Study of the Effect of Current Pulse Frequency on Ti-6Al-4V Alloy Coating Formation by Micro Arc Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Production
2.2. Coating Characterization
2.3. Coating Corrosion Resistance Test
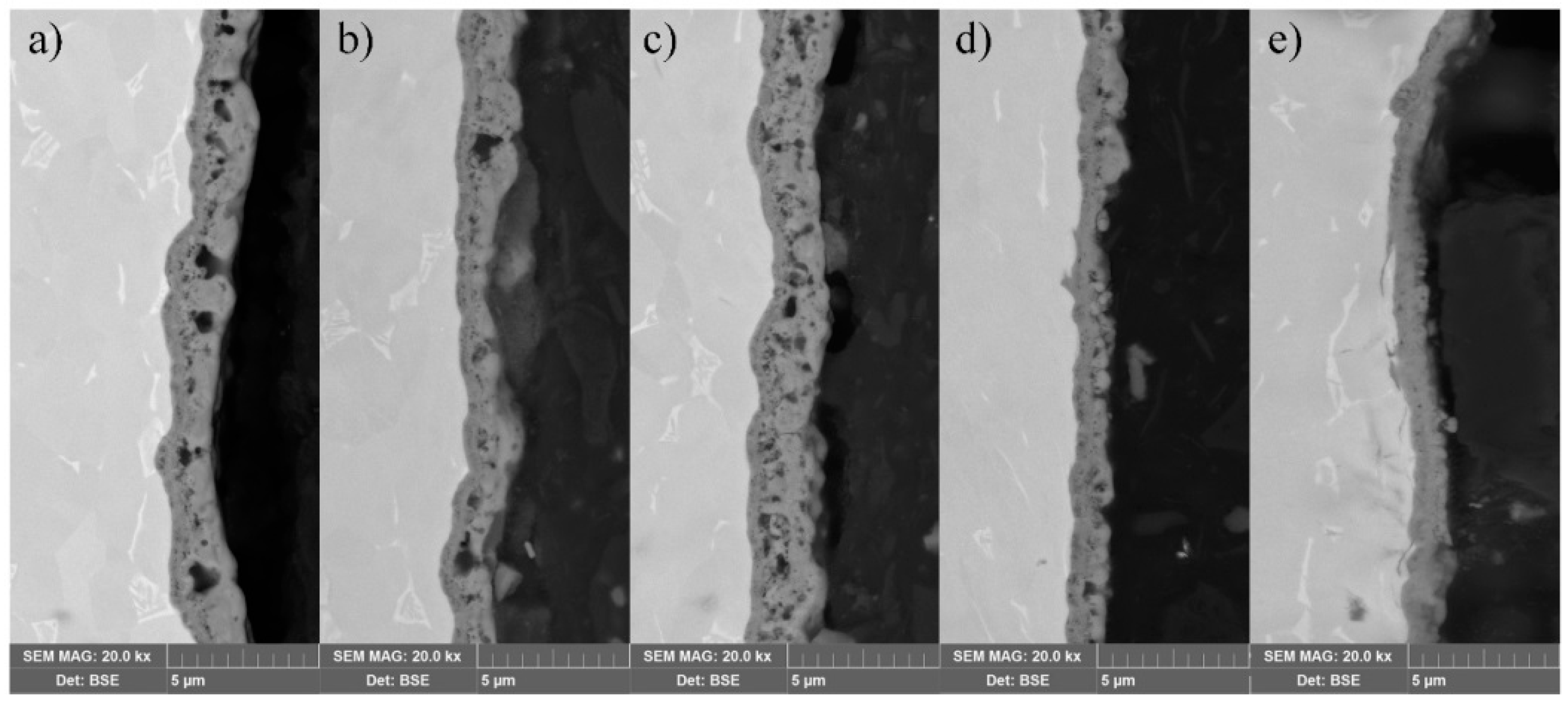
3. Results and Discussion
3.1. Process Characterization
3.2. Surface Characterization
3.3. Chemical and Phase Analysis
3.4. Corrosion Resistance Investigation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gu, Y.; Ma, A.; Jiang, J.; Li, H.; Song, D.; Wu, H.; Yuan, Y. Simultaneously improving mechanical properties and corrosion resistance of pure Ti by continuous ECAP plus short-duration annealing. Mater. Charact. 2018, 138, 38–47. [Google Scholar] [CrossRef]
- Gurrappa, I. Characterization of titanium alloy Ti-6Al-4V for chemical, marine and industrial applications. Mater. Charact. 2003, 51, 131–139. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Zhu, R.-F.; Lu, Y.-P.; Xiao, G.-Y.; Zhao, X.-C.; He, K.; Ma, X.-N. Preparation and properties of plasma electrolytic oxidation coating on sandblasted pure titanium by a combination treatment. Mater. Sci. Eng. C 2014, 42, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, A.; Wolicki, I.; Kossenko, A.; Zinigrad, M.; Borodianskiy, K. Coating formation on Ti-6Al-4V alloy by micro arc oxidation in molten salt. Materials 2018, 11, 1611. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A. Characterization of oxide films produced by plasma electrolytic oxidation of a Ti–6Al–4V alloy. Surf. Coat. Technol. 2000, 130, 195–206. [Google Scholar] [CrossRef]
- Nominé, A.; Martin, J.; Henrion, G.; Belmonte, T. Effect of cathodic micro-discharges on oxide growth during plasma electrolytic oxidation (PEO). Surf. Coat. Technol. 2015, 269, 131–137. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, X.-Q.; Xue, Z.; Matykina, E.; Skeldon, P.; Thompson, G.E. Microstructure, corrosion and wear performance of plasma electrolytic oxidation coatings formed on Ti–6Al–4V alloy in silicate-hexametaphosphate electrolyte. Surf. Coat. Technol. 2013, 217, 129–139. [Google Scholar] [CrossRef]
- Zhu, L.; Petrova, R.S.; Gashinski, J.P.; Yang, Z. The effect of surface roughness on PEO-treated Ti-6Al-4V alloy and corrosion resistance. Surf. Coat. Technol. 2017, 325, 22–29. [Google Scholar] [CrossRef]
- Sobolev, A.; Valkov, A.; Kossenko, A.; Wolicki, I.; Zinigrad, M.; Borodianskiy, K. Bioactive coating on titanium alloy with high osseointegration and antibacterial Ag nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 39534–39544. [Google Scholar] [CrossRef] [PubMed]
- Mierzejewska, Ż.; Hudák, R.; Sidun, J. Mechanical Properties and Microstructure of DMLS Ti6Al4V Alloy Dedicated to Biomedical Applications. Materials 2019, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Santos-Coquillat, A.; Gonzalez Tenorio, R.; Mohedano, M.; Martinez-Campos, E.; Arrabal, R.; Matykina, E. Tailoring of antibacterial and osteogenic properties of Ti6Al4V by plasma electrolytic oxidation. Appl. Surf. Sci. 2018, 454, 157–172. [Google Scholar] [CrossRef]
- Shah, F.A.; Trobos, M.; Thomsen, P.; Palmquist, A. Commercially pure titanium (cp-Ti) versus titanium alloy (Ti6Al4V) materials as bone anchored implants—Is one truly better than the other? Mater. Sci. Eng. C 2016, 62, 960–966. [Google Scholar] [CrossRef]
- Durdu, S.; Usta, M.; Berkem, A.S. Bioactive coatings on Ti6Al4V alloy formed by plasma electrolytic oxidation. Surf. Coat. Technol. 2016, 301, 85–93. [Google Scholar] [CrossRef]
- Wheeler, J.M.; Collier, C.A.; Paillard, J.M.; Curran, J.A. Evaluation of micromechanical behaviour of plasma electrolytic oxidation (PEO) coatings on Ti–6Al–4V. Surf. Coat. Technol. 2010, 204, 3399–3409. [Google Scholar] [CrossRef]
- Aliasghari, S.; Němcová, A.; Čížek, J.; Gholinia, A.; Skeldon, P.; Thompson, G.E. Effects of reagent purity on plasma electrolytic oxidation of titanium in an aluminate—Phosphate electrolyte. Int. J. Surf. Eng. Coat. 2016, 94, 32–42. [Google Scholar] [CrossRef]
- Stojadinović, S.; Vasilić, R.; Petković, M.; Kasalica, B.; Belča, I.; Žekić, A.; Zeković, L. Characterization of the plasma electrolytic oxidation of titanium in sodium metasilicate. Appl. Surf. Sci. 2013, 265, 226–233. [Google Scholar] [CrossRef]
- Shokouhfar, M.; Dehghanian, C.; Baradaran, A. Preparation of ceramic coating on Ti substrate by Plasma electrolytic oxidation in different electrolytes and evaluation of its corrosion resistance: Part 2. Appl. Surf. Sci. 2012, 258, 2416–2423. [Google Scholar] [CrossRef]
- Songur, F.; Dikici, B.; Niinomi, M.; Arslan, E. The plasma electrolytic oxidation (PEO) coatings to enhance in-vitro corrosion resistance of Ti–29Nb–13Ta–4.6Zr alloys: The combined effect of duty cycle and the deposition frequency. Surf. Coat. Technol. 2019, 374, 345–354. [Google Scholar] [CrossRef]
- Torres-Ceron, D.A.; Restrepo-Parra, E.; Acosta-Medina, C.D.; Escobar-Rincon, D.; Ospina-Ospina, R. Study of duty cycle influence on the band gap energy of TiO2/P coatings obtained by PEO process. Surf. Coat. Technol. 2019, 375, 221–228. [Google Scholar] [CrossRef]
- Snizhko, L.O.; Yerokhin, A.L.; Pilkington, A.; Gurevina, N.L.; Misnyankin, D.O.; Leyland, A.; Matthews, A. Anodic processes in plasma electrolytic oxidation of aluminium in alkaline solutions. Electrochem. Acta 2004, 49, 2085–2095. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O.; Yerokhin, A.; Matthews, A. Spectroscopic study of electrolytic plasma and discharging behaviour during the plasma electrolytic oxidation (PEO) process. J. Phys. D Appl. Phys. 2010, 43, 13. [Google Scholar] [CrossRef]
- Mortazavi, G.; Jiang, J.; Meletis, E.I. Investigation of the plasma electrolytic oxidation mechanism of titanium. Appl. Surf. Sci. 2019, 488, 370–382. [Google Scholar] [CrossRef]
- Ahounbar, E.; Khoei, S.M.M.; Omidvar, H. Characteristics of in-situ synthesized hydroxyapatite on TiO2 ceramic via plasma electrolytic oxidation. Ceram. Int. 2018, 45, 3118–3125. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Balachandran, U.; Eror, N.G. Raman spectra of titanium dioxide. J. Solid State Chem. 1982, 42, 276–282. [Google Scholar] [CrossRef]






| Name of the Sample | Frequency Applied (Hz) |
|---|---|
| Sam–200 Hz | 200 Hz |
| Sam–400 Hz | 400 Hz |
| Sam–600 Hz | 600 Hz |
| Sam–800 Hz | 800 Hz |
| Sam–1000 Hz | 1000 Hz |
| Sample | Ti | Al | V | Si | O |
|---|---|---|---|---|---|
| Sam–200 Hz | 24.2 ± 1.3 | 2.3 ± 0.5 | 0.9 ± 0.1 | 5.1 ± 0.9 | Balance |
| Sam–400 Hz | 24.1 ± 1.5 | 2.5 ± 0.3 | 0.9 ± 0.1 | 3.5 ± 0.7 | Balance |
| Sam–600 Hz | 24.4 ± 2.1 | 2.8 ± 0.3 | 1.0 ± 0.2 | 3.7 ± 0.7 | Balance |
| Sam–800 Hz | 27.4 ± 2.3 | 3.1 ± 0.7 | 1.1 ± 0.3 | 2.2 ± 0.6 | Balance |
| Sam–1000Hz | 24.0 ± 1.2 | 2.5 ± 0.5 | 0.9 ± 0.2 | 4.4 ± 1.0 | Balance |
| Samples | Ecorr (mV) | icorr (nA/cm2) | βa (mV/decade) | βc (mV/decade) | Rp (kΩ cm2) | Corrosion Rate (mm/year) |
|---|---|---|---|---|---|---|
| Untreated alloy | −198.386 | 2141.5 | 310.368 | 88.89 | 14 | 0.018644 |
| Sam–200Hz | 270.387 | 49.319 | 333.266 | 130.897 | 832 | 0.000429 |
| Sam–400Hz | 269.917 | 33.000 | 304.585 | 92.802 | 937 | 0.000287 |
| Sam–600Hz | 303.337 | 30.534 | 225.418 | 117.102 | 1097 | 0.000266 |
| Sam–800Hz | 282.228 | 33.364 | 308.202 | 129.618 | 1189 | 0.000290 |
| Sam–1000Hz | 300.085 | 17.260 | 216.575 | 113.596 | 1877 | 0.000150 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobolev, A.; Kossenko, A.; Borodianskiy, K. Study of the Effect of Current Pulse Frequency on Ti-6Al-4V Alloy Coating Formation by Micro Arc Oxidation. Materials 2019, 12, 3983. https://doi.org/10.3390/ma12233983
Sobolev A, Kossenko A, Borodianskiy K. Study of the Effect of Current Pulse Frequency on Ti-6Al-4V Alloy Coating Formation by Micro Arc Oxidation. Materials. 2019; 12(23):3983. https://doi.org/10.3390/ma12233983
Chicago/Turabian StyleSobolev, Alexander, Alexey Kossenko, and Konstantin Borodianskiy. 2019. "Study of the Effect of Current Pulse Frequency on Ti-6Al-4V Alloy Coating Formation by Micro Arc Oxidation" Materials 12, no. 23: 3983. https://doi.org/10.3390/ma12233983





