Silver-Copper Oxide Heteronanostructures for the Plasmonic-Enhanced Photocatalytic Oxidation of N-Hexane in the Visible-NIR Range
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Photocatalysts
2.2. Characterization Techniques
2.3. Photocatalytic Reaction Setup
3. Results
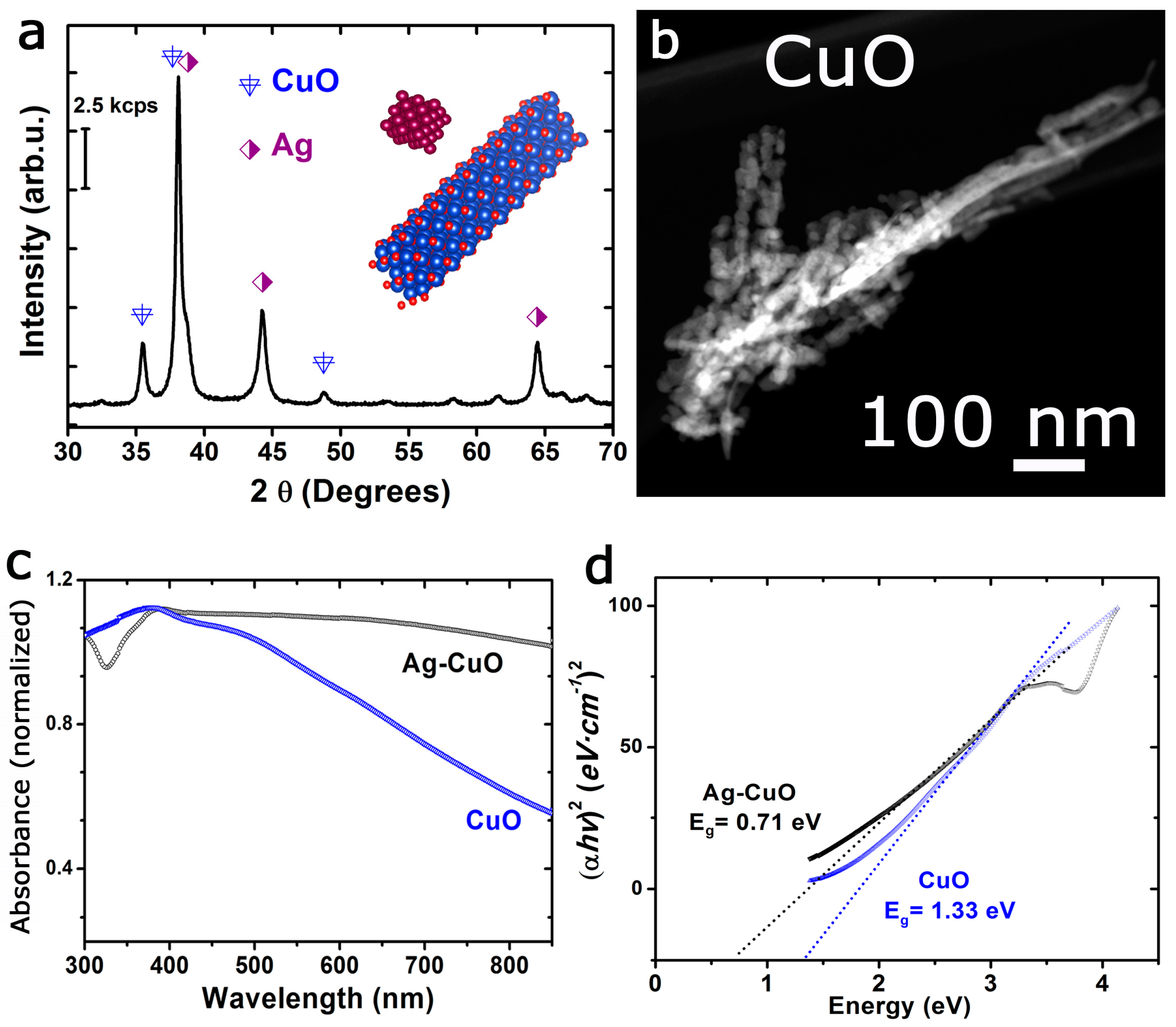
3.1. Characterization of the Silver-Copper Oxide Plasmonic Photocatalyst
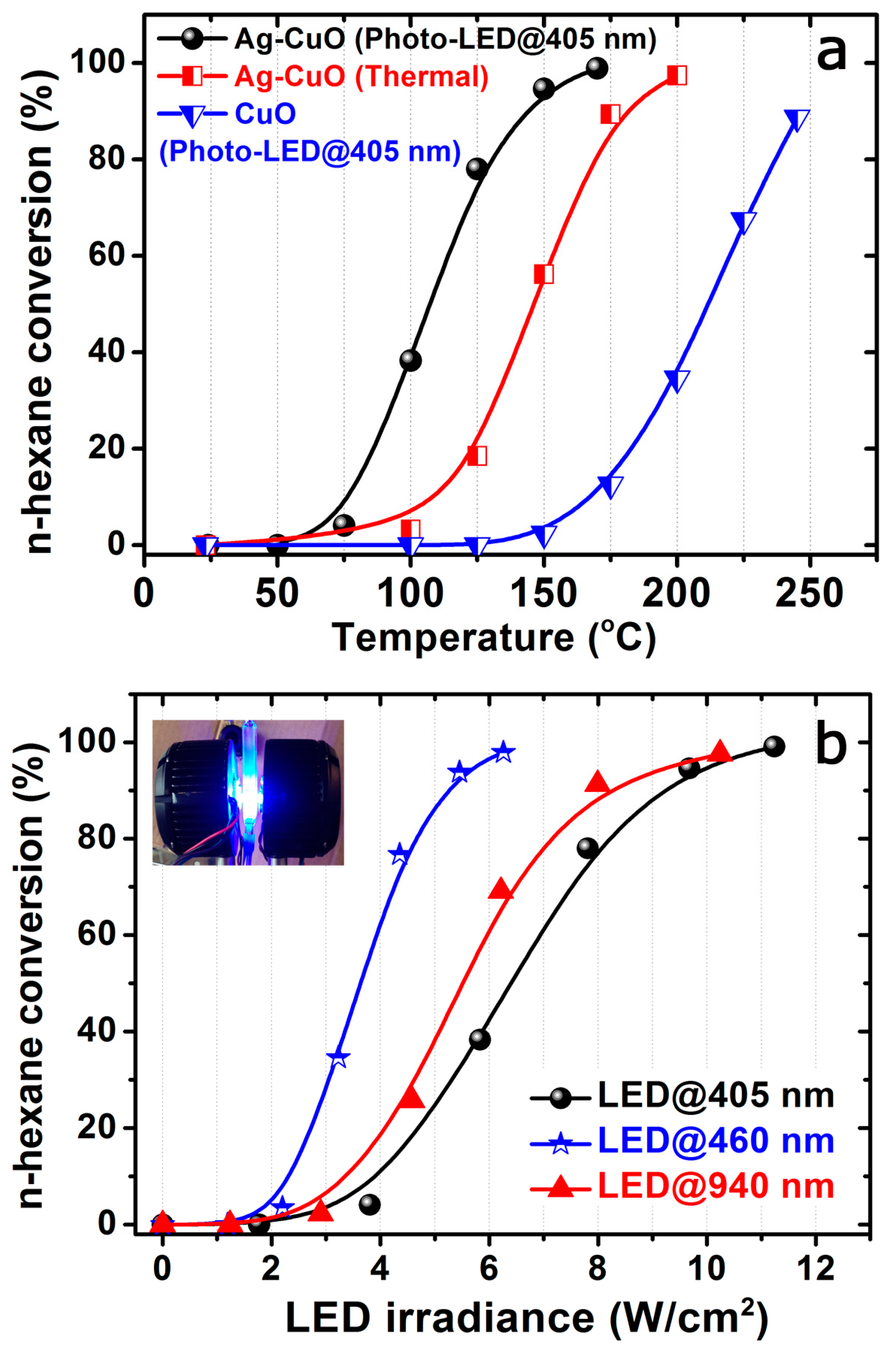
3.2. Photocatalytic Performance of the Ag-CuO Heterostructures for N-Hexane Total Oxidation
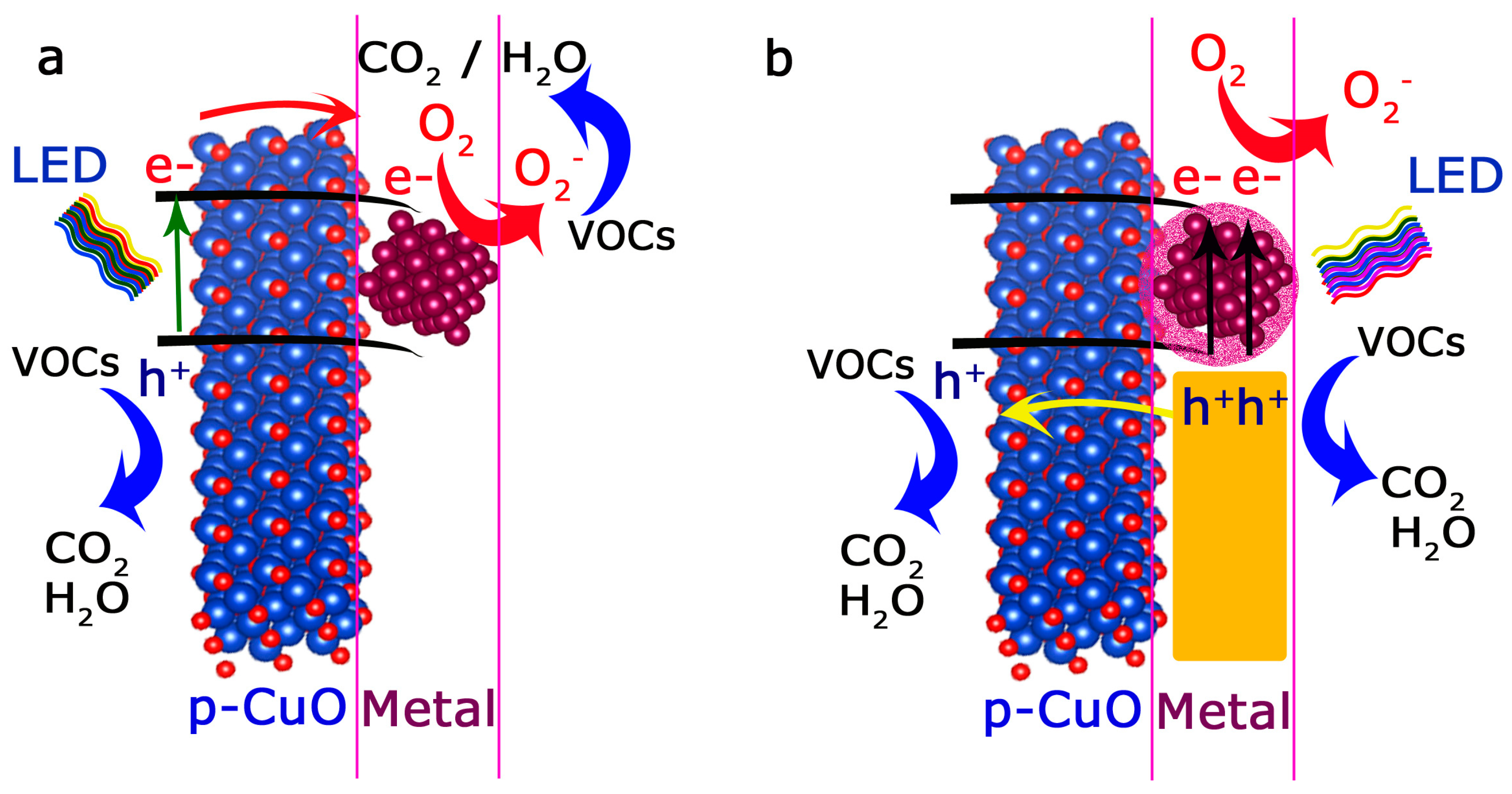
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z.P. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Huang, H.B.; Xu, Y.; Feng, Q.Y.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Smielowska, M.; Marc, M.; Zabiegala, B. Indoor air quality in public utility environments—A review. Environ. Sci. Pollut. Res. 2017, 24, 11166–11176. [Google Scholar] [CrossRef] [PubMed]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Kim, H.H.; Teramoto, Y.; Negishi, N.; Ogata, A. A multidisciplinary approach to understand the interactions of nonthermal plasma and catalyst: A review. Catal. Today 2015, 256, 13–22. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Jiang, Z.; Shangguan, W.F. Low-temperature catalysis for VOCs removal in technology and application: A state-of-the-art review. Catal. Today 2016, 264, 270–278. [Google Scholar] [CrossRef]
- Hueso, J.L.; Cotrino, J.; Caballero, A.; Espinos, J.P.; Gonzalez-Elipe, A.R. Plasma catalysis with perovskite-type catalysts for the removal of NO and CH4 from combustion exhausts. J. Catal. 2007, 247, 288–297. [Google Scholar] [CrossRef]
- Mao, L.G.; Chen, Z.Z.; Wu, X.Y.; Tang, X.J.; Yao, S.L.; Zhang, X.M.; Jiang, B.Q.; Han, J.Y.; Wu, Z.L.; Lu, H.; et al. Plasma-catalyst hybrid reactor with CeO2/gamma-Al2O3 for benzene decomposition with synergetic effect and nano particle by-product reduction. J. Hazard. Mater. 2018, 347, 150–159. [Google Scholar] [CrossRef]
- Nigar, H.; Sturm, G.S.J.; Garcia-Banos, B.; Penaranda-Foix, F.L.; Catala-Civera, J.M.; Mallada, R.; Stankiewicz, A.; Santamaria, J. Numerical analysis of microwave heating cavity: Combining electromagnetic energy, heat transfer and fluid dynamics for a NaY zeolite fixed-bed. Appl. Therm. Eng. 2019, 155, 226–238. [Google Scholar] [CrossRef]
- Nigar, H.; Julian, I.; Mallada, R.; Santamaria, J. Microwave-Assisted Catalytic Combustion for the Efficient Continuous Cleaning of VOC-Containing Air Streams. Environ. Sci. Technol. 2018, 52, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Nigar, H.; Navascues, N.; De la Iglesia, O.; Mallada, R.; Santamaria, J. Removal of VOCs at trace concentration levels from humid air by Microwave Swing Adsorption, kinetics and proper sorbent selection. Sep. Purif. Technol. 2015, 151, 193–200. [Google Scholar] [CrossRef]
- Yang, X.G.; Wang, D.W. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energ. Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Kumari, G.; Zhang, X.Q.; Devasia, D.; Heo, J.; Jain, P.K. Watching Visible Light-Driven CO2 Reduction on a Plasmonic Nanoparticle Catalyst. ACS Nano 2018, 12, 8330–8340. [Google Scholar] [CrossRef] [PubMed]
- Brigden, C.T.; Poulston, S.; Twigg, M.V.; Walker, A.P.; Wilkins, A.J.J. Photo-oxidation of short-chain hydrocarbons over titania. Appl. Catal. B Environ. 2001, 32, 63–71. [Google Scholar] [CrossRef]
- Chen, J.Y.; He, Z.G.; Li, G.Y.; An, T.C.; Shi, H.X.; Li, Y.Z. Visible-light-enhanced photothermocatalytic activity of ABO(3)-type perovskites for the decontamination of gaseous styrene. Appl. Catal. B Environ. 2017, 209, 146–154. [Google Scholar] [CrossRef]
- Deng, X.Y.; Yue, Y.H.; Gao, Z. Gas-phase photo-oxidation of organic compounds over nanosized TiO2 photocatalysts by various preparations. Appl. Catal. B Environ. 2002, 39, 135–147. [Google Scholar] [CrossRef]
- Boulamanti, A.K.; Philippopoulos, C.J. Photocatalytic degradation of C-5-C-7 alkanes in the gas-phase. Atmos. Environ. 2009, 43, 3168–3174. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Sun, H.Q.; Liu, J.; Pareek, V.K.; Wang, S.B. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Bueno-Alejo, C.J.; Hueso, J.L.; Mallada, R.; Julian, I.; Santamaria, J. High-radiance LED-driven fluidized bed photoreactor for the complete oxidation of n-hexane in air. Chem. Eng. J. 2019, 358, 1363–1370. [Google Scholar] [CrossRef]
- Liotta, L.F.; Ousmane, M.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Marci, G.; Retailleau, L.; Giroir-Fendler, A. Total oxidation of propene at low temperature over Co3O4-CeO2 mixed oxides: Role of surface oxygen vacancies and bulk oxygen mobility in the catalytic activity. Appl. Catal. A Gen. 2008, 347, 81–88. [Google Scholar] [CrossRef]
- Ousmane, M.; Liotta, L.F.; Di Carlo, G.; Pantaleo, G.; Venezia, A.M.; Deganello, G.; Retailleau, L.; Boreave, A.; Giroir-Fendler, A. Supported Au catalysts for low-temperature abatement of propene and toluene, as model VOCs: Support effect. Appl. Catal. B Environ. 2011, 101, 629–637. [Google Scholar] [CrossRef]
- Scire, S.; Liotta, L.F. Supported gold catalysts for the total oxidation of volatile organic compounds. Appl. Catal. B Environ. 2012, 125, 222–246. [Google Scholar] [CrossRef]
- Liotta, L.F.; Wu, H.J.; Pantaleo, G.; Venezia, A.M. Co3O4 nanocrystals and Co3O4-MOx binary oxides for CO, CH4 and VOC oxidation at low temperatures: A review. Catal. Sci. Technol. 2013, 3, 3085–3102. [Google Scholar] [CrossRef]
- Ousmane, M.; Liotta, L.F.; Pantaleo, G.; Venezia, A.M.; Di Carlo, G.; Aouine, M.; Retailleau, L.; Giroir-Fendler, A. Supported Au catalysts for propene total oxidation: Study of support morphology and gold particle size effects. Catal. Today 2011, 176, 7–13. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Mikheeva, N.N.; Mamontov, G.V.; Salaev, M.A.; Liotta, L.F.; Vodyankina, O.V. Ag/CeO2 Composites for Catalytic Abatement of CO, Soot and VOCs. Catalysts 2018, 8, 285. [Google Scholar] [CrossRef]
- Sihaib, Z.; Puleo, F.; Pantaleo, G.; La Parola, V.; Valverde, J.L.; Gil, S.; Liotta, L.F.; Giroir-Fendler, A. The Effect of Citric Acid Concentration on the Properties of LaMnO3 as a Catalyst for Hydrocarbon Oxidation. Catalysts 2019, 9, 226. [Google Scholar] [CrossRef]
- Liu, L.C.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Yang, C.T.; Miao, G.; Pi, Y.H.; Xia, Q.B.; Wu, J.L.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Uson, L.; Hueso, J.L.; Sebastian, V.; Arenal, R.; Florea, I.; Irusta, S.; Arruebo, M.; Santamaria, J. In-situ preparation of ultra-small Pt nanoparticles within rod-shaped mesoporous silica particles: 3-D tomography and catalytic oxidation of n-hexane. Catal. Commun. 2017, 100, 93–97. [Google Scholar] [CrossRef]
- Uson, L.; Colmenares, M.G.; Hueso, J.L.; Sebastian, V.; Balas, F.; Arruebo, M.; Santamaria, J. VOCs abatement using thick eggshell Pt/SBA-15 pellets with hierarchical porosity. Catal. Today 2014, 227, 179–186. [Google Scholar] [CrossRef]
- Hueso, J.L.; Sebastian, V.; Mayoral, A.; Uson, L.; Arruebo, M.; Santamaria, J. Beyond gold: rediscovering tetrakis-(hydroxymethyl)-phosphonium chloride (THPC) as an effective agent for the synthesis of ultra-small noble metal nanoparticles and Pt-containing nanoalloys. RSC Adv. 2013, 3, 10427–10433. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Nikolla, E.; Linic, S.; Medlin, J.W.; Janik, M.J. Multicomponent Catalysts: Limitations and Prospects. ACS Catal. 2018, 8, 3202–3208. [Google Scholar] [CrossRef]
- Cellier, C.; Lambert, S.; Gaigneaux, E.M.; Poleunis, C.; Ruaux, V.; Eloy, P.; Lahousse, C.; Bertrand, P.; Pirard, J.P.; Grange, P. Investigation of the preparation and activity of gold catalysts in the total oxidation of n-hexane. Appl. Catal. B Environ. 2007, 70, 406–416. [Google Scholar] [CrossRef]
- Liotta, L.F. Catalytic oxidation of volatile organic compounds on supported noble metals. Appl. Catal. B-Environ. 2010, 100, 403–412. [Google Scholar] [CrossRef]
- Guo, J.H.; Lin, C.X.; Jiang, C.J.; Zhang, P.Y. Review on noble metal-based catalysts for formaldehyde oxidation at room temperature. Appl. Surf. Sci. 2019, 475, 237–255. [Google Scholar] [CrossRef]
- Liotta, L.F.; Ousmane, M.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Boreave, A.; Giroir-Fendler, A. Catalytic Removal of Toluene over Co3O4-CeO2 Mixed Oxide Catalysts: Comparison with Pt/Al2O3. Catal. Lett. 2009, 127, 270–276. [Google Scholar] [CrossRef]
- Pereniguez, R.; Hueso, J.L.; Gaillard, F.; Holgado, J.P.; Caballero, A. Study of Oxygen Reactivity in La1-x Sr (x) CoO3-delta Perovskites for Total Oxidation of Toluene. Catal. Lett. 2012, 142, 408–416. [Google Scholar] [CrossRef]
- Pereniguez, R.; Hueso, J.L.; Holgado, J.P.; Gaillard, F.; Caballero, A. Reactivity of LaNi1-y Co (y) O3-delta Perovskite Systems in the Deep Oxidation of Toluene. Catal. Lett. 2009, 131, 164–169. [Google Scholar] [CrossRef]
- Szabo, V.; Bassir, M.; Gallot, J.E.; Van Neste, A.; Kaliaguine, S. Perovskite-type oxides synthesised by reactive grinding–Part III. Kinetics of n-hexane oxidation over LaCo(1-x)FexO3. Appl. Catal. B Environ. 2003, 42, 265–277. [Google Scholar] [CrossRef]
- Rhodes, C.J. Perovskites - some snapshots of recent developments. Sci. Prog. 2018, 101, 384–396. [Google Scholar] [CrossRef]
- Njagi, E.C.; Genuino, H.C.; King’ondu, C.K.; Dharmarathna, S.; Suib, S.L. Catalytic oxidation of ethylene at low temperatures using porous copper manganese oxides. Appl. Catal. A Gen. 2012, 421, 154–160. [Google Scholar] [CrossRef]
- Genuino, H.C.; Dharmarathna, S.; Njagi, E.C.; Mei, M.C.; Suib, S.L. Gas-Phase Total Oxidation of Benzene, Toluene, Ethylbenzene, and Xylenes Using Shape-Selective Manganese Oxide and Copper Manganese Oxide Catalysts. J. Phys. Chem. C 2012, 116, 12066–12078. [Google Scholar] [CrossRef]
- Cordi, E.M.; O’Neill, P.J.; Falconer, J.L. Transient oxidation of volatile organic compounds on a CuO/Al2O3 catalyst. Appl. Catal. B-Environ. 1997, 14, 23–36. [Google Scholar] [CrossRef]
- Li, T.Y.; Chiang, S.J.; Liaw, B.J.; Chen, Y.Z. Catalytic oxidation of benzene over CuO/Ce1-xMnxO2 catalysts. Appl. Catal. B Environ. 2011, 103, 143–148. [Google Scholar] [CrossRef]
- Liu, B.S.; Wu, H.; Parkin, I.P. Gaseous Photocatalytic Oxidation of Formic Acid over TiO2: A Comparison between the Charge Carrier Transfer and Light-Assisted Mars-van Krevelen Pathways. J. Phys. Chem. C 2019, 123, 22261–22272. [Google Scholar] [CrossRef]
- Shah, K.W.; Li, W.X. A Review on Catalytic Nanomaterials for Volatile Organic Compounds VOC Removal and Their Applications for Healthy Buildings. Nanomaterials 2019, 9, 910. [Google Scholar] [CrossRef] [Green Version]
- Kontos, A.G.; Katsanaki, A.; Maggos, T.; Likodimos, V.; Ghicov, A.; Kim, D.; Kunze, J.; Vasilakos, C.; Schmuki, P.; Falaras, P. Photocatalytic degradation of gas pollutants on self-assembled titania nanotubes. Chem. Phys. Lett. 2010, 490, 58–62. [Google Scholar] [CrossRef]
- Van Gerven, T.; Mul, G.; Moulijn, J.; Stankiewicz, A. A review of intensification of photocatalytic processes. Chem. Eng. Process. 2007, 46, 781–789. [Google Scholar] [CrossRef]
- Da Costa, B.M.; Araujo, A.L.P.; Silva, G.V.; Boaventura, R.A.R.; Dias, M.M.; Lopes, J.C.B.; Vilar, V.J.P. Intensification of heterogeneous TiO2 photocatalysis using an innovative micro-meso-structured-photoreactor for n-decane oxidation at gas phase. Chem. Eng. J. 2017, 310, 331–341. [Google Scholar] [CrossRef]
- Moulis, F.; Krysa, J. Photocatalytic degradation of several VOCs (n-hexane, n-butyl acetate and toluene) on TiO2 layer in a closed-loop reactor. Catal. Today 2013, 209, 153–158. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.Z.; Fang, S.M.; Yang, Y.; Zhao, X.J. UV-Vis-infrared light-driven thermocatalytic abatement of benzene on Fe doped OMS-2 nanorods enhanced by a novel photoactivation. Chem. Eng. J. 2018, 332, 205–215. [Google Scholar] [CrossRef]
- Wang, L.J.; Zhou, Q.; Zhang, G.L.; Liang, Y.J.; Wang, B.S.; Zhang, W.W.; Lei, B.; Wang, W.Z. A facile room temperature solution-phase route to synthesize CuO nanowires with enhanced photocatalytic performance. Mater. Lett. 2012, 74, 217–219. [Google Scholar] [CrossRef]
- Wu, S.M.; Li, F.; Zhang, L.J.; Li, Z. Enhanced field emission properties of CuO nanoribbons decorated with Ag nanoparticles. Mater. Lett. 2016, 171, 220–223. [Google Scholar] [CrossRef]
- Yang, J.B.; Li, Z.; Zhao, W.; Zhao, C.X.; Wang, Y.; Liu, X.Q. Controllable synthesis of Ag-CuO composite nanosheets with enhanced photocatalytic property. Mater. Lett. 2014, 120, 16–19. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.Z.; Zhang, Q.; Zeng, M.; Wu, S.W.; Lan, L.; Zhao, X.J. Novel photoactivation and solar-light-driven thermocatalysis on epsilon-MnO2 nanosheets lead to highly efficient catalytic abatement of ethyl acetate without acetaldehyde as unfavorable by-product. J. Mater. Chem. A 2018, 6, 14195–14206. [Google Scholar] [CrossRef]
- Chang, Y.C.; Guo, J.Y. Double-sided plasmonic silver nanoparticles decorated copper oxide/zinc oxide heterostructured nanomaces with improving photocatalytic performance. J. Photochem. Photobiol. A Chem. 2019, 378, 184–191. [Google Scholar] [CrossRef]
- Zhang, X.D.; Yang, Y.; Li, H.X.; Zou, X.J.; Wang, Y.X. Non-TiO2 Photocatalysts Used for Degradation of Gaseous VOCs. Prog. Chem. 2016, 28, 1550–1559. [Google Scholar]
- Liu, X.Q.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.H.; Zhao, S.Q.; Li, Z.; Lin, Z.Q. Noble metal-metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Almquist, C.B.; Biswas, P. The photo-oxidation of cyclohexane on titanium dioxide: An investigation of competitive adsorption and its effects on product formation and selectivity. Appl. Catal. A Gen. 2001, 214, 259–271. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Issa, G.; Blasco, T.; Dimitrov, M.; Popova, M.; Hernandez, S.; Kovacheva, D.; Atanasova, G.; Nieto, J.M.L. Catalytic VOCs elimination over copper and cerium oxide modified mesoporous SBA-15 silica. Appl. Catal. A Gen. 2013, 453, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, A.M.; Carriazo, J.G. Cu and Co oxides supported on halloysite for the total oxidation of toluene. Appl. Catal. B Environ. 2015, 164, 443–452. [Google Scholar] [CrossRef]
- Cui, E.T.; Hou, G.H.; Chen, X.H.; Zhang, F.; Deng, Y.X.; Yu, G.Y.; Li, B.B.; Wu, Y.Q. In-situ hydrothermal fabrication of Sr2FeTaO6/NaTaO3 heterojunction photocatalyst aimed at the effective promotion of electron-hole separation and visible-light absorption. Appl. Catal. B Environ. 2019, 241, 52–65. [Google Scholar] [CrossRef]
- Li, J.J.; Yu, E.Q.; Cai, S.C.; Chen, X.; Chen, J.; Jia, H.P.; Xu, Y.J. Noble metal free, CeO2/LaMnO3 hybrid achieving efficient photo-thermal catalytic decomposition of volatile organic compounds under IR light. Appl. Catal. B Environ. 2019, 240, 141–152. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.Y.; Fang, Y.R.; Liu, B.K.; Huang, J.D.; Miao, F.J.; Bao, Y.A.; Dong, B. IR-Driven strong plasmonic-coupling on Ag nanorices/W18O49 nanowires heterostructures for photo/thermal synergistic enhancement of H-2 evolution from ammonia borane. Appl. Catal. B Environ. 2019, 252, 164–173. [Google Scholar] [CrossRef]
- Lee, Y.E.; Chung, W.C.; Chang, M.B. Photocatalytic oxidation of toluene and isopropanol by LaFeO3/black-TiO2. Environ. Sci. Pollut. Res. 2019, 26, 20908–20919. [Google Scholar] [CrossRef]
- Ray, C.; Pal, T. Recent advances of metal-metal oxide nanocomposites and their tailored nanostructures in numerous catalytic applications. J. Mater. Chem. A 2017, 5, 9465–9487. [Google Scholar] [CrossRef]
- Valenti, M.; Jonsson, M.P.; Biskos, G.; Schmidt-Ott, A.; Smith, W.A. Plasmonic nanoparticle-semiconductor composites for efficient solar water splitting. J. Mater. Chem. A 2016, 4, 17891–17912. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.Q. Plasmonic metal-semiconductor photocatalysts and photoelectrochemical cells: A review. Nanoscale 2018, 10, 2679–2696. [Google Scholar] [CrossRef]
- Mendez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Bahena, D.; Briois, V.; Colbeau-Justin, C.; Rodriguez-Lopez, J.L.; Remita, H. Surface Modification of TiO2 with Ag Nanoparticles and CuO Nanoclusters for Application in Photocatalysis. J. Phys. Chem. C 2016, 120, 5143–5154. [Google Scholar] [CrossRef]
- Boriskina, S.V.; Ghasemi, H.; Chen, G. Plasmonic materials for energy: From physics to applications. Mater. Today 2013, 16, 375–386. [Google Scholar] [CrossRef]
- Zhang, X.D.; Yang, Y.; Song, L.; Wang, Y.X.; He, C.; Wang, Z.; Cui, L.F. High and stable catalytic activity of Ag/Fe2O3 catalysts derived from MOFs for CO oxidation. Mol. Catal. 2018, 447, 80–89. [Google Scholar] [CrossRef]
- DuChene, J.S.; Tagliabue, G.; Welch, A.J.; Cheng, W.H.; Atwater, H.A. Hot Hole Collection and Photoelectrochemical CO2 Reduction with Plasmonic Au/p-GaN Photocathodes. Nano Lett. 2018, 18, 2545–2550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Torres, D.D.; Jain, P.K. Activation Energies of Plasmonic Catalysts. Nano Lett. 2016, 16, 3399–3407. [Google Scholar] [CrossRef]
- Khiavi, N.D.; Katal, R.; Eshkalak, S.K.; Masudy-Panah, S.; Ramakrishna, S.; Hu, J.Y. Visible Light Driven Heterojunction Photocatalyst of CuO-Cu2O Thin Films for Photocatalytic Degradation of Organic Pollutants. Nanomaterials 2019, 9, 1011. [Google Scholar] [CrossRef] [Green Version]
- Christopher, P.; Xin, H.L.; Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 2011, 3, 467–472. [Google Scholar] [CrossRef]
- Leong, K.H.; Abd Aziz, A.; Sim, L.C.; Saravanan, P.; Jang, M.; Bahnemann, D. Mechanistic insights into plasmonic photocatalysts in utilizing visible light. Beilstein J. Nanotechnol. 2018, 9, 628–648. [Google Scholar] [CrossRef]
- Ma, L.; Chen, S.; Shao, Y.; Chen, Y.L.; Liu, M.X.; Li, H.X.; Mao, Y.L.; Ding, S.J. Recent Progress in Constructing Plasmonic Metal/Semiconductor Hetero-Nanostructures for Improved Photocatalysis. Catalysts 2018, 8, 634. [Google Scholar] [CrossRef] [Green Version]
- Truppi, A.; Petronella, F.; Placido, T.; Striccoli, M.; Agostiano, A.; Curri, M.L.; Comparelli, R. Visible-Light-Active TiO2-Based Hybrid Nanocatalysts for Environmental Applications. Catalysts 2017, 7, 100. [Google Scholar] [CrossRef]
- Zhang, Y.C.; He, S.; Guo, W.X.; Hu, Y.; Huang, J.W.; Mulcahy, J.R.; Wei, W.D. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 2018, 118, 2927–2954. [Google Scholar] [CrossRef]
- Tatsuma, T.; Nishi, H.; Ishida, T. Plasmon-induced charge separation: chemistry and wide applications. Chem. Sci. 2017, 8, 3325–3337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erwin, W.R.; Zarick, H.F.; Talbert, E.M.; Bardhan, R. Light trapping in mesoporous solar cells with plasmonic nanostructures. Energy Environ. Sci. 2016, 9, 1577–1601. [Google Scholar] [CrossRef] [Green Version]
- Araujo, T.P.; Quiroz, J.; Barbosa, E.C.M.; Camargo, P.H.C. Understanding plasmonic catalysis with controlled nanomaterials based on catalytic and plasmonic metals. Curr. Opin. Colloid Interface Sci. 2019, 39, 110–122. [Google Scholar] [CrossRef]
- Mao, M.Y.; Li, Y.Z.; Lv, H.Q.; Hou, J.T.; Zeng, M.; Ren, L.; Huang, H.; Zhao, X.J. Efficient UV-vis-IR light-driven thermocatalytic purification of benzene on a Pt/CeO2 nanocomposite significantly promoted by hot electron-induced photoactivation. Environ. Sci. Nano 2017, 4, 373–384. [Google Scholar] [CrossRef]
- Fu, S.F.; Zheng, Y.; Zhou, X.B.; Ni, Z.M.; Xia, S.J. Visible light promoted degradation of gaseous volatile organic compounds catalyzed by Au supported layered double hydroxides: Influencing factors, kinetics and mechanism. J. Hazard. Mater. 2019, 363, 41–54. [Google Scholar] [CrossRef]
- Ma, X.C.; Dai, Y.; Yu, L.; Huang, B.B. Energy transfer in plasmonic photocatalytic composites. Light Sci. Appl. 2016, 5, e16017. [Google Scholar] [CrossRef] [Green Version]
- Gomez, L.; Hueso, J.L.; Ortega-Liebana, M.C.; Santamaria, J.; Cronin, S.B. Evaluation of gold-decorated halloysite nanotubes as plasmonic photocatalysts. Catal. Commun. 2014, 56, 115–118. [Google Scholar] [CrossRef]
- Graus, J.; Bueno-Alejo, C.J.; Hueso, J.L. In-Situ Deposition of Plasmonic Gold Nanotriangles and Nanoprisms onto Layered Hydroxides for Full-Range Photocatalytic Response towards the Selective Reduction of p-Nitrophenol. Catalysts 2018, 8, 354. [Google Scholar] [CrossRef] [Green Version]
- Uson, L.; Sebastian, V.; Mayoral, A.; Hueso, J.L.; Eguizabal, A.; Arruebo, M.; Santamaria, J. Spontaneous formation of Au-Pt alloyed nanoparticles using pure nano-counterparts as starters: A ligand and size dependent process. Nanoscale 2015, 7, 10152–10161. [Google Scholar] [CrossRef] [Green Version]
- Zieba, M.; Hueso, J.L.; Arruebo, M.; Martinez, G.; Santamaria, J. Gold-coated halloysite nanotubes as tunable plasmonic platforms. New J. Chem. 2014, 38, 2037–2042. [Google Scholar] [CrossRef]
- Halas, N.J.; Lal, S.; Chang, W.S.; Link, S.; Nordlander, P. Plasmons in Strongly Coupled Metallic Nanostructures. Chem. Rev. 2011, 111, 3913–3961. [Google Scholar] [CrossRef] [PubMed]
- Aslam, U.; Rao, V.G.; Chavez, S.; Linic, S. Catalytic conversion of solar to chemical energy on plasmonic metal nanostructures. Nat. Catal. 2018, 1, 656–665. [Google Scholar] [CrossRef]
- Kim, Y.; Smith, J.G.; Jain, P.K. Harvesting multiple electron-hole pairs generated through plasmonic excitation of Au nanoparticles. Nat. Chem. 2018, 10, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Mustafa, J.; Neaton, J.B.; Louie, S.G. Theory and computation of hot carriers generated by surface plasmon polaritons in noble metals. Nat. Commun. 2015, 6, 7044. [Google Scholar] [CrossRef] [PubMed]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef]
- Kriegel, I.; Scotognella, F.; Manna, L. Plasmonic doped semiconductor nanocrystals: Properties, fabrication, applications and perspectives. Phys. Rep. Rev. Sec. Phys. Lett. 2017, 674, 1–52. [Google Scholar] [CrossRef]
- Rao, V.G.; Aslam, U.; Linic, S. Chemical Requirement for Extracting Energetic Charge Carriers from Plasmonic Metal Nanoparticles to Perform Electron-Transfer Reactions. J. Am. Chem. Soc. 2019, 141, 643–647. [Google Scholar] [CrossRef]
- Aslam, U.; Chavez, S.; Linic, S. Controlling energy flow in multimetallic nanostructures for plasmonic catalysis. Nat. Nanotechnol. 2017, 12, 1000–1005. [Google Scholar] [CrossRef]
- Boerigter, C.; Aslam, U.; Linic, S. Mechanism of Charge Transfer from Plasmonic Nanostructures to Chemically Attached Materials. ACS Nano 2016, 10, 6108–6115. [Google Scholar] [CrossRef]
- Linic, S.; Aslam, U.; Boerigter, C.; Morabito, M. Photochemical transformations on plasmonic metal nanoparticles. Nat. Mater. 2015, 14, 567–576. [Google Scholar] [CrossRef]
- Gargiulo, J.; Berte, R.; Li, Y.; Maier, S.A.; Cortes, E. From Optical to Chemical Hot Spots in Plasmonics. Acc. Chem. Res. 2019, 52, 2525–2535. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Xin, H.L.; Marimuthu, A. Catalytic and Photocatalytic Transformations on Metal Nanoparticles with Targeted Geometric and Plasmonic Properties. Acc. Chem. Res. 2013, 46, 1890–1899. [Google Scholar] [CrossRef]
- Brus, L. Noble Metal Nanocrystals: Plasmon Electron Transfer Photochemistry and Single-Molecule Raman Spectroscopy. Acc. Chem. Res. 2008, 41, 1742–1749. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef]
- Marimuthu, A.; Zhang, J.W.; Linic, S. Tuning Selectivity in Propylene Epoxidation by Plasmon Mediated Photo-Switching of Cu Oxidation State. Science 2013, 339, 1590–1593. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.L.; Zhou, Q.X.; Wang, X.; Wood, T.E.; Wang, L.; Duchesne, P.N.; Guo, J.L.; Yan, X.L.; Xia, M.K.; Lie, Y.F.; et al. Cu2O nanocubes with mixed oxidation-state facets for (photo)catalytic hydrogenation of carbon dioxide. Nat. Catal. 2019, 2, 889–898. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Zhang, K.L.; Xu, D.G.; Yang, G.C.; Huang, H.; Nie, F.D.; Liu, C.M.; Yang, S.H. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Kumar, M.K.; Bhavani, K.; Naresh, G.; Srinivas, B.; Venugopal, A. Plasmonic resonance nature of Ag-Cu/TiO2 photocatalyst under solar and artificial light: Synthesis, characterization and evaluation of H2O splitting activity. Appl. Catal. B Environ. 2016, 199, 282–291. [Google Scholar]
- Nguyen, N.L.; de Gironcoli, S.; Piccinin, S. Ag-Cu catalysts for ethylene epoxidation: Selectivity and activity descriptors. J. Chem. Phys. 2013, 138, 184707. [Google Scholar] [CrossRef]
- Rapallo, A.; Rossi, G.; Ferrando, R.; Fortunelli, A.; Curley, B.C.; Lloyd, L.D.; Tarbuck, G.M.; Johnston, R.L. Global optimization of bimetallic cluster structures. I. Size-mismatched Ag-Cu, Ag-Ni, and Au-Cu systems. J. Chem. Phys. 2005, 122, 194308. [Google Scholar] [CrossRef]
- Verma, A.; Gupta, R.K.; Shukla, M.; Malviya, M.; Sinha, I. Ag-Cu Bimetallic Nanoparticles as Efficient Oxygen Reduction Reaction Electrocatalysts in Alkaline Media. J. Nanosci. Nanotechnol. 2020, 20, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Piccinin, S.; Zafeiratos, S.; Stampfl, C.; Hansen, T.W.; Havecker, M.; Teschner, D.; Bukhtiyarov, V.I.; Girgsdies, F.; Knop-Gericke, A.; Schlogl, R.; et al. Alloy Catalyst in a Reactive Environment: The Example of Ag-Cu Particles for Ethylene Epoxidation. Phys. Rev. Lett. 2010, 104, 035503. [Google Scholar] [CrossRef] [PubMed]
- Tchaplyguine, M.; Zhang, C.F.; Andersson, T.; Bjorneholm, O. Ag-Cu oxide nanoparticles with high oxidation states: towards new high T-c materials. Dalton Trans. 2018, 47, 16660–16667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Wang, L.L.; Kong, X.Y.; Jiang, H.Y.; Zhang, F.; Shi, J.S. Novel Ag-Cu bimetallic alloy decorated near-infrared responsive three-dimensional rod-like architectures for efficient photocatalytic water purification. J. Colloid Interface Sci. 2018, 522, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Arcelli, L.; Ikoma, T.; Tanaka, J.; Mann, S. Dextran templating for the synthesis of metallic and metal oxide sponges. Nat. Mater. 2003, 2, 386. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, Z.; Yao, W.; Wang, P.Y.; Yu, S.J.; Wang, X.K. Decorating of Ag and CuO on Cu Nanoparticles for Enhanced High Catalytic Activity to the Degradation of Organic Pollutants. Langmuir 2017, 33, 7606–7614. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Ogeleka, D.F.; Mbonu, J.I. Phyto-assisted Preparation of Ag and Ag-CuO Nanoparticles Using Aqueous Extracts of Mimosa pigra and their Catalytic Activities in the Degradation of Some Common Pollutants. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1798–1806. [Google Scholar] [CrossRef]
- Ji, W.K.; Shen, T.; Kong, J.J.; Rui, Z.B.; Tong, Y.X. Synergistic Performance between Visible-Light Photocatalysis and Thermocatalysis for VOCs Oxidation over Robust Ag/F-Codoped SrTiO3. Ind. Eng. Chem. Res. 2018, 57, 12766–12773. [Google Scholar] [CrossRef]
- Wan, X.; Yang, J.; Huang, X.Y.; Tie, S.L.; Lan, S. A high-performance room temperature thermocatalyst Cu2O/Ag-0@Ag-NPs for dye degradation under dark condition. J. Alloys Compd. 2019, 785, 398–409. [Google Scholar] [CrossRef]
- Kung, M.L.; Tai, M.H.; Lin, P.Y.; Wu, D.C.; Wu, W.J.; Yeh, B.W.; Hung, H.S.; Kuo, C.H.; Chen, Y.W.; Hsieh, S.L.; et al. Silver decorated copper oxide (Ag@CuO) nanocomposite enhances ROS-mediated bacterial architecture collapse. Colloid Surf. B Biointerfaces 2017, 155, 399–407. [Google Scholar] [CrossRef]
- Ramirez, A.; Hueso, J.L.; Suarez, H.; Mallada, R.; Ibarra, A.; Irusta, S.; Santamaria, J. A Nanoarchitecture Based on Silver and Copper Oxide with an Exceptional Response in the Chlorine-Promoted Epoxidation of Ethylene. Angew. Chem. Int. Edit. 2016, 55, 11158–11161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottega-Pergher, B.; Graus, J.; Bueno-Alejo, C.J.; Hueso, J.L. Triangular and Prism-Shaped Gold-Zinc Oxide Plasmonic Nanostructures: In situ Reduction, Assembly, and Full-Range Photocatalytic Performance. Eur. J. Inorg. Chem. 2019, 2019, 3228–3234. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, A.; Hueso, J.L.; Mallada, R.; Santamaria, J. Ethylene epoxidation in microwave heated structured reactors. Catal. Today 2016, 273, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, A.; Hueso, J.L.; Mallada, R.; Santamaria, J. In situ temperature measurements in microwave-heated gas-solid catalytic systems. Detection of hot spots and solid-fluid temperature gradients in the ethylene epoxidation reaction. Chem. Eng. J. 2017, 316, 50–60. [Google Scholar] [CrossRef]
- Gomez-Romero, P.; Tejada-Rosales, E.M.; Palacin, M.R. Ag2Cu2O3: The first silver copper oxide. Angew. Chem. Int. Edit. 1999, 38, 524–525. [Google Scholar] [CrossRef]
- Tejada-Rosales, E.M.; Rodriguez-Carvajal, J.; Casan-Pastor, N.; Alemany, P.; Ruiz, E.; El-Fallah, M.S.; Alvarez, S.; Gomez-Romero, P. Room-temperature synthesis and crystal, magnetic, and electronic structure of the first silver copper oxide. Inorg. Chem. 2002, 41, 6604–6613. [Google Scholar] [CrossRef]
- Carreras, A.; Conejeros, S.; Camon, A.; Garcia, A.; Casan-Pastor, N.; Alemany, P.; Canadell, E. Charge Delocalization, Oxidation States, and Silver Mobility in the Mixed Silver-Copper Oxide AgCuO2. Inorg. Chem. 2019, 58, 7026–7035. [Google Scholar] [CrossRef]
- Navaladian, S.; Viswanathan, B.; Viswanath, R.P.; Varadarajan, T.K. Thermal decomposition as route for silver nanoparticles. Nanoscale Res. Lett. 2007, 2, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Boerigter, C.; Campana, R.; Morabito, M.; Linic, S. Evidence and implications of direct charge excitation as the dominant mechanism in plasmon-mediated photocatalysis. Nat. Commun. 2016, 7, 10545. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Wang, S.J.; Zhang, X.Y.; Su, D.; Yang, Y.; Wu, J.Y.; Xu, Y.Y.; Zhao, N. Progress in the Utilization Efficiency Improvement of Hot Carriers in Plasmon-Mediated Heterostructure Photocatalysis. Appl. Sci. 2019, 9, 2093. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.A.; Swearer, D.F.; Zhang, C.; Robatjazi, H.; Zhao, H.Q.; Henderson, L.; Dong, L.L.; Christopher, P.; Carter, E.A.; Nordlander, P.; et al. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 2018, 362, 69–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.F.; Zhang, M.; Zhao, L.B.; Feng, J.M.; Wu, D.Y.; Ren, B.; Tian, Z.Q. Activation of Oxygen on Gold and Silver Nanoparticles Assisted by Surface Plasmon Resonances. Angew. Chem. Int. Edit. 2014, 53, 2353–2357. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, J.H.; Nam, J.M. Plasmonic Photothermal Nanoparticles for Biomedical Applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.M.; Sundararaman, R.; Narang, P.; Goddard, W.A.; Atwater, H.A. Nonradiative Plasmon Decay and Hot Carrier Dynamics: Effects of Phonons, Surfaces, and Geometry. ACS Nano 2016, 10, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Jermyn, A.S.; Tagliabue, G.; Atwater, H.A.; Goddard, W.A.; Narang, P.; Sundararaman, R. Transport of hot carriers in plasmonic nanostructures. Phys. Rev. Mater. 2019, 3, 075201. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.; Li, X.Y.; Kong, Q.; Long, R.; Wang, C.M.; Jiang, J.; Xiong, Y.J. Toward Enhanced Photocatalytic Oxygen Evolution: Synergetic Utilization of Plasmonic Effect and Schottky Junction via Interfacing Facet Selection. Adv. Mater. 2015, 27, 3444–3452. [Google Scholar] [CrossRef]
- Ghosh, P.; Kar, A.; Khandelwal, S.; Vyas, D.; Mir, A.; Chakraborty, A.L.; Hegde, R.S.; Sharma, S.; Dutta, A.; Khatua, S. Plasmonic CoO-Decorated Au Nanorods for Photoelectrocatalytic Water Oxidation. ACS Appl. Nano Mater. 2019, 2, 5795–5803. [Google Scholar] [CrossRef]
- Al-Zubeidi, A.; Hoener, B.S.; Collins, S.S.E.; Wang, W.X.; Kirchner, S.R.; Jebeli, S.A.H.; Joplin, A.; Chang, W.S.; Link, S.; Landes, C.F. Hot Holes Assist Plasmonic Nanoelectrode Dissolution. Nano Lett. 2019, 19, 1301–1306. [Google Scholar] [CrossRef]
- Pensa, E.; Gargiulo, J.; Lauri, A.; Schlucker, S.; Cortes, E.; Maier, S.A. Spectral Screening of the Energy of Hot Holes over a Particle Plasmon Resonance. Nano Lett. 2019, 19, 1867–1874. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suarez, H.; Ramirez, A.; Bueno-Alejo, C.J.; Hueso, J.L. Silver-Copper Oxide Heteronanostructures for the Plasmonic-Enhanced Photocatalytic Oxidation of N-Hexane in the Visible-NIR Range. Materials 2019, 12, 3858. https://doi.org/10.3390/ma12233858
Suarez H, Ramirez A, Bueno-Alejo CJ, Hueso JL. Silver-Copper Oxide Heteronanostructures for the Plasmonic-Enhanced Photocatalytic Oxidation of N-Hexane in the Visible-NIR Range. Materials. 2019; 12(23):3858. https://doi.org/10.3390/ma12233858
Chicago/Turabian StyleSuarez, Hugo, Adrian Ramirez, Carlos J. Bueno-Alejo, and Jose L. Hueso. 2019. "Silver-Copper Oxide Heteronanostructures for the Plasmonic-Enhanced Photocatalytic Oxidation of N-Hexane in the Visible-NIR Range" Materials 12, no. 23: 3858. https://doi.org/10.3390/ma12233858




