Thermal Reactivity in Metal Organic Materials (MOMs): From Single-Crystal-to-Single-Crystal Reactions and Beyond
Abstract
:1. Introduction
2. Hybrid Metal Organic Materials: Coordination Polymers (CPs)/Metal Organic Frameworks (MOFs) and Second Sphere Metal-Organic Adducts
2.1. Coordination Polymers/Metal Organic Frameworks
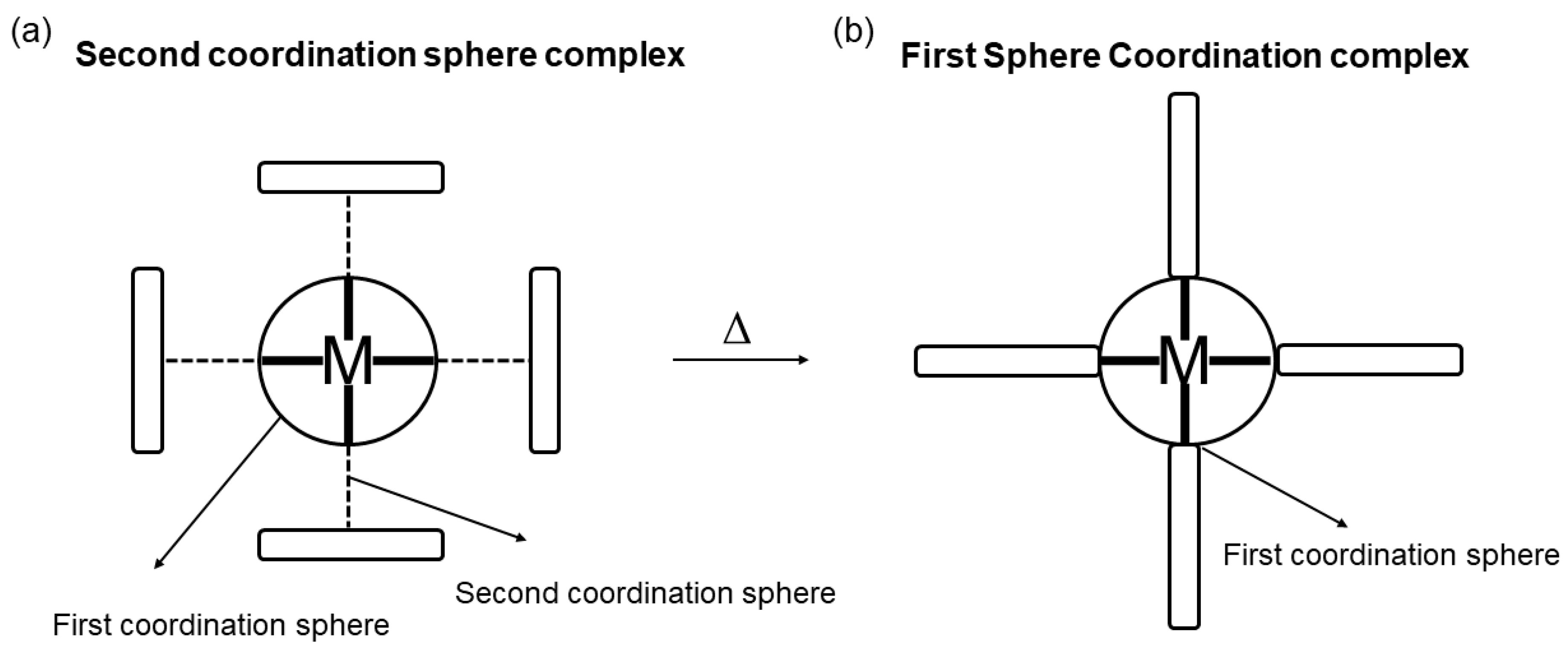
2.2. Second Sphere Coordination Adducts
3. Structural Transformations Classified upon the Degree of Crystallinity: Crystal-to-Crystal vs Crystal-to-Polycrystalline Processes
4. Thermally Induced Solid-State Reactivity in CP/MOFs
4.1. Thermally Induced Single-Crystal-to-Single-Crystal (SCSC) Reactions in CPs/MOFs
4.2. Thermally Induced Crystal to Polycrystalline Transformations in CPs/MOFs
5. Thermally Induced Solid-State Reactivity in SSC
5.1. Thermally Induced Single-Crystal-to-Single-Crystal (SCSC) Reactions in SSCs
5.2. Thermally Induced Crystal to Polycrystalline Transformations in SSCs
6. Beyond the Limits of Single-Crystal-to-Single-Crystal Transformations in Hybrid Metal Organic Materials
7. Conclusions
Funding
Conflicts of Interest
References
- Thomas, J.M. Diffusionless chemical reactions and crystal engineering. Nature 1981, 289, 633–634. [Google Scholar] [CrossRef]
- Schmidt, G.M.J. Solid state Photochemistry; Verlag Chemie: New York, NY, USA, 1976. [Google Scholar]
- Schmidt, G.M.J. Photodimerization in the solid-state. Pure Appl. Chem. 1971, 27, 647. [Google Scholar] [CrossRef] [Green Version]
- Enkelmann, V.; Wegner, G.; Novak, K.; Wagener, K.B. Single-crystal-to-single-crystal photodimerization of cinnamic acid. J. Am. Chem. Soc. 1993, 115, 10390–10391. [Google Scholar] [CrossRef]
- Elacqua, E.; Kaushik, P.; Groeneman, R.H.; Sumrak, J.C.; Bučar, D.-K.; MacGillivray, L.R. Supramolecular Protecting Group Strategy Introduced to the Organic Solid State: Enhanced Reactivity through Molecular Pedal Motion. Angew. Chem. Int. Ed. 2012, 51, 1037–1041. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem. Int. Ed. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Caronna, T.; Liantonio, R.; Logothetis, T.A.; Metrangolo, P.; Pilati, T.; Resnati, G. Halogen bonding and π···π stacking control reactivity in the solid state. J. Am. Chem. Soc. 2004, 126, 4500–4501. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Vittal, J.J.; Ramanan, A. Crystal Engineering: A Textbook; World Scientific: Singapore, 2011. [Google Scholar]
- Guo, F.; Martí-Rujas, J.; Pan, Z.; Hughes, C.E.; Harris, K.D.M. Direct Structural Understanding of a Topochemical Solid State Photopolymerization Reaction. J. Phys. Chem. C 2008, 112, 19793–19796. [Google Scholar] [CrossRef]
- Mir, M.H.; Koh, L.L.; Tan, G.K.; Vittal, J.J. Single-Crystal to Single-Crystal Photochemical Structural Transformations of Interpenetrated 3 D Coordination Polymers by [2+2] Cycloaddition Reactions. Angew. Chem. Int. Ed. 2010, 49, 390–393. [Google Scholar] [CrossRef]
- Kole, G.K.; Kojima, T.; Kawano, M.; Vittal, J.J. Reversible single-crystal-to-single-crystal photochemical formation and thermal cleavage of a cyclobutane ring. Angew. Chem. Int. Ed. 2014, 53, 2143–2146. [Google Scholar] [CrossRef]
- MacGillivray, L.R.; Papaefstathiou, G.S.; Friščić, T.; Hamilton, T.D.; Bučar, D.K.; Chu, Q.; Varshney, D.B.; Georgiev, I.G. Supramolecular control of reactivity in the solid-state: From templates to ladderanes to Metal-Organic Frameworks. Acc. Chem. Res. 2008, 41, 280–291. [Google Scholar] [CrossRef]
- Biradha, K.; Santra, R. Crystal engineering of topochemical solid state reactions. Chem. Soc. Rev. 2013, 42, 950–967. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, W.; Friščić, T.; Apperley, D.; James, S.L. High reactivity of Metal-Organic Frameworks under grinding conditions: Parallels with Organic Molecular Materials. Angew. Chem. Int. Ed. 2010, 49, 3916–3919. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.; Villacampa, M.; Menendez, J.C. Multicomponent mechanochemical synthesis. Chem. Sci. 2018, 9, 2042–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marelli, E.; Casati, N.; Gozzo, F.; Macchi, P.; Simoncic, P.; Sironi, A. High pressure modification of organic NLO materials: Large conformational re-arrangement of 4-aminobenzophenone. CrystEngComm 2011, 13, 6845–6849. [Google Scholar] [CrossRef]
- Wehinger, B.; Fiolka, C.; Lanza, A.; Scatena, R.; Kubus, M.; Grockowiak, A.; Coniglio, W.A.; Graf, D.; Skoulatos, M.; Chen, J.H.; et al. Giant pressure dependence and dimensionality switching in a metal-organic quantum antiferromagnet. Phys. Rev. Lett. 2018, 121, 117201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurd, J.A.; Vaidhyanathan, R.; Thangadurai, V.; Ratcliffe, C.I.; Moudrakovski, I.L.; Shimizu, G.K.H. Anhydrous proton conduction at 150 °C in a crystalline metal-organic framework. Nat. Chem. 2009, 1, 705–710. [Google Scholar] [CrossRef]
- Yang, C.; Kaipa, U.; Mather, Q.Z.; Wang, X.; Nesterov, V.; Venero, A.F.; Omary, M.A. Fluorous Metal-organic frameworks with superior adsorption and hydrophobic properties toward oil spill cleanup and hydrocarbon storage. J. Am. Chem. Soc. 2011, 133, 18094–18097. [Google Scholar] [CrossRef]
- Martí-Rujas, J.; Meazza, L.; Lim, G.K.; Terraneo, G.; Pilati, T.; Harris, K.D.M.; Metrangolo, P.; Resnati, G. An adaptable and dynamically porous organic salt traps unique tetrahalide dianions. Angew. Chem. Int. Ed. 2013, 52, 13444–13448. [Google Scholar] [CrossRef] [Green Version]
- Lavenn, C.; Okhrimenko, L.; Guillou, N.; Monge, M.; Ledoux, G.; Dujardin, C.; Chiriac, R.; Fateeva, A.; Demessence, A. A luminescent double helical gold(I)–thiophenolate coordination polymer obtained by hydrothermal synthesis or by thermal solid-state amorphous-to-crystalline isomerization. J. Mater. Chem. C 2015, 3, 4115–4125. [Google Scholar] [CrossRef]
- Zhang, C.; Li, T.; Xu, H.; Ma, J.; Zheng, H. The mutation in the single-crystal structural transformation process, induced by the combined stimuli of temperature and solvent. Chem. Eur. J. 2018, 24, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.Y.; Harris, K.D.M. How to determine structures when single crystals cannot be grown: Opportunities for structure determination of molecular materials using powder diffraction data. Chem. Soc. Rev. 2005, 33, 526–538. [Google Scholar]
- Harris, K.D.M.; Tremayne, M.J.; Lightfoot, P.; Bruce, P.G. Crystal-structure determination from powder diffraction data by Monte-Carlo methods. J. Am. Chem. Soc. 1994, 114, 3543–3547. [Google Scholar] [CrossRef]
- Oszlányi, G.; Sütő, A. Ab initio structure solution by charge flipping. Acta Crystallogr. Sect. A 2004, 60, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Masciocchi, N.; Ardizzoia, G.A.; LaMonica, G.; Maspero, A.; Galli, S.; Sironi, A. Metal imizazolato complexes: Synthesis, characterization, and X-ray powder diffraction studies of Group 10 coordination polymers. Inorg. Chem. 2001, 40, 6983–6989. [Google Scholar] [CrossRef]
- Courvoisier, E.; Williams, P.A.; Lim, G.K.; Hughes, C.E.; Harris, K.D.M. The crystal structure of L-arginine. Chem. Commun. 2012, 48, 2761–2763. [Google Scholar] [CrossRef] [Green Version]
- Al Rahal, O.; Hughes, C.E.; Williams, P.A.; Logsdail, A.J.; Diskin-Posner, Y.; Harris, K.D.M. Polymorphism of L-Triptophan. Angew. Chem. Int. Ed. 2019. [Google Scholar] [CrossRef]
- Matsuda, R.; Kitaura, R.; Kitagawa, S.; Kubota, Y.; Belosludov, R.V.; Kobayashi, T.C.; Sakamoto, H.; Chiba, T.; Takata, M.; Kawazoe, Y.; et al. Highly controlled acetylene accomodation in a metal-organic microporous material. Nature 2005, 436, 238–241. [Google Scholar] [CrossRef]
- Kawano, M.; Haneda, T.; Hashizume, D.; Izumi, F.; Fujita, M. A selective instant synthesis of a coordination network and its ab initio powder structure determination. Angew. Chem. Int. Ed. 2008, 47, 1269–1271. [Google Scholar] [CrossRef]
- Gándara, F.; Uribe-Romo, F.J.; Britt, D.K.; Furukawa, H.; Lei, L.; Cheng, R.; Duan, X.; O’Keeffe, M.; Yaghi, O.M. Porous, conductive metal-triazolates and their structural elucidation by the charge flipping method. Chem. Eur. J. 2012, 18, 10595–10601. [Google Scholar] [CrossRef]
- Martí-Rujas, J.; Kawano, M. Kinetic Products in Coordination Networks: Ab Initio X-ray Powder Diffraction Analysis. Acc. Chem. Res. 2013, 46, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Beake, E.O.R.; Dove, M.T.; Phillips, A.E.; Keen, D.A.; Tucker, M.G.; Goodwin, A.L.; Bennet, T.D.; Cheetham, A.K. Flexibility of zeolitic imidazolate framework structures studied by neutron total scattering and the reverse Monte Carlo method. J. Phys. Condens. Matter 2013, 25, 395403–395409. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Mohammad, A.; Mobin, S.M. Recent Advances in Single-Crystal-to-Single-Crystal Transformation at the Discrete Molecular Level. Cryst. Growth Des. 2017, 17, 2893–2910. [Google Scholar] [CrossRef]
- He, W.W.; Li, S.L.; Lan, Y.Q. Liquid-free single-crystal-to-single-crystal transformation in coordination polymers. Inorg. Chem. Front. 2018, 5, 279–300. [Google Scholar] [CrossRef]
- Halder, A.; Ghosh, D. Structure and properties of dynamic metal–organic frameworks: A brief accounts of crystalline-to-crystalline and crystalline-to-amorphous-transformations. CrystEngComm 2018, 20, 1322–1345. [Google Scholar] [CrossRef]
- Kawano, M.; Fujita, M. Direct observation of crystalline-state guest exchange in coordination networks. Coord. Chem. Rev. 2007, 251, 2592–2605. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal−Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Férey, G. Hybrid porous solids: Past, present and future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yao, Z.Z.; Xiang, S.; Chen, B. Perspective of microporous metal-organic framework for CO2 capture and separation. Energy Environ. Sci. 2014, 7, 2868–2899. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.L.; Yaghi, O.M. Carbon capture and conversion using metal-organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, D.A.; Loeb, S.J. Hydrogen-bonded networks through second sphere coordination. Chem. Eur. J. 2002, 8, 5084–5088. [Google Scholar] [CrossRef]
- Mercer, D.J.; Loeb, S.J. Metal-based anion receptors: An application of second sphere coordination. Chem. Soc. Rev. 2010, 39, 3612–3620. [Google Scholar] [CrossRef]
- Guo, F.; Martí-Rujas, J. Second sphere coordination of hybrid metal–organic materials: Solid state reactivity. Dalton Trans. 2016, 45, 13648–13662. [Google Scholar] [CrossRef]
- Pichlo, L.; Juetten, F.; Wojtalla, M.; Schacherl, D. Diaz and U.; Baumann, Molecular determinants of the mechanism and substrate specificity of Clostridium difficile proline-proline endopeptidase-1. J. Biol. Chem. 2019, 294, 11525–11535. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, H.B.; Ji, L.N.; Mao, Z.W. Insights into metalloenzyme microenvironments: Biomimetic metal complexes with a functional second coordination sphere. Chem. Soc. Rev. 2013, 42, 8360–8375. [Google Scholar] [CrossRef]
- Sharma, B.; Striegler, S. Tailored Interactions of the Secondary Coordination Sphere Enhance the Hydrolytic Activity of Cross-Linked Microgels. ACS Catal. 2019, 9, 1686–1691. [Google Scholar] [CrossRef]
- Gotico, P.; Botirel, B.; Guillot, R.; Sircoglou, M.; Quaranta, A.; Halime, Z.; Leibl, W.; Aukauloo, A. Second-Sphere Biomimetic Multipoint Hydrogen-Bonding Patterns to Boost CO2 Reduction of Iron Porphyrins. Angew. Chem. Int. Ed. 2019, 58, 4504–4509. [Google Scholar] [CrossRef]
- Liu, Z.; Frasconi, M.; Lei, J.; Brown, Z.J.; Zhu, Z.; Cao, D.; Iehl, J.; Liu, G.; Fahrenbach, A.C.; Botros, Y.Y.; et al. Selective isolation of gold facilitated by second-sphere coordination with α-cyclodextrin. Nat. Commun. 2013, 4, 1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalrymple, S.A.; Shimizu, G.K.H. Crystal Engineering of a Permanently Porous Network Sustained Exclusively by Charge-Assisted Hydrogen Bonds. J. Am. Chem. Soc. 2007, 129, 12114–12116. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Gimenez-Marques, M.; Espallargas, G.M.; Brammer, L. Tuning the magneto-structural properties of non-porous coordination polymers by HCl chemisorption. Nat. Commun. 2012, 3, 828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortecchia, D.; Soci, C.; Cametti, M.; Petrozza, A.; Martí-Rujas, J. Crystal Engineering of a Two-Dimensional Lead-Free Perovskite with Functional Organic Cations by Second-Sphere Coordination. ChemPlusChem 2017, 82, 681–685. [Google Scholar] [CrossRef] [Green Version]
- Adams, C.J.; Colquhoun, H.M.; Crawford, P.C.; Lusi, M.; Orpen, A.G. Solid-state interconversion of coordination networks and hydrogen-bonded salts. Angew. Chem. Int. Ed. 2007, 46, 1124–1128. [Google Scholar] [CrossRef]
- Inokuma, Y.; Yoshioka, S.; Ariyoshi, J.; Arai, T.; Hitora, Y.; Takada, K.; Matsunaga, S.; Rissanen, K.; Fujita, M. X-ray analysis on the nanogram to microgram scale using porous complexes. Nature 2013, 495, 461–466. [Google Scholar] [CrossRef]
- Inokuma, Y.; Yoshioka, S.; Ariyoshi, J.; Arai, T.; Fujita, M. Preparation and Guest-Uptake Protocol for a Porous Complex Useful for ‘Crystal-Free’ Crystallography. Nat. Protoc. 2014, 9, 246–252. [Google Scholar] [CrossRef]
- Hou, J.; Ashling, C.W.; Collins, S.M.; Krajnc, A.; Zhou, C.; Longley, L.; Johnstone, D.N.; Chater, P.A.; Li, S.; Coulet, M.V.; et al. Metal-organic framework crystal-glass composites. Nat. Commun. 2019, 18, 2580–2590. [Google Scholar] [CrossRef]
- Horike, S.; Nagarkar, S.S.; Ogawa, T.; Kitagawa, S. New dimension of coordination polymers and metal organic frameworks toward functional glasses and liquids. Angew. Chem. Int. Ed. 2019. [Google Scholar] [CrossRef]
- Biradha, K.; Fujita, M. A Springlike 3D-Coordination Network That Shrinks or Swells in a Crystal-to-Crystal Manner upon Guest Removal or Readsorption. Angew. Chem. Int. Ed. 2002, 41, 3392–3395. [Google Scholar] [CrossRef]
- Zhang, J.P.; Lin, Y.Y.; Zhang, W.X.; Chen, X.M. Temperature- or guest-induced drastic single-crystal-to-single-crystal transformations of a nanoporous coordination polymer. J. Am. Chem. Soc. 2005, 127, 14162–14163. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, K.; Kawano, M.; Hozumi, T.; Ohkoshi, S.I.; Fujita, M. 2D hydrogen-bonded square-grid coordination networks with a substitution-active metal site. Inorg. Chem. 2006, 45, 3976–3982. [Google Scholar] [CrossRef] [PubMed]
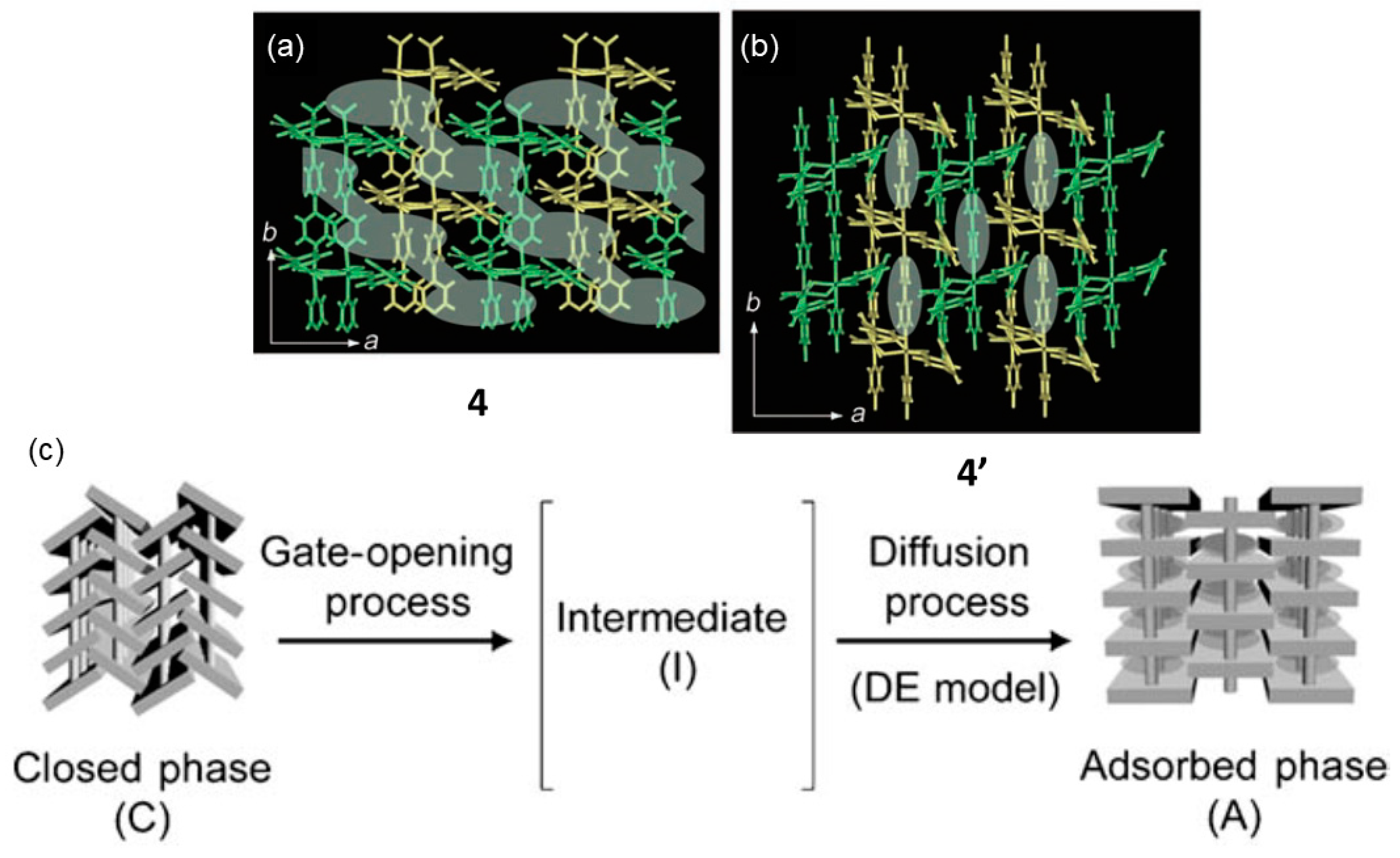
- Tanaka, D.; Nakagawa, K.; Higuchi, M.; Horike, S.; Kubota, Y.; Kobayashi, T.C.; Takata, M.; Kitagawa, S. Kinetic gate-opening process in a flexible porous coordination polymer. Angew. Chem. Int. Ed. 2008, 47, 3914–3918. [Google Scholar] [CrossRef] [PubMed]
- Bernini, M.C.; Gandara, F.; Iglesias, M.; Snejko, N.; Gutierrez-Puebla, E.; Brusau, E.V.; Narda, G.E.; Monge, M.A. Reversible Breaking and Forming of Metal-Ligand Coordination bonds: Temperature-Triggered Single-Crystal to Single-Crystal Transformation in a Metal Organic Framework. Chem. Eur. J. 2009, 15, 4896–4905. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; He, W.W.; Du, D.Y.; Jiang, H.L.; Li, S.L.; Lang, Z.L.; Su, Z.M.; Fu, Q.; Lan, Y.Q. Solid-state structural transformation doubly triggered by reaction temperature and time in 3D metal-organic frameworks: Great enhancement of stability and gas adsorption. Chem. Sci. 2014, 5, 1368–1374. [Google Scholar] [CrossRef]
- Shivanna, M.; Yang, Q.Y.; Bajpai, A.; Patyk-Kazmierczak, E.; Zaworotko, M.J. A dynamic and multi-responsive porous metal-organic material. Nat. Commun. 2018, 9, 3080. [Google Scholar] [CrossRef] [PubMed]
- Ohara, K.; Martí-Rujas, J.; Haneda, T.; Kawano, M.; Hashizume, D.; Izumi, F.; Fujita, M. Formation of a Thermally Stable, Porous Coordination Network via a Crystalline-to-Amorphous-to-Crystalline Phase Transition. J. Am. Chem. Soc. 2009, 131, 3860–3861. [Google Scholar] [CrossRef]
- David, W.I.F.; Shankland, K.; van de Streek, J.; Pidcock, E.; Motherwell, W.D.S.; Cole, J.C. DASH: A program for crystal structure determination from powder diffraction data. J. Appl. Crystallogr. 2006, 39, 910–915. [Google Scholar] [CrossRef]
- Martí-Rujas, J.; Islam, N.; Hashizume, D.; Izumi, F.; Fujita, M.; Kawano, M. Dramatic structural rearrangements in porous coordination networks. J. Am. Chem. Soc. 2011, 133, 5853–5860. [Google Scholar] [CrossRef]
- Martí-Rujas, J.; Kawano, M. Structural memory effect in solid state transformation of kinetic coordination networks. Spring 8 Res. Front. 2011, 86–87. [Google Scholar]
- Bennett, T.D.; Keen, D.A.; Tan, J.C.; Barney, E.R.; Goodwin, A.L.; Cheetham, A.K. Thermal amorphization of Zeolitic Imidazolate Frameworks. Angew. Chem. Int. Ed. 2011, 50, 3067–3071. [Google Scholar] [CrossRef] [PubMed]
- Bennett, T.D.; Cheetham, A.K. Amorphous Metal-Organic Frameworks. Acc. Chem. Res. 2014, 47, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Lässig, D.; Lincke, J.; Gerhardt, R.; Krautscheid, H. Solid-State Syntheses of Coordination Polymers by Thermal Conversion of Molecular Building Blocks and Polymeric Precursors. Inorg. Chem. 2012, 51, 6180–6189. [Google Scholar] [CrossRef] [PubMed]
- Ohtsu, H.; Bennet, T.D.; Kojima, T.; Keen, D.A.; Niwa, Y.; Kawano, M. Amorphous-amorphous transition in a porous coordination polymer. Chem. Commun. 2017, 53, 7060–7063. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Kurawa, M.A.; Lusi, M.; Orpen, A.G. Solid state synthesis of coordination compounds from basic metal salts. CrystEngComm 2008, 10, 1790–1795. [Google Scholar] [CrossRef]
- Mínguez-Espallargas, G.; Brammer, L.; van de Streek, J.; Shankland, K.; Florence, A.J.; Adams, H. Reversible Extrusion and Uptake of HCl Molecules by Crystalline Solids Involving Coordination Bond Cleavage and Formation. J. Am. Chem. Soc. 2006, 128, 9584–9585. [Google Scholar] [CrossRef]
- Cai, L.Z.; Jiang, X.M.; Zhang, Z.J.; Guo, P.Y.; Jin, A.P.; Wang, M.S.; Guo, G.C. Reversible Single-Crystal-to-Single-Crystal Transformation and Magnetic Change of Nonporous Copper(II) Complexes by the Chemisorption/Desorption of HCl and H2O. Inorg. Chem. 2017, 56, 1036–1040. [Google Scholar] [CrossRef]
- Miguel-Donet, J.; López-Cabrelles, J.; Calvo Galve, N.; Coronado, E.; Mínguez Espallargas, G. Two consecutive magneto-structural gas-solid transformations in non-porous molecular materials. Chem. Eur. J. 2018, 24, 12426–12432. [Google Scholar] [CrossRef]
- Guo, F.; Wang, H.C.; Famulari, A.; Lu, H.D.; Martí-Rujas, J. Dynamic behavior un nonporous hybrid metal-organic materials via mechanochemical and gas solid reactions. CrystEngComm. 2018, 20, 6721–6726. [Google Scholar] [CrossRef]
- Guo, F.; Wang, X.; Guan, H.; Yu, H.B.; Li, L.; Chen, S.S.; Famulari, A.; Martí-Rujas, J. Tuning the Inclusion Properties and Solid-State Reactivity of Second Sphere Adducts Using Conformationally Flexible Bidentate Ligands. Cryst. Growth Des. 2015, 15, 2842–2852. [Google Scholar]











| Starting MOM | Thermal Product | Process | Temperature | Structural Transformation | References |
|---|---|---|---|---|---|
| 1 | 1′ | SCSC1 | 446 K | Guest release, network sliding. | [62] |
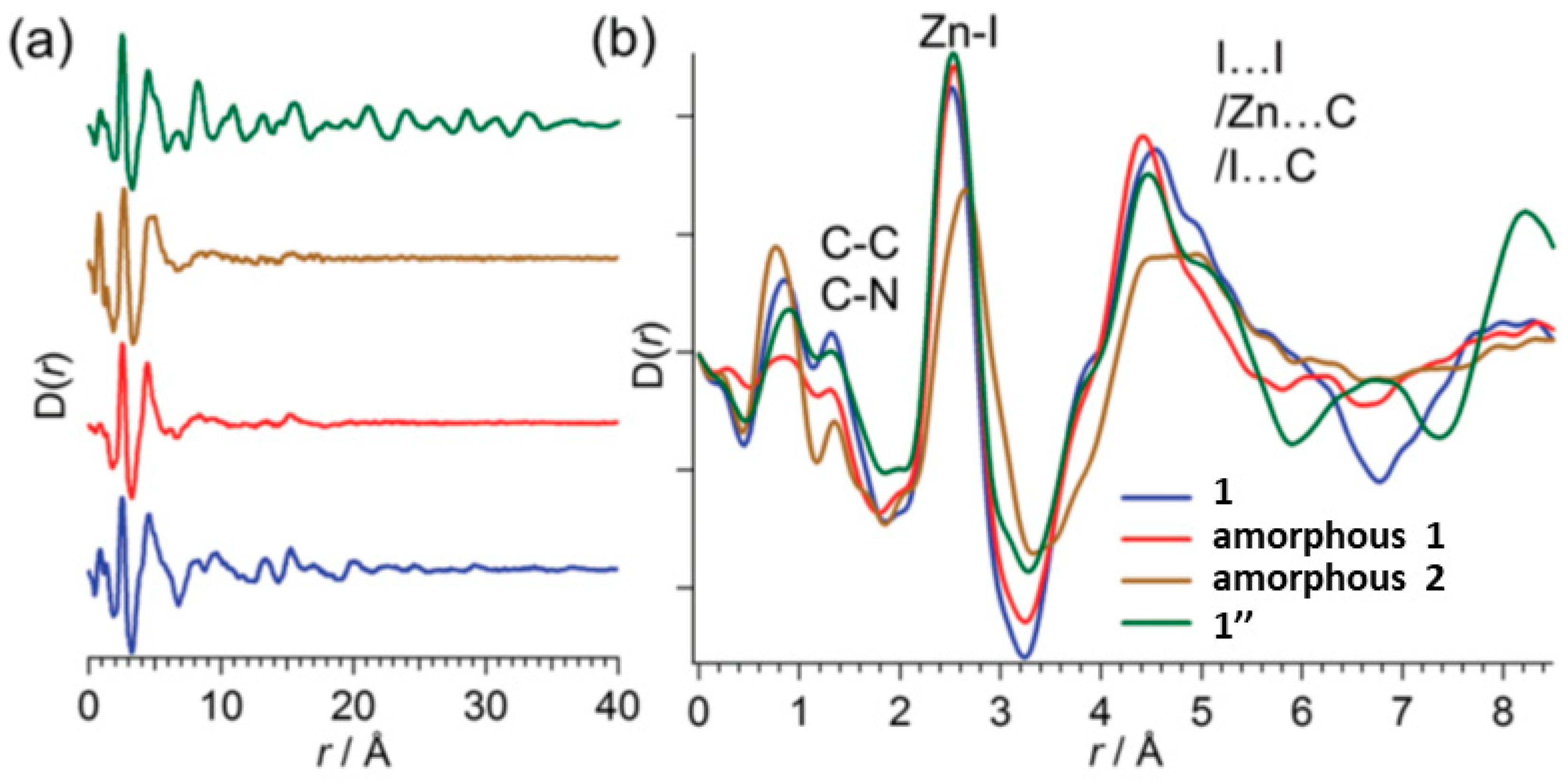
| 1′ | 1″ | SC-PC2 | 573 K | Interpenetrated to non-intepenetrated networks; CAC3; bond cleavage and formation. | [69,71,72] |
| 2 | 2′ | SCSC | 375 K | 5-fold→6-fold intepenetration; bond cleavage and formation. | [63] |
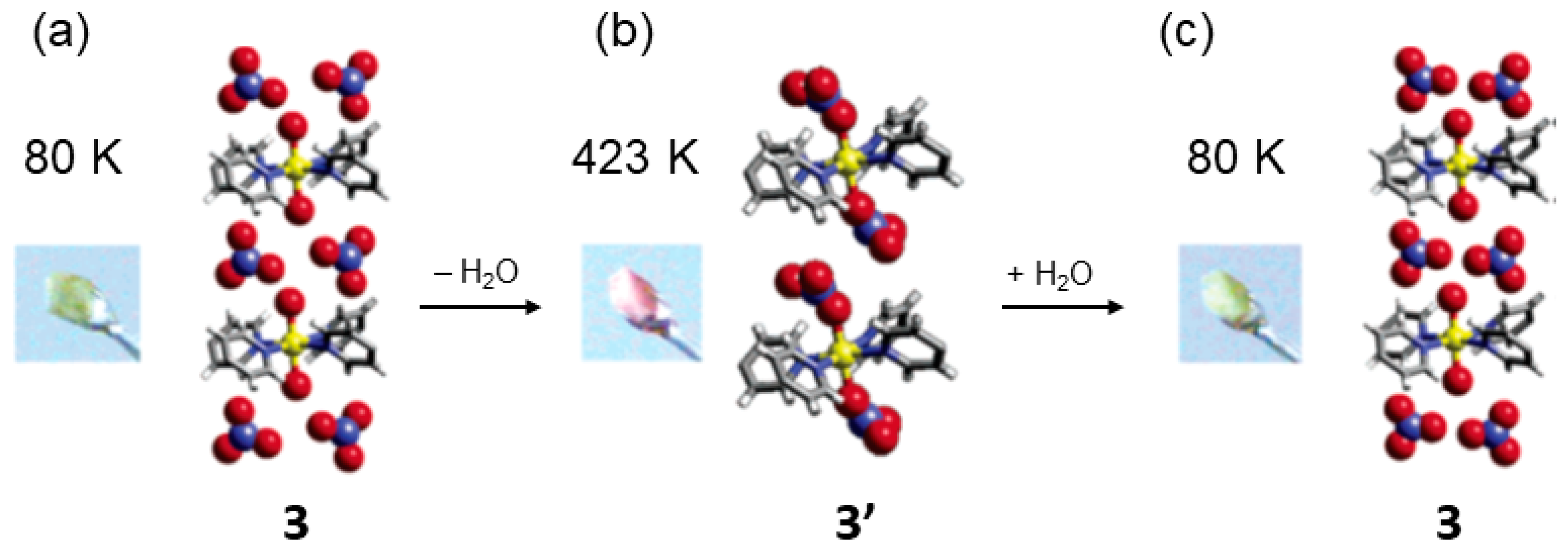
| 3 | 3′ | SCSC | 473 K | Apical H2O for NO32− ligand exchange; cationic→neutral network change. | [64] |
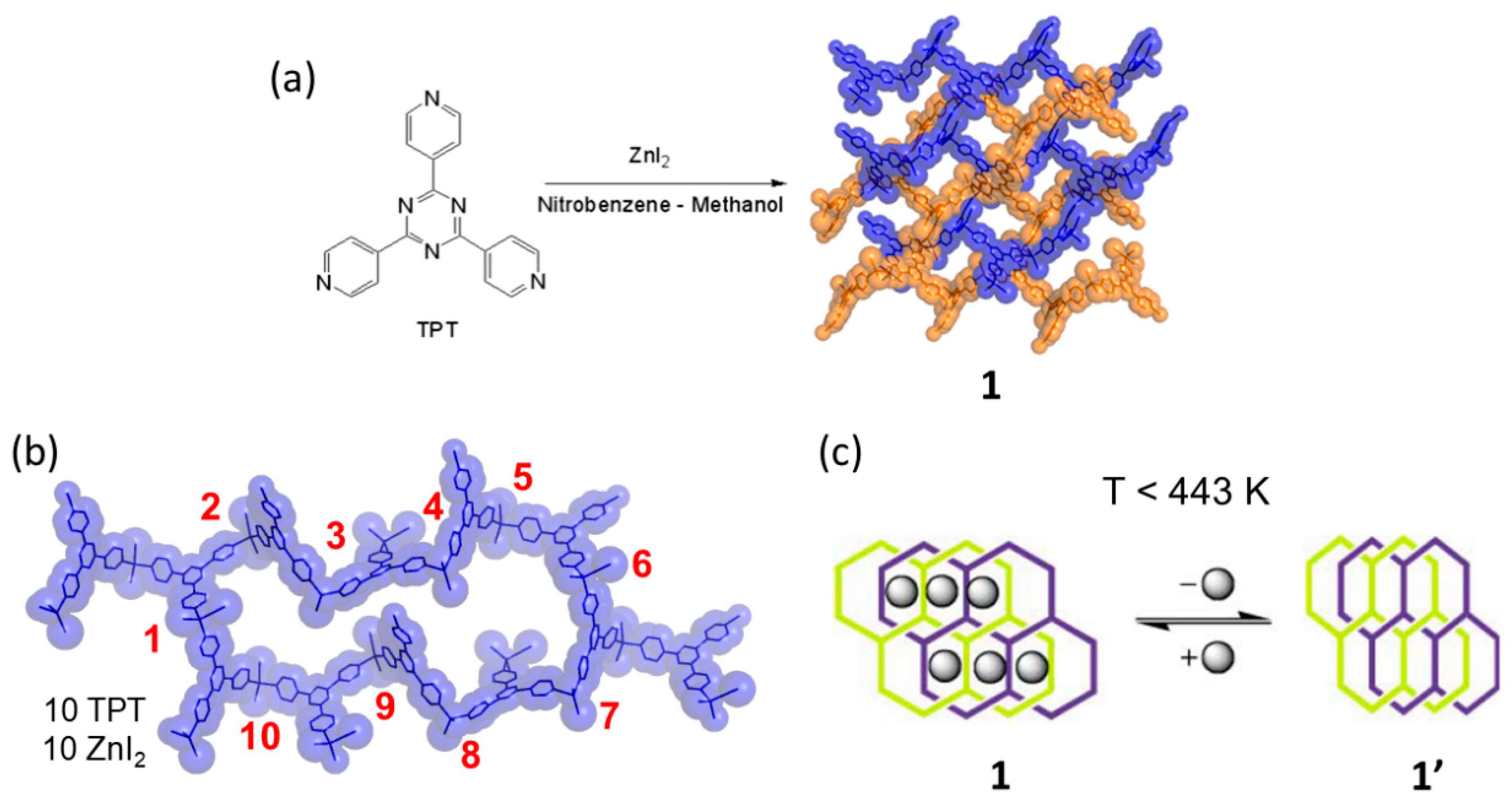
| 4 | 4′ | SCSC | 413 K | Guest release, network sliding; gate opening behavior. | [65] |
| 5 | 5′ | SCSC | 429 K | Bond breaking and formation; change from triangular dodecahedron YbO8 to pentagonal bipyramidal YbO7. | [66] |
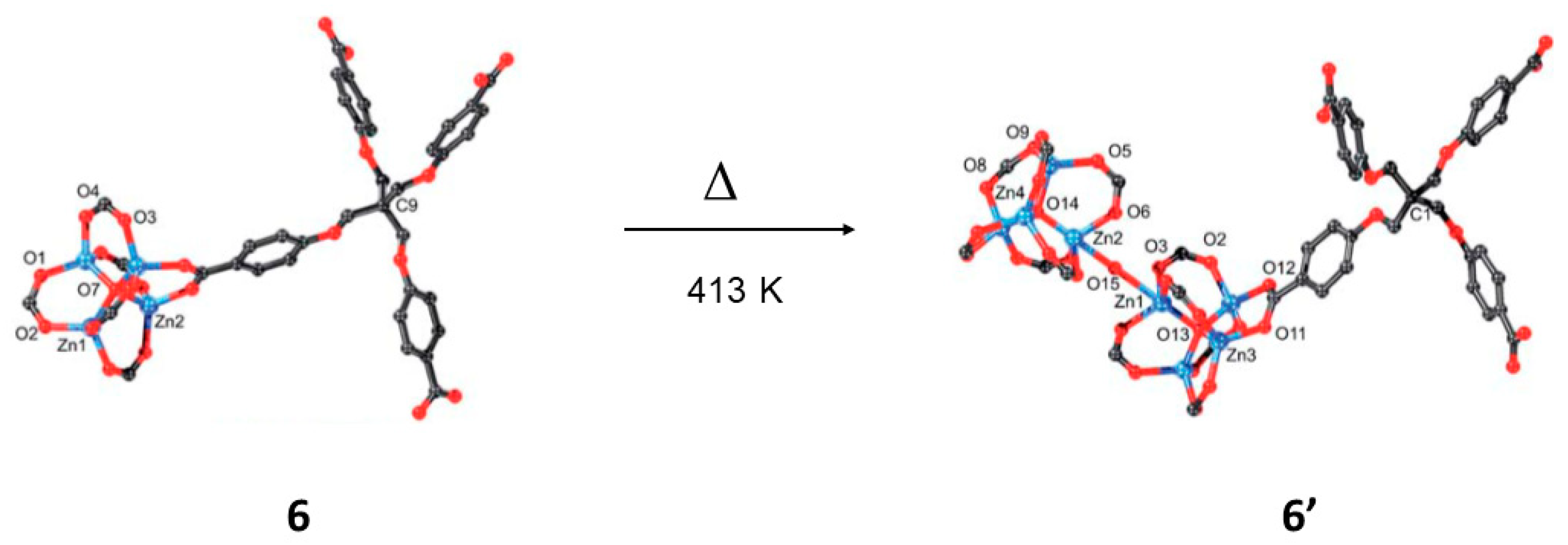
| 6 | 6′ | SCSC | 438 K | Non-intepenetrated to interpenetrated nets; dimerization of secondary building blocks via H2O; bond formation. | [67] |
| 7 | 7′ | SCSC | 403 K | Bond cleavage and formation; 3-fold→4-fold interpenetration. | [68] |
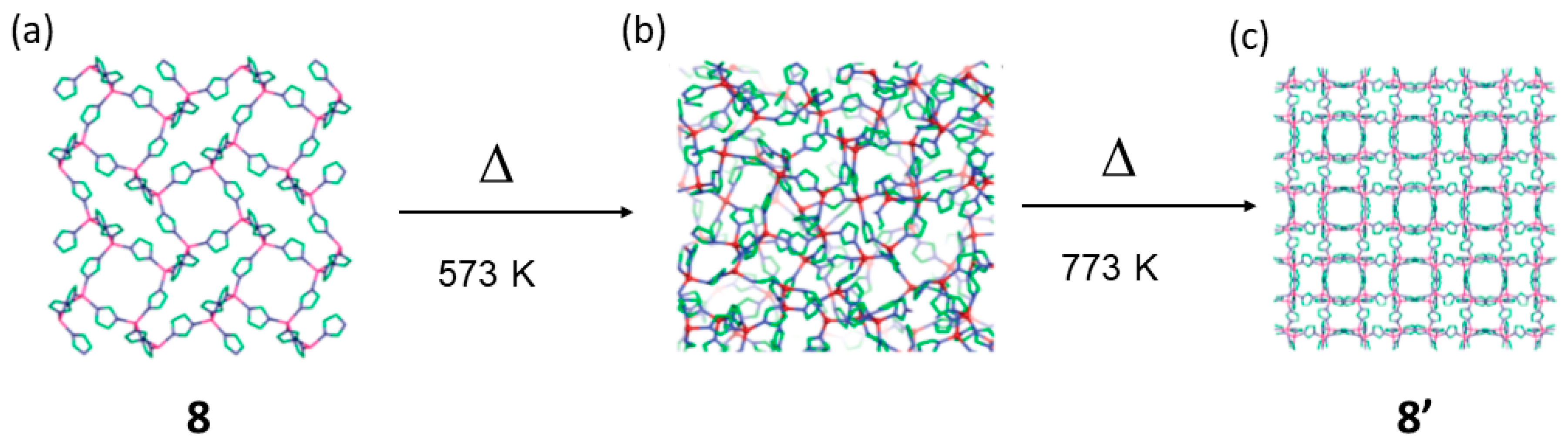
| 8 | 8′ | SCPC | 573 K–733 K | CAC; bond-cleavage and formation. | [73,74] |
| 9 | 9′ | SCPC | 506 K–583 K | Discrete (0D) complex→3D CPs; 1D→3D CPs; 3D→3D CPs CAC; bond cleavage and formation. | [75] |
| 10 | 10′ | SCSC | 373 K | Second sphere adduct→second sphere adduct; chemisorption of HCl, bond-cleavage and formation. | [79] |
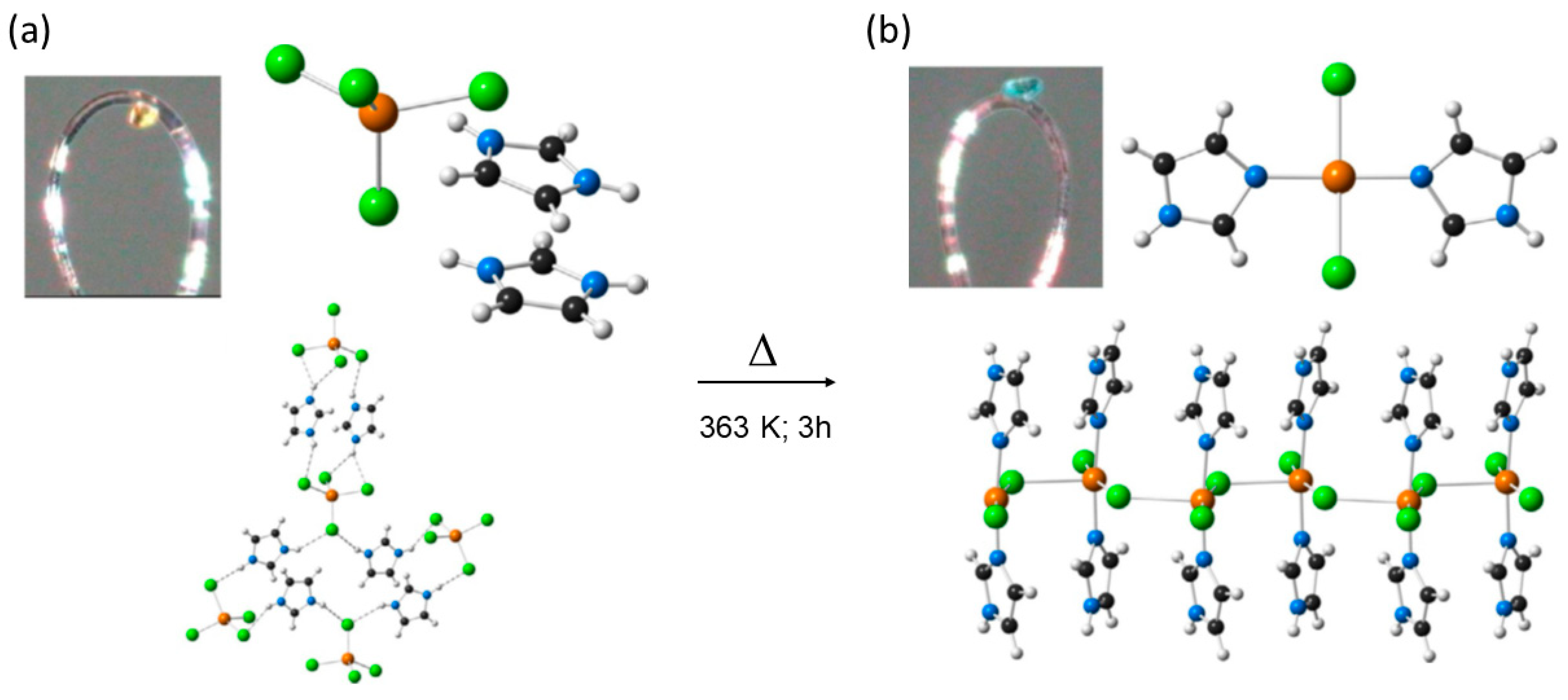
| 11 | 11′ | SCSC | 363 K | Second sphere adduct→1D CP; chemisorption of HCl bond-cleavage and formation. | [80] |
| 12 | 12′ | SCPC | 423 K | Second sphere adduct→1D CP; chemisorption of HCl bond-cleavage and formation. | [81] |
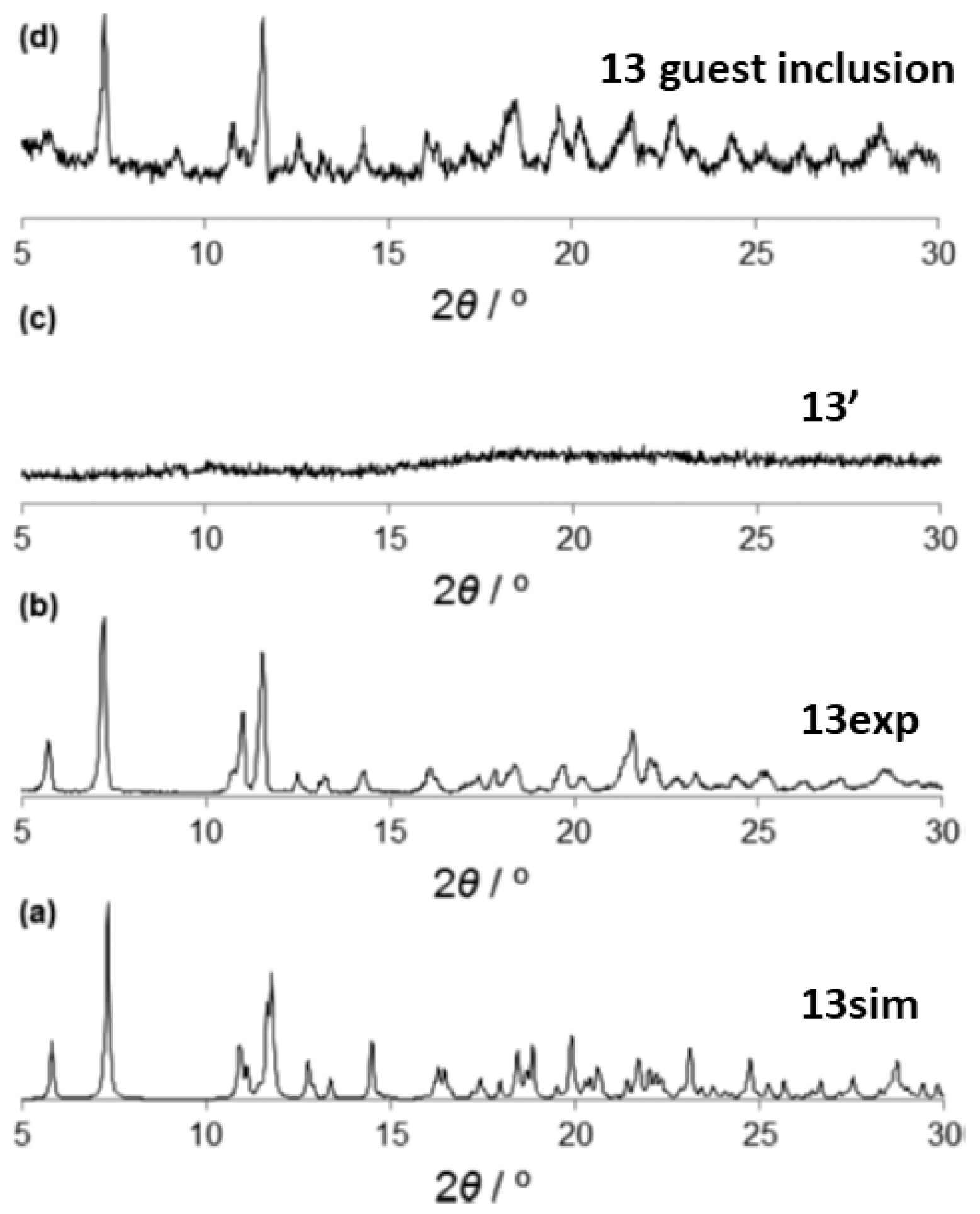
| 13 | 13′ | SCPC | 373 K | Second sphere adduct guest release; CAC. | [82] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martí-Rujas, J. Thermal Reactivity in Metal Organic Materials (MOMs): From Single-Crystal-to-Single-Crystal Reactions and Beyond. Materials 2019, 12, 4088. https://doi.org/10.3390/ma12244088
Martí-Rujas J. Thermal Reactivity in Metal Organic Materials (MOMs): From Single-Crystal-to-Single-Crystal Reactions and Beyond. Materials. 2019; 12(24):4088. https://doi.org/10.3390/ma12244088
Chicago/Turabian StyleMartí-Rujas, Javier. 2019. "Thermal Reactivity in Metal Organic Materials (MOMs): From Single-Crystal-to-Single-Crystal Reactions and Beyond" Materials 12, no. 24: 4088. https://doi.org/10.3390/ma12244088





