; Garces, H.F.; Ding, T.; Pang, S.; Zeng, X.C.; Padture, N.P.; Sun, X.W. Heterojunction-Depleted Lead-Free Perovskite Solar Cells with Coarse-Grained B-γ-CsSnI3 Thin Films. Adv. Energy Mater. 2016, 6, 1601130. [Google Scholar] [CrossRef]
- Bernasconi, A.; Rizzo, A.; Listorti, A.; Mahata, A.; Mosconi, E.; De Angelis, F.; Malavasi, L. Synthesis, Properties, and Modeling of Cs 1-x Rb x SnBr 3 Solid Solution: A New Mixed-Cation Lead-Free All-Inorganic Perovskite System. Chem. Mater. 2019, 31, 3527–3533. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, J.; Rong, X.; Wei, P.; Molokeev, M.S.; Huang, Y.; Zhao, J.; Liu, Q.; Zhang, X.; Tang, J.; et al. Lead-Free Perovskite Derivative Cs2SnCl6−xBrx Single Crystals for Narrowband Photodetectors. Adv. Opt. Mater. 2019, 7, 1900139. [Google Scholar] [CrossRef]
- Han, X.; Liang, J.; Yang, J.-H.; Soni, K.; Fang, Q.; Wang, W.; Zhang, J.; Jia, S.; Martí, A.A.; Zhao, Y.; et al. Lead-Free Double Perovskite Cs2SnX6: Facile Solution Synthesis and Excellent Stability. Small 2019, 15, 1901650. [Google Scholar] [CrossRef]
- Qiu, J.; Xia, Y.; Chen, Y.; Huang, W. Management of Crystallization Kinetics for Efficient and Stable Low-Dimensional Ruddlesden–Popper (LDRP) Lead-Free Perovskite Solar Cells. Adv. Sci. 2019, 6, 1800793. [Google Scholar] [CrossRef] [PubMed]
- Kerner, R.A.; Rand, B.P. Electrochemical and Thermal Etching of Indium Tin Oxide by Solid-State Hybrid Organic–Inorganic Perovskites. ACS Appl. Energy Mater. 2019, 2, 6097–6101. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, J.-F.; Huang, Z.-G.; Wei, J.-H.; Wang, X.-D.; Li, W.-G.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. A Highly Red-Emissive Lead-Free Indium-Based Perovskite Single Crystal for Sensitive Water Detection. Angew. Chem. Int. Ed. 2019, 58, 5277–5281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Xia, X.; Wang, Z.; Huang, Z.; Lei, B.; Gao, Y. High-Quality (CH3NH3)3Bi2I9 Film-Based Solar Cells: Pushing Efficiency up to 1.64%. J. Phys. Chem. Lett. 2017, 8, 4300–4307. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Gao, L.-L.; Ding, B.; Chu, Q.-Q.; Li, Z.; Yang, G.-J. (C6H5NH3)BiI4: A lead-free perovskite with >330 days humidity stability for optoelectronic applications. J. Mater. Chem. A 2019, 7, 15722–15730. [Google Scholar] [CrossRef]
- Xie, J.-L.; Huang, Z.-Q.; Wang, B.; Chen, W.-J.; Lu, W.-X.; Liu, X.; Song, J.-L. New lead-free perovskite Rb7Bi3Cl16 nanocrystals with blue luminescence and excellent moisture-stability. Nanoscale 2019, 11, 6719–6726. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liang, M.; Chang, B.; Sun, H.; Zheng, K.; Pullerits, T.; Chi, Q. Lead-free double halide perovskite Cs3BiBr6 with well-defined crystal structure and high thermal stability for optoelectronics. J. Mater. Chem. C 2019, 7, 3369–3374. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, K.; Ansari, S.N.; Natarajan, K.; Mobin, S.M. A (CH3NH3)3Bi2I9 Perovskite Based on a Two-Step Deposition Method: Lead-Free, Highly Stable, and with Enhanced Photovoltaic Performance. ChemElectroChem 2019, 6, 1192–1198. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, M.; Wan, L.; Liu, Y.; Wang, Y.; Gan, Y.; Guo, Z.; Eder, D.; Wang, S. Anti-solvent spin-coating for improving morphology of lead-free (CH3NH3)3Bi2I9 perovskite films. SN Appl. Sci. 2019, 1, 706. [Google Scholar] [CrossRef] [Green Version]
- Zuo, C.; Ding, L. Lead-free Perovskite Materials (NH4)3Sb2IxBr9−x. Angew. Chem. Int. Ed. 2017, 56, 6528–6532. [Google Scholar] [CrossRef]
- Du, K.Z.; Meng, W.; Wang, X.; Yan, Y.; Mitzi, D.B. Bandgap Engineering of Lead-Free Double Perovskite Cs2AgBiBr6 through Trivalent Metal Alloying. Angew. Chem. Int. Ed. 2017, 56, 8158–8162. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.C.; Osowiecki, W.T.; Cai, Y.; Swabeck, J.K.; Bekenstein, Y.; Asta, M.; Chan, E.M.; Alivisatos, A.P. Probing the Stability and Band Gaps of Cs2AgInCl6 and Cs2AgSbCl6 Lead-Free Double Perovskite Nanocrystals. Chem. Mater. 2019, 31, 3134–3143. [Google Scholar] [CrossRef] [Green Version]
- Igbari, F.; Wang, R.; Wang, Z.-K.; Ma, X.-J.; Wang, Q.; Wang, K.-L.; Zhang, Y.; Liao, L.-S.; Yang, Y. Composition Stoichiometry of Cs2AgBiBr6 Films for Highly Efficient Lead-Free Perovskite Solar Cells. Nano Lett. 2019, 19, 2066–2073. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jing, Y.; Zhao, J.; Liu, Q.; Xia, Z. Design Optimization of Lead-Free Perovskite Cs2AgInCl6:Bi Nanocrystals with 11.4% Photoluminescence Quantum Yield. Chem. Mater. 2019, 31, 3333–3339. [Google Scholar] [CrossRef]
- Wei, F.; Deng, Z.; Sun, S.; Hartono, N.T.P.; Seng, H.L.; Buonassisi, T.; Bristowe, P.D.; Cheetham, A.K. Enhanced visible light absorption for lead-free double perovskite Cs2AgSbBr6. Chem. Commun. 2019, 55, 3721–3724. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, Y.; Huang, H.; Liu, X.; Li, Q.; Chen, L.; Xu, D. Stable and Highly Efficient Photocatalysis with Lead-Free Double-Perovskite of Cs2AgBiBr6. Angew. Chem. Int. Ed. 2019, 58, 7263–7267. [Google Scholar] [CrossRef]
- Weng, Z.; Qin, J.; Umar, A.A.; Wang, J.; Zhang, X.; Wang, H.; Cui, X.; Li, X.; Zheng, L.; Zhan, Y. Lead-Free Cs2BiAgBr6 Double Perovskite-Based Humidity Sensor with Superfast Recovery Time. Adv. Funct. Mater. 2019, 29, 1902234. [Google Scholar] [CrossRef]
- Yang, J.; Bao, C.; Ning, W.; Wu, B.; Ji, F.; Yan, Z.; Tao, Y.; Liu, J.-M.; Sum, T.C.; Bai, S.; et al. Stable, High-Sensitivity and Fast-Response Photodetectors Based on Lead-Free Cs2AgBiBr6 Double Perovskite Films. Adv. Opt. Mater. 2019, 7, 1801732. [Google Scholar] [CrossRef]
- Worley, C.; Yangui, A.; Roccanova, R.; Du, M.-H.; Saparov, B. (CH3NH3)AuX4⋅H2O (X = Cl, Br) and (CH3NH3)AuCl4: Low-Band Gap Lead-Free Layered Gold Halide Perovskite Materials. Chem.–A Eur. J. 2019, 25, 9875–9884. [Google Scholar] [CrossRef]
- Pious, J.K.; Katre, A.; Muthu, C.; Chakraborty, S.; Krishna, S.; Nair, V.C. Zero-Dimensional Lead-Free Hybrid Perovskite-like Material with a Quantum-Well Structure. Chem. Mater. 2019, 31, 1941–1945. [Google Scholar] [CrossRef]
- Zhang, R.; Mao, X.; Yang, Y.; Yang, S.; Zhao, W.; Wumaier, T.; Wei, D.; Deng, W.; Han, K. Air-Stable, Lead-Free Zero-Dimensional Mixed Bismuth-Antimony Perovskite Single Crystals with Ultra-broadband Emission. Angew. Chem. 2019, 131, 2751–2755. [Google Scholar] [CrossRef]
- Ding, N.; Zhou, D.; Pan, G.; Xu, W.; Chen, X.; Li, D.; Zhang, X.; Zhu, J.; Ji, Y.; Song, H. Europium-Doped Lead-Free Cs3Bi2Br9 Perovskite Quantum Dots and Ultrasensitive Cu2+ Detection. ACS Sustain. Chem. Eng. 2019, 7, 8397–8404. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Li, C.; Lu, Y.; Tong, X.-W.; Liang, F.-X.; Zhao, X.-Y.; Wu, D.; Xie, C.; Luo, L.-B. Sensitive Deep Ultraviolet Photodetector and Image Sensor Composed of Inorganic Lead-Free Cs3Cu2I5 Perovskite with Wide Bandgap. J. Phys. Chem. Lett. 2019, 10, 5343–5350. [Google Scholar] [CrossRef]
- Moon, B.J.; Kim, S.J.; Lee, S.; Lee, A.; Lee, H.; Lee, D.S.; Kim, T.-W.; Lee, S.-K.; Bae, S.; Lee, S.H. Rare-Earth-Element-Ytterbium-Substituted Lead-Free Inorganic Perovskite Nanocrystals for Optoelectronic Applications. Adv. Mater. 2019, 31, 1901716. [Google Scholar] [CrossRef]
- Chen, M.; Ju, M.G.; Garces, H.F.; Carl, A.D.; Ono, L.K.; Hawash, Z.; Zhang, Y.; Shen, T.; Qi, Y.; Grimm, R.L.; et al. Highly stable and efficient all-inorganic lead-free perovskite solar cells with native-oxide passivation. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Zhou, T.; Fang, X.; Zhang, W.; Li, X.; Guan, Z.; Li, J.; Wang, L.; Hark, S.; Zhang, Z. Temperature dependent geometry in perovskite microcrystals for whispering gallery and Fabry-Pérot mode lasing. J. Mater. Chem. C 2019, 7, 4102–4108. [Google Scholar] [CrossRef]
- Geng, C.; Xu, S.; Zhong, H.; Rogach, A.L.; Bi, W. Aqueous synthesis of methylammonium lead halide perovskite nanocrystals. Angew. Chem. Int. Ed. 2018, 57, 9650–9654. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, P.; Pan, A.; Yan, K.; Zhu, Y.; Yang, M.; He, L. Unusual Stability and Temperature-Dependent Properties of Highly Emissive CsPbBr3 Perovskite Nanocrystals Obtained from in Situ Crystallization in Poly(vinylidene difluoride). ACS Appl. Mater. Interfaces 2019, 11, 22786–22793. [Google Scholar] [CrossRef]
- Malgras, V.; Tominaka, S.; Ryan, J.W.; Henzie, J.; Takei, T.; Ohara, K.; Yamauchi, Y. Observation of Quantum Confinement in Monodisperse Methylammonium Lead Halide Perovskite Nanocrystals Embedded in Mesoporous Silica. J. Am. Chem. Soc. 2016, 138, 13874–13881. [Google Scholar] [CrossRef]
- Tan, Y.; Zou, Y.; Wu, L.; Huang, Q.; Yang, D.; Chen, M.; Ban, M.; Wu, C.; Wu, T.; Bai, S.; et al. Highly Luminescent and Stable Perovskite Nanocrystals with Octylphosphonic Acid as a Ligand for Efficient Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 3784–3792. [Google Scholar] [CrossRef]
- Krieg, F.; Ochsenbein, S.T.; Yakunin, S.; Ten Brinck, S.; Aellen, P.; Süess, A.; Clerc, B.; Guggisberg, D.; Nazarenko, O.; Shynkarenko, Y.; et al. Colloidal CsPbX3 (X = Cl, Br, I) Nanocrystals 2.0: Zwitterionic Capping Ligands for Improved Durability and Stability. ACS Energy Lett. 2018, 3, 641–646. [Google Scholar] [CrossRef] [Green Version]
- Ushakova, E.V.; Matuhina, A.I.; Sokolova, A.V.; Cherevkov, S.A.; Dubavik, A.; Medvedev, O.S.; Litvin, A.P.; Kurdyukov, D.A.; Golubev, V.G.; Baranov, A.V. Enhanced stability of the optical responses from all-inorganic perovskite nanocrystals embedded in a synthetic opal matrix. Nanotechnology 2019, 30, 405206. [Google Scholar] [CrossRef]
- Tong, X.W.; Kong, W.Y.; Wang, Y.Y.; Zhu, J.M.; Luo, L.B.; Wang, Z.H. High-Performance Red-Light Photodetector Based on Lead-Free Bismuth Halide Perovskite Film. ACS Appl. Mater. Interfaces 2017, 9, 18977–18985. [Google Scholar] [CrossRef]
- Roknuzzaman, M.; Ostrikov, K.K.; Wang, H.; Du, A.; Tesfamichael, T. Towards lead-free perovskite photovoltaics and optoelectronics by ab-initio simulations. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Khanna, V.K. Fundamentals of Solid-State Lighting: LEDs, OLEDs, and Their Applications in Illumination and Displays; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014. [Google Scholar]
- Song, J.; Fang, T.; Li, J.; Xu, L.; Zhang, F.; Han, B.; Shan, Q.; Zeng, H. Organic–Inorganic Hybrid Passivation Enables Perovskite QLEDs with an EQE of 16.48%. Adv. Mater. 2018, 30, 1805409. [Google Scholar] [CrossRef]
- Van Le, Q.; Jang, H.W.; Kim, S.Y. Recent Advances toward High-Efficiency Halide Perovskite Light-Emitting Diodes: Review and Perspective. Small Methods 2018, 2, 1700419. [Google Scholar] [CrossRef]
- Veldhuis, S.A.; Boix, P.P.; Yantara, N.; Li, M.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G. Perovskite Materials for Light-Emitting Diodes and Lasers. Adv. Mater. 2016, 28, 6804–6834. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, Z.; Lin, J. Correction: An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chem. Soc. Rev. 2019, 48, 405. [Google Scholar] [CrossRef]
- Quan, L.N.; Rand, B.P.; Friend, R.H.; Mhaisalkar, S.G.; Lee, T.-W.; Sargent, E.H. Perovskites for Next-Generation Optical Sources. Chem. Rev. 2019, 119, 7444–7477. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, W.; Wang, W.; Xu, B.; Liu, S.; Dai, H.; Chen, S.; Wang, K.; Sun, X.W. Efficient light-emitting diodes based on green perovskite nanocrystals with mixed-metal cations. Nano Energy 2016, 30, 511–516. [Google Scholar] [CrossRef]
- Singh, A.; Chiu, N.-C.; Boopathi, K.M.; Lu, Y.-J.; Mohapatra, A.; Li, G.; Chen, Y.-F.; Guo, T.-F.; Chu, C.-W. Lead-Free Antimony-Based Light-Emitting Diodes through the Vapor–Anion-Exchange Method. ACS Appl. Mater. Interfaces 2019, 11, 35088–35094. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Li, S.; Liu, J.; Guo, Y.; Niu, G.; Yao, L.; Fu, Y.; Gao, L.; Dong, Q.; et al. Efficient and stable emission of warm-white light from lead-free halide double perovskites. Nature 2018, 563, 541–545. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Zhang, Y.; Zhang, X.; Wang, S.; Lu, M.; Cui, H.; Kershaw, S.V.; Yu, W.W.; Rogach, A.L. Bright Orange Electroluminescence from Lead-Free Two-Dimensional Perovskites. ACS Energy Lett. 2019, 4, 242–248. [Google Scholar] [CrossRef]
- Wang, A.; Guo, Y.; Zhou, Z.; Niu, X.; Wang, Y.; Muhammad, F.; Li, H.; Zhang, T.; Wang, J.; Nie, S.; et al. Aqueous acid-based synthesis of lead-free tin halide perovskites with near-unity photoluminescence quantum efficiency. Chem. Sci. 2019, 10, 4573–4579. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Jiang, Z.; Liu, X.; Zhou, X.; Zhang, J.; Li, W. Bright Blue Light-Emitting Doped Cesium Bromide Nanocrystals: Alternatives of Lead-Free Perovskite Nanocrystals for White LEDs. Adv. Opt. Mater. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Schawlow, A.L.; Townes, C.H. Infrared and optical masers. Phys. Rev. 1958, 112, 1940–1949. [Google Scholar] [CrossRef] [Green Version]
- Maiman, T.H.; Hoskins, R.H.; D’Haenens, I.J.; Asawa, C.K.; Evtuhov, V. Stimulated optical emission in fluorescent solids. II. Spectroscopy and stimulated emission in ruby. Phys. Rev. 1961, 123, 1151–1157. [Google Scholar] [CrossRef]
- Maiman, T.H. Stimulated optical radiation in Ruby. Nature 1960, 187, 493–494. [Google Scholar] [CrossRef]
- Wang, K.; Wang, S.; Xiao, S.; Zhang, N.; Wang, Y.; Yang, W.; Wang, Y.; Zhang, C.; Sun, W.; Song, Q. Single-Crystalline Perovskite Microlasers for High-Contrast and Sub-Diffraction Imaging. Adv. Funct. Mater. 2019, 29, 1904868. [Google Scholar] [CrossRef]
- Wang, J.; Da, P.; Zhang, Z.; Luo, S.; Liao, L.; Sun, Z.; Shen, X.; Wu, S.; Zheng, G.; Chen, Z. Lasing from lead halide perovskite semiconductor microcavity system. Nanoscale 2018, 10, 10371–10376. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, J.; Du, J.; Hu, Z.; Shi, T.; Zhang, Z.; Liu, Y.; Tang, X.; Leng, Y.; Li, R. Robust Subwavelength Single-Mode Perovskite Nanocuboid Laser. ACS Nano 2018, 12, 5923–5931. [Google Scholar] [CrossRef] [PubMed]
- Weng, G.; Xue, J.; Tian, J.; Hu, X.; Bao, X.; Lin, H.; Chen, S.; Zhu, Z.; Chu, J. Picosecond Random Lasing Based on Three-Photon Absorption in Organometallic Halide CH 3 NH 3 PbBr 3 Perovskite Thin Films. ACS Photonics 2018, 5, 2951–2959. [Google Scholar] [CrossRef]
- Du, W.; Zhang, S.; Wu, Z.; Shang, Q.; Mi, Y.; Chen, J.; Qin, C.; Qiu, X.; Zhang, Q.; Liu, X. Unveiling lasing mechanism in CsPbBr 3 microsphere cavities. Nanoscale 2019, 11, 3138–3144. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Azuma, T.; Yuasa, T.; Ito, R. Biexciton lasing in the layered perovskite-type material (C6H13NH3) 2PbI4. Solid State Commun. 1998, 105, 253–255. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Ding, C.; Kobayashi, S.; Izuishi, T.; Nakazawa, N.; Toyoda, T.; Ohta, T.; Hayase, S.; Minemoto, T.; et al. Highly Luminescent Phase-Stable CsPbI 3 Perovskite Quantum Dots Achieving Near 100% Absolute Photoluminescence Quantum Yield. ACS Nano 2017, 11, 10373–10383. [Google Scholar] [CrossRef]
- Safdar, A.; Wang, Y.; Krauss, T.F. Random lasing in solution-processed perovskite thin films. Opt. InfoBase Conf. Pap. 2017, 26, 75–84. [Google Scholar]
- Li, Z.; Moon, J.; Gharajeh, A.; Haroldson, R.; Hawkins, R.; Hu, W.; Zakhidov, A.; Gu, Q. Room-temperature continuous-wave operation of organometal halide perovskite lasers. ACS Nano 2018, 12, 10968–10976. [Google Scholar] [CrossRef] [Green Version]
- Mathies, F.; Brenner, P.; Hernandez-Sosa, G.; Howard, I.A.; Paetzold, U.W.; Lemmer, U. Inkjet-printed perovskite distributed feedback lasers. Opt. Express 2018, 26, A144. [Google Scholar] [CrossRef]
- Jäckle, M.; Linnenbank, H.; Hentschel, M.; Saliba, M.; Tikhodeev, S.G.; Giessen, H. Tunable green lasing from circular grating distributed feedback based on CH 3 NH 3 PbBr 3 perovskite. Opt. Mater. Express 2019, 9, 2006–2021. [Google Scholar] [CrossRef]
- Weng, G.; Tian, J.; Chen, S.; Xue, J.; Yan, J.; Hu, X.; Chen, S.; Zhu, Z.; Chu, J. Giant reduction of the random lasing threshold in CH3NH3PbBr3 perovskite thin films by using a patterned sapphire substrate. Nanoscale 2019, 11, 10636–10645. [Google Scholar] [CrossRef]
- Guo, P.; Hossain, M.K.; Shen, X.; Sun, H.; Yang, W.; Liu, C.; Ho, C.Y.; Kwok, C.K.; Tsang, S.W.; Luo, Y.; et al. Room-Temperature Red–Green–Blue Whispering-Gallery Mode Lasing and White-Light Emission from Cesium Lead Halide Perovskite (CsPbX 3, X = Cl, Br, I) Microstructures. Adv. Opt. Mater. 2018, 6, 1700993. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, Z.; Bian, Y.; Liu, D.; Tang, X.; Hu, W.; Zang, Z.; Zhou, M.; Sun, L.; Tang, J.; et al. Robust Cesium Lead Halide Perovskite Microcubes for Frequency Upconversion Lasing. Adv. Opt. Mater. 2017, 5, 1700419. [Google Scholar] [CrossRef]
- Zhou, B.; Dong, H.; Jiang, M.; Zheng, W.; Sun, L.; Zhao, B.; Tang, B.; Pan, A.; Zhang, L. Single-mode lasing and 3D confinement from perovskite micro-cubic cavity. J. Mater. Chem. C 2018, 6, 11740–11748. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, M.; Dong, H.; Zheng, W.; Huang, Y.; Han, J.; Pan, A.; Zhang, L. High-Temperature Upconverted Single-Mode Lasing in 3D Fully Inorganic Perovskite Microcubic Cavity. ACS Photonics 2019, 6, 793–801. [Google Scholar] [CrossRef]
- Mi, Y.; Liu, Z.; Shang, Q.; Niu, X.; Shi, J.; Zhang, S.; Chen, J.; Du, W.; Wu, Z.; Wang, R.; et al. Fabry–Pérot Oscillation and Room Temperature Lasing in Perovskite Cube-Corner Pyramid Cavities. Small 2018, 14, 1703136. [Google Scholar] [CrossRef]
- Zhao, X.; He, D.-W.; Wang, Y.-S.; Hu, Y.; Fu, C.; Li, X. Label-free tungsten disulfide quantum dots as a fluorescent sensing platform for highly efficient detection of copper (II) ions. Chin. Phys. B 2017, 26, 066102. [Google Scholar] [CrossRef]
- Zhang, N.; Fan, Y.; Wang, K.; Gu, Z.; Wang, Y.; Ge, L.; Xiao, S.; Song, Q. All-optical control of lead halide perovskite microlasers. Nat. Commun. 2019, 10, 1770. [Google Scholar] [CrossRef]
- Liu, X.; Niu, L.; Wu, C.; Cong, C.; Wang, H.; Zeng, Q.; He, H.; Fu, Q.; Fu, W.; Yu, T.; et al. Periodic Organic–Inorganic Halide Perovskite Microplatelet Arrays on Silicon Substrates for Room-Temperature Lasing. Adv. Sci. 2016, 3, 1600137. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Zhu, H.; Stoumpos, C.C.; Ding, Q.; Wang, J.; Kanatzidis, M.G.; Zhu, X.; Jin, S. Broad Wavelength Tunable Robust Lasing from Single-Crystal Nanowires of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). ACS Nano 2016, 10, 7963–7972. [Google Scholar] [CrossRef]
- Evans, T.J.S.; Schlaus, A.; Fu, Y.; Zhong, X.; Atallah, T.L.; Spencer, M.S.; Brus, L.E.; Jin, S.; Zhu, X.Y. Continuous-Wave Lasing in Cesium Lead Bromide Perovskite Nanowires. Adv. Opt. Mater. 2018, 6, 1700982. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, Y.; Wei, C.; Zhang, W.; Gao, Z.; Zhao, Y.S. Switchable Single-Mode Perovskite Microlasers Modulated by Responsive Organic Microdisks. Nano Lett. 2018, 18, 1241–1245. [Google Scholar] [CrossRef] [PubMed]
- Schlaus, A.P.; Spencer, M.S.; Miyata, K.; Liu, F.; Wang, X.; Datta, I.; Lipson, M.; Pan, A.; Zhu, X.Y. How lasing happens in CsPbBr 3 perovskite nanowires. Nat. Commun. 2019, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Dong, H.; Sun, L.; Zheng, W.; Wang, Q.; Sun, F.; Jiang, X.; Pan, A.; Zhang, L. Single-Mode Lasers Based on Cesium Lead Halide Perovskite Submicron Spheres. ACS Nano 2017, 11, 10681–10688. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Wang, X.; Shen, Y.; Luo, Z.; Li, L.; Gan, L.; Ma, Y.; Li, H.; Pan, A.; Zhai, T. Space-Confined Synthesis of 2D All-Inorganic CsPbI 3 Perovskite Nanosheets for Multiphoton-Pumped Lasing. Adv. Opt. Mater. 2018, 6, 1800879. [Google Scholar] [CrossRef]
- Huang, C.; Sun, W.; Liu, S.; Li, S.; Wang, S.; Wang, Y.; Zhang, N.; Fu, H.; Xiao, S.; Song, Q. Highly Controllable Lasing Actions in Lead Halide Perovskite–Si 3 N 4 Hybrid Micro-Resonators. Laser Photonics Rev. 2019, 13, 1800189. [Google Scholar] [CrossRef]
- Zhizhchenko, A.; Syubaev, S.; Berestennikov, A.; Yulin, A.V.; Porfirev, A.; Pushkarev, A.; Shishkin, I.; Golokhvast, K.; Bogdanov, A.A.; Zakhidov, A.A.; et al. Single-Mode Lasing from Imprinted Halide-Perovskite Microdisks. ACS Nano 2019, 13, 4140–4147. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.; Ren, Y.; Wang, Z.; Yao, B.; Dong, Z.; Adamo, G.; Zeng, H.; Sun, H. Perovskite-Ion Beam Interactions: Toward Controllable Light Emission and Lasing. ACS Appl. Mater. Interfaces 2019, 11, 15756–15763. [Google Scholar] [CrossRef]
- Booker, E.P.; Price, M.B.; Budden, P.J.; Abolins, H.; del Valle-Inclan Redondo, Y.; Eyre, L.; Nasrallah, I.; Phillips, R.T.; Friend, R.H.; Deschler, F.; et al. Vertical Cavity Biexciton Lasing in 2D Dodecylammonium Lead Iodide Perovskites. Adv. Opt. Mater. 2018, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, C.M.; Chen, T.P.; Li, S.S.; Chen, W.L.; Lo, C.Y.; Liao, Y.M.; Haider, G.; Lin, C.C.; Chen, C.C.; Sankar, R.; et al. Low-Threshold Lasing from 2D Homologous Organic-Inorganic Hybrid Ruddlesden-Popper Perovskite Single Crystals. Nano Lett. 2018, 18, 3221–3228. [Google Scholar] [CrossRef]
- Liang, Y.; Shang, Q.; Wei, Q.; Zhao, L.; Liu, Z.; Shi, J.; Zhong, Y.; Chen, J.; Gao, Y.; Li, M.; et al. Lasing from Mechanically Exfoliated 2D Homologous Ruddlesden–Popper Perovskite Engineered by Inorganic Layer Thickness. Adv. Mater. 2019, 31, 1903030. [Google Scholar] [CrossRef]
- Zhai, W.; Tian, C.; Yuan, K.; Ge, C.; Zhao, S.; Yu, H.; Li, Y.; Chen, W.; Ran, G. Optically pumped lasing of segregated quasi-2D perovskite microcrystals in vertical microcavity at room temperature. Appl. Phys. Lett. 2019, 114, 131107. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Nalla, V.; Zeng, H.; Sun, H. Solution-Processed Low Threshold Vertical Cavity Surface Emitting Lasers from All-Inorganic Perovskite Nanocrystals. Adv. Funct. Mater. 2017, 27, 1605088. [Google Scholar] [CrossRef]
- Lin, C.H.; Zeng, Q.; Lafalce, E.; Yu, S.; Smith, M.J.; Yoon, Y.J.; Chang, Y.; Jiang, Y.; Lin, Z.; Vardeny, Z.V.; et al. Large-Area Lasing and Multicolor Perovskite Quantum Dot Patterns. Adv. Opt. Mater. 2018, 6, 1800474. [Google Scholar] [CrossRef]
- Fan, T.; Lü, J.; Chen, Y.; Yuan, W.; Huang, Y. Random lasing in cesium lead bromine perovskite quantum dots film. J. Mater. Sci. Mater. Electron. 2019, 30, 1084–1088. [Google Scholar] [CrossRef]
- Tang, X.; Hu, Z.; Chen, W.; Xing, X.; Zang, Z.; Hu, W.; Qiu, J.; Du, J.; Leng, Y.; Jiang, X.; et al. Room temperature single-photon emission and lasing for all-inorganic colloidal perovskite quantum dots. Nano Energy 2016, 28, 462–468. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, D.; Li, X.; Zhong, J.; Xu, X. In Situ Crystallization Synthesis of CsPbBr3 Perovskite Quantum Dot-Embedded Glasses with Improved Stability for Solid-State Lighting and Random Upconverted Lasing. ACS Appl. Mater. Interfaces 2018, 10, 18918–18926. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Meng, L.; Chen, L.; Huang, S.; Wu, X.; Dai, G.; Deng, L.; Han, J.; Zou, B.; Zhang, C.; et al. Ultralow-Threshold and Color-Tunable Continuous-Wave Lasing at Room-Temperature from in Situ Fabricated Perovskite Quantum Dots. J. Phys. Chem. Lett. 2019, 10, 3248–3253. [Google Scholar] [CrossRef]
- Pourdavoud, N.; Haeger, T.; Mayer, A.; Cegielski, P.J.; Giesecke, A.L.; Heiderhoff, R.; Olthof, S.; Zaefferer, S.; Shutsko, I.; Henkel, A.; et al. Room-Temperature Stimulated Emission and Lasing in Recrystallized Cesium Lead Bromide Perovskite Thin Films. Adv. Mater. 2019, 31, 1903717. [Google Scholar] [CrossRef] [Green Version]
- Brenner, P.; Bar-On, O.; Jakoby, M.; Allegro, I.; Richards, B.S.; Paetzold, U.W.; Howard, I.A.; Scheuer, J.; Lemmer, U. Continuous wave amplified spontaneous emission in phase-stable lead halide perovskites. Nat. Commun. 2019, 10, 988. [Google Scholar] [CrossRef]
- Li, G.; Price, M.; Deschler, F. Research Update: Challenges for high-efficiency hybrid lead-halide perovskite LEDs and the path towards electrically pumped lasing. APL Mater. 2016, 4, 091507. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Wei, Q.; Muduli, S.K.; Yantara, N.; Xu, Q.; Mathews, N.; Mhaisalkar, S.G.; Xing, G.; Sum, T.C. Enhanced Exciton and Photon Confinement in Ruddlesden–Popper Perovskite Microplatelets for Highly Stable Low-Threshold Polarized Lasing. Adv. Mater. 2018, 30, 1707235. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Cheng, X.; Wang, Y.; Liu, Y.; Rong, K.; Li, Z.; Wan, Y.; Gong, W.; Watanabe, K.; Taniguchi, T.; et al. Waterproof Perovskite-Hexagonal Boron Nitride Hybrid Nanolasers with Low Lasing Thresholds and High Operating Temperature. ACS Photonics 2018, 5, 4520–4528. [Google Scholar] [CrossRef]
- Huang, L.; Gao, Q.; Sun, L.D.; Dong, H.; Shi, S.; Cai, T.; Liao, Q.; Yan, C.H. Composition-Graded Cesium Lead Halide Perovskite Nanowires with Tunable Dual-Color Lasing Performance. Adv. Mater. 2018, 30, 1800596. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Kumar, M.H.; Chong, W.K.; Liu, X.; Cai, Y.; Ding, H.; Asta, M.; Grätzel, M.; Mhaisalkar, S.; Mathews, N.; et al. Solution-Processed Tin-Based Perovskite for Near-Infrared Lasing. Adv. Mater. 2016, 28, 8191–8196. [Google Scholar] [CrossRef]
- Chen, L.J.; Dai, J.H.; Lin, J.D.; Mo, T.S.; Lin, H.P.; Yeh, H.C.; Chuang, Y.C.; Jiang, S.A.; Lee, C.R. Wavelength-Tunable and Highly Stable Perovskite-Quantum-Dot-Doped Lasers with Liquid Crystal Lasing Cavities. ACS Appl. Mater. Interfaces 2018, 10, 33307–33315. [Google Scholar] [CrossRef]

| Chemical Formula | Morphology | Size * | Type of Synthesis | Abs, nm | PL, nm | PL FWHM, nm | PLQY, % | Reference |
|---|---|---|---|---|---|---|---|---|
| Tin-Based Perovskites | ||||||||
| Cs1–xRbxSnBr3 | Film | N/A | Annealing | 670–750 | 680–710 | 50 | N/A | [29] |
| Cs2SnCl6−xBrx | Single crystal | 1–2 cm | Hydrothermal | 350–750 | N/A | [30] | ||
| Cs2SnX6 (X = Br, I) | Single crystal | 3–5 µm | Hydrothermal | 900 | 675 | 50 | N/A | [31] |
| BA2MA3Sn4I13 | Film | GS 9 µm | Spin-coating | 850 | 990 | 75 | N/A | [32] |
| Bismuth-Based Perovskites | ||||||||
| (C6H5NH3)BiI4 | Film | area > 20 cm2 | Spin-coating | 525 | 690 | 30 | N/A | [36] |
| Rb7Bi3Cl16 | Single crystal | 18.4 × 7.2 × 6.0 mm | Hydrothermal | 280 | 437 | 93 | 28.4 | [37] |
| Rb7Bi3Cl16 | NCs | 1.85 nm | LARP | 280 | 437 | 93 | 28.4 | [37] |
| Cs3BiBr6 | Single crystal | 30 µm | Annealing | 485 | 475 | 52 | 19.4 | [38] |
| (CH3NH3)3Bi2I9 | Film | GS 7.57 μm | Spin-coating | 650 | N/A | [39] | ||
| (CH3NH3)3Bi2I9 | Film | GS 200 nm | Spin-coating | 600 | 575 and 605 | N/A | [40] | |
| (PrAm)2Bi2I10·2H2O | Single crystal | 1–10 mm | Antisolvent diffusion | 600 | 530 and 690 | N/A | [51] | |
| Cs3Bi2Br9 | NCs | 3.3 nm | Hot injection | 390 | 600 and 620 | 42.4 | [53] | |
| Other Elements (In, Au, Cu, Yb) | ||||||||
| Cs2InBr5⋅H2O | Single crystal | 2 mm | Hydrothermal | 450 | 695 | 200 | 33 | [34] |
| (CH3NH3)AuX4⋅H2O, X = Cl, Br | Single crystal | 5 mm | Slow evaporation | 650 | 425 | 75 | N/A | [50] |
| Cs3Cu2I5 | Film | GS 40 µm | Vapor-assisted | 400 | 442 | 100 | N/A | [54] |
| CsYbI3 | NCs | 9.5 nm | Hot injection | 660 | 671 | 50 | 58 | [55] |
| Double Perovskites | ||||||||
| Cs2AgInCl6 | NCs | 10 nm | Hot injection | 300 | 550 | 250 | N/A | [43] |
| Cs2AgSbCl6 | NCs | 10 nm | Hot injection | 360 | 550 | 250 | N/A | [43] |
| Cs2AgBiBr6 | Film | T 200 nm | Spin-coating | 440 | 620 | 50 | N/A | [44] |
| Cs2AgInCl6 | NCs | 10 nm | Hot injection | 390 | 580 | 125 | 11.4 | [45] |
| Cs2AgSbBr6 | Single crystal | 1 mm | Hydrothermal | 550 | N/A | [46] | ||
| Cs2AgBiBr6 | Single crystal | 1–10 µm | Hydrothermal | 650 | N/A | [47] | ||
| Cs2AgBiBr6 | Film | GS 500 nm | Spin-coating | N/A | [48] | |||
| Cs2AgBiBr6 | Single crystal | 250 nm | Hydrothermal | 440 | 630 | 145 | N/A | [49] |
| Alloyed Perovskites | ||||||||
| CsSn0.5Ge0.5I3 | Film | T 200 nm, GS 80 nm | Pyrex tubes annealing | 840 | 830 | 52 | N/A | [56] |
| (C8NH12)4Bi0.57Sb0.43Br7⋅H2O | Single crystals | 3 mm | Cooling-induced | 850 | 450 and 640 | N/A | [52] | |
| Chemical Formula | Type of Perovskite | Type of Resonator | Pump Source | Lasing Threshold (CW—kW/cm2; P—µJ/cm2) | Spontaneous Emission | Stimulated Emission | Q max | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PL Peak, nm; PLQY | FWHM, nm | PL Peak, nm | FWHM, nm | |||||||
| (C6H13NH3)2PbI4 | thin film | DBR | 337 nm, 3 ns | 20 @ 16 K | 543 | N/A | 544 | 2 | 272 | [86] |
| MAPbI3 | nanoimprinted thin film | DFB | CW 355 nm | 13·10−3 | 780 | N/A | 780 | 1.16 | 672 | [89] |
| MAPbI3 | thin film on the grating | DFB | 532 nm, 1 ns | 235 | 780 | 50 | 784 | 0.4 | 1960 | [90] |
| MAPbX3 | NCs | DFB | CW 405 nm | 15–58·10−3 | 515/540; 95–97% | 30 | 538.7 | 0.45 | 1200 | [119] |
| CsPbX3 | thin films | VCSEL | 355 nm, 0.3 ns, 1 kHz | 2.2 | 538; 68% | 20 | 538.3 | 0.07 | 5400 | [120] |
| MAPbBr3 | microcrystals | F-P | 400 nm, 100 fs, 1 kHz | 9.1 | 530 | 40 | 548 | 0.21 | 2500 | [81] |
| CsPbBr3 | nanocuboid | F-P | 400/800 nm, 35 fs, 1k Hz | 40.2 | 530 | 18 | 539.2 | 0.29 | 2075/1859 | [83] |
| CsPbBr3 | nanowires | F-P | CW 450 nm | 6 @ 77 K | 530 | 15 | 533 | 0.25 | 2300 | [102] |
| (OA)2(MA)n−1PbnBr3n+1 | 2D R–P layers | F-P | 400 nm, fs | 8 | 530; 65% | 30 | 545 | 0.3–0.6 | 1815 | [123] |
| CsPbBr3 | microcuboids | WGM | 400 nm, 40 fs, 10 kHz | 16.9 | 525 | 15.6 | 540.9 | 0.064 | 8500 | [95] |
| CsPbBr3 | microcuboids | WGM | 800 nm, 40 fs, 10 kHz | 210 | 528 | 20 | 536.8 | 0.053 | 10,100 | [96] |
| CsPbBr3 | microspheres | WGM | 800 nm, 120 fs, 76 MHz | 3.5 @ 300 K 0.4 @ 77 K | 530 | 20 | 535–540 | 0.15 | 3600 | [85] |
| (C4H9NH3)2(CH3NH3)n−1 PbnI3n+1 (n = 1, 2, and 3) | 2D R–P layers | RL | 374 nm, 55 ps, 40 MHz | 2.85 | 520–630 | 20 | 520–630 | <0.5 | 1040 | [111] |
| (BA)2(MA)n−1PbnI3n+1 | 2D layers | RL | 400 nm, 80 fs, 1 kHz | 2.3 @ 70 K | 520–680 | 15 | 520–680 | 0.9 | 755 | [112] |
| MAPbBr3 | thin films | RL | 800/400 nm, 35 fs, 1 kHz | 0.15 @ 300 K | 535/545 | 30 | 550 | 5 | 110 | [92] |
| CsSnX3 (X = Br, I) doped with SnF2 | Film | DFB | 650 nm, 50 fs, 1 kHz | 6 | 680–950; 13% | 50–100 | 950 | 1.93 | 500 | [126] |
| CsSnI3 | NCs in CLCC | DFB | 532 nm, 8 ns, 10 Hz | 800 | 594 | 35 | 579–606 | 0.2 | 2895 | [127] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ushakova, E.V.; Cherevkov, S.A.; Kuznetsova, V.A.; Baranov, A.V. Lead-Free Perovskites for Lighting and Lasing Applications: A Minireview. Materials 2019, 12, 3845. https://doi.org/10.3390/ma12233845
Ushakova EV, Cherevkov SA, Kuznetsova VA, Baranov AV. Lead-Free Perovskites for Lighting and Lasing Applications: A Minireview. Materials. 2019; 12(23):3845. https://doi.org/10.3390/ma12233845
Chicago/Turabian StyleUshakova, Elena V., Sergei A. Cherevkov, Vera A. Kuznetsova, and Alexander V. Baranov. 2019. "Lead-Free Perovskites for Lighting and Lasing Applications: A Minireview" Materials 12, no. 23: 3845. https://doi.org/10.3390/ma12233845






