Development of Marine Antifouling Epoxy Coating Enhanced with Clay Nanotubes
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Preparation of DCOIT Loaded into Halloysite Nanotubes
2.3. Antifouling Assay
2.4. Characterization
3. Results and Discussion
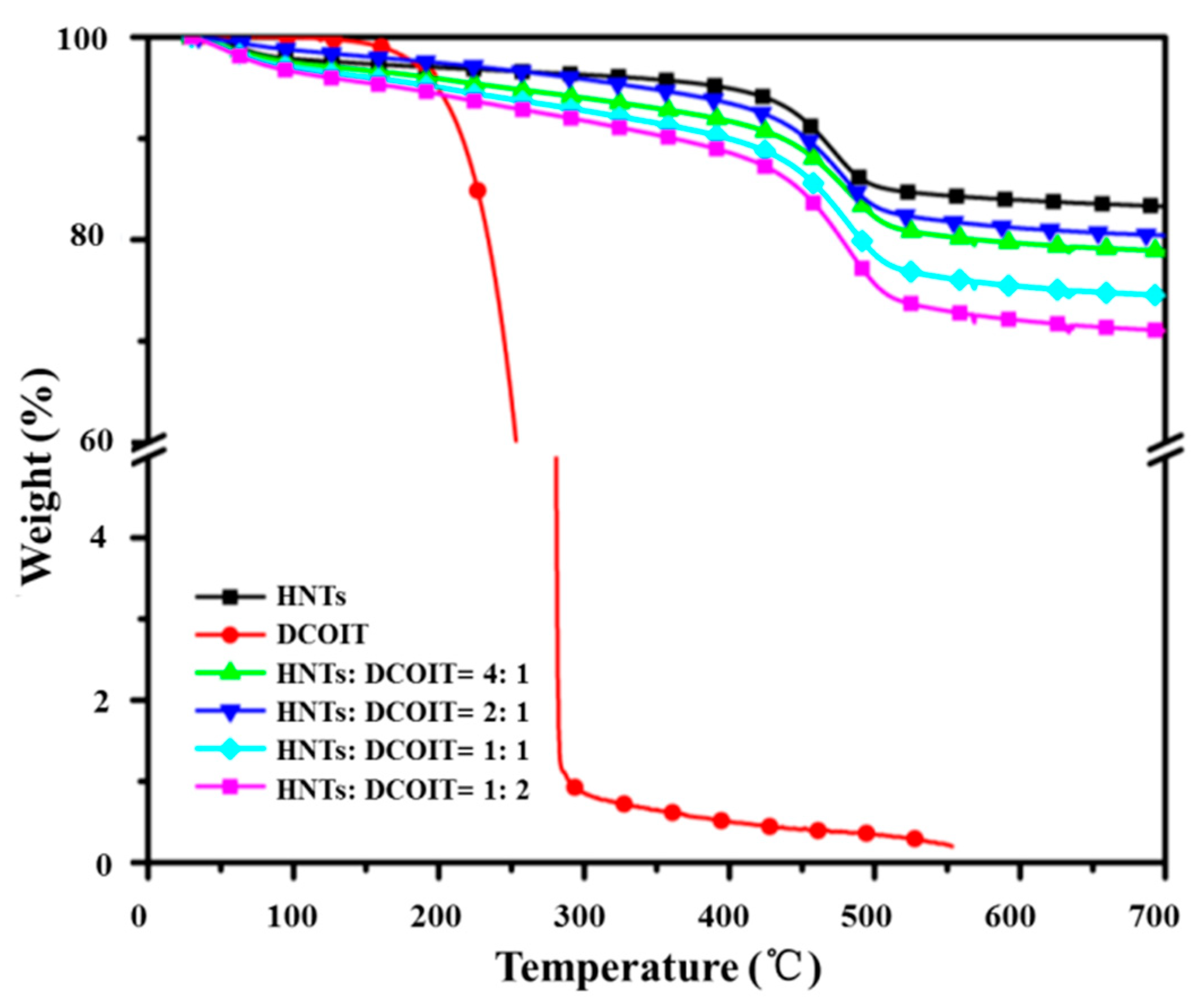
3.1. Halloysite Lumens Encapsulating Antifoulant-DCOIT
3.2. Release Kinetics of the Antifoulant
3.3. Silver Nanoparticles on Halloysite and Its Epoxy Composites
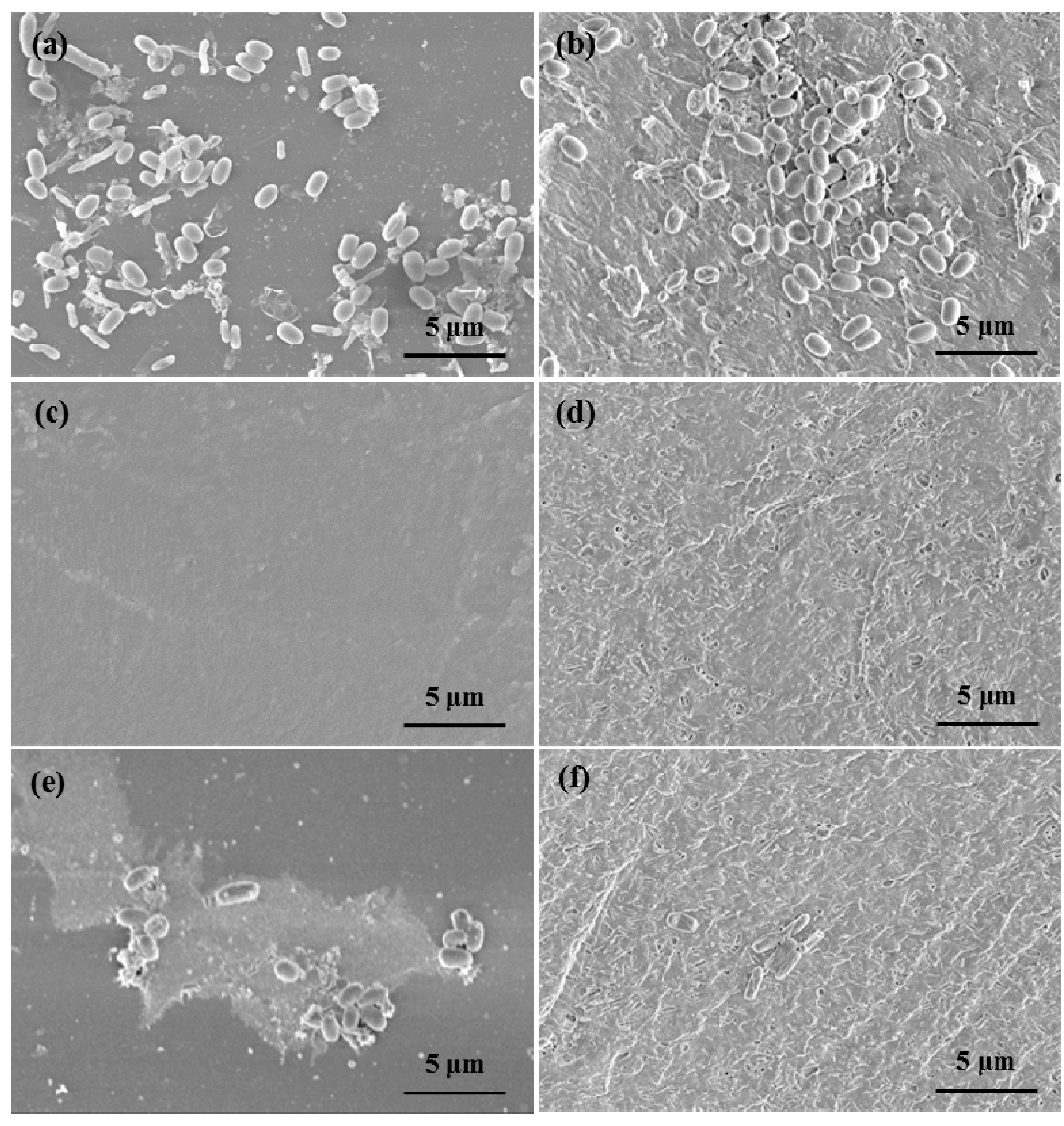
3.4. Antibacterial Test of Epoxy Resin/Halloysite Coating Formualtions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mieszkin, S.; Callow, M.E.; Callow, J.A. Interactions between Microbial Biofilms and Marine Fouling Algae: A Mini Review. Biofouling 2013, 29, 1097–1113. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.; Diamantino, T.C.; de Sousa, O. Marine Paints: The Particular Case of Antifouling Paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic Impact of Biofouling on a Naval Surface Ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Abioye, O.P.; Loto, C.A.; Fayomi, O.S.I. Evaluation of Anti-Biofouling Progresses in Marine Application. J. Bio Tribo Corros. 2019, 5, 22. [Google Scholar] [CrossRef]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of Biofilm Structures by the Novel Computer Program COMSTAT. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selim, M.S.; Shenashen, M.A.; El-Safty, S.A.; Higazy, S.A.; Selim, M.M.; Isago, H.; Elmarakbi, A. Recent Progress in Marine Foul-Release Polymeric Nanocomposite Coatings. Prog. Mater. Sci. 2017, 87, 1–32. [Google Scholar] [CrossRef]
- Ciriminna, R.; Bright, F.V.; Pagliaro, M. Ecofriendly Antifouling Marine Coatings. ACS Publ. 2015, 3, 559–565. [Google Scholar] [CrossRef]
- Kotrikla, A. Environmental Management Aspects for Tbt Antifouling Wastes from the Shipyards. J. Environ. Manag. 2009, 90, S77–S85. [Google Scholar] [CrossRef]
- Elshaarawy, R.F.M.; Mustafa, F.H.A.; Sofy, A.R.; Hmed, A.A.; Janiak, C. A New Synthetic Antifouling Coatings Integrated Novel Aminothiazole-Functionalized Ionic Liquids Motifs with Enhanced Antibacterial Performance. J. Environ. Chem. Eng. 2019, 7, 102800. [Google Scholar] [CrossRef]
- Yandi, W.; Mieszkin, S.; Martin-Tanchereau, P.; Callow, M.E.; Callow, J.A.; Tyson, L.; Liedberg, B.; Ederth, T. Hydration and Chain Entanglement Determines the Optimum Thickness of Poly (Hema-Co-Peg10ma) Brushes for Effective Resistance to Settlement and Adhesion of Marine Fouling Organisms. ACS Appl. Mater. Interfaces 2014, 6, 11448–11458. [Google Scholar] [CrossRef] [Green Version]
- Fang, L.; Huang, J.; Liu, Y.; Zhang, B.; Li, H. Cored-Wire Arc Spray Fabrication of Novel Aluminium-Copper Coatings for Anti-Corrosion/Fouling Hybrid Performances. Surf. Coat. Technol. 2019, 357, 794–801. [Google Scholar] [CrossRef]
- Ramalhosa, P.; Gestoso, I.; Duarte, B.; Caçador, I.; Canning-Clode, J. Metal Pollution Affects Both Native and Non-Indigenous Biofouling Recruitment in a Subtropical Island System. Mar. Pollut. Bull. 2019, 141, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Paradas, W.C.; Amado Filho, G.M. Are Metals of Antifouling Paints Transferred to Marine Biota? Braz. J. Oceanogr. 2007, 55, 51–56. [Google Scholar] [CrossRef]
- Amara, I.; Miled, W.; Slama, R.B.; Ladhari, N. Antifouling Processes and Toxicity Effects of Antifouling Paints on Marine Environment. A Review. Environ. Toxicol. Pharmacol. 2018, 57, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, J.A.; McCallum, K.; Davis, A.A. Phylogenetic Diversity of Subsurface Marine Microbial Communities from the Atlantic and Pacific Oceans. Appl. Environ. Microbiol. 1993, 59, 1294–1302. [Google Scholar] [PubMed]
- Shivapooja, P.; Cao, C.; Orihuela, B.; Levering, V.; Zhao, X.; Rittschof, D.; López, G.P. Incorporation of Silicone Oil into Elastomers Enhances Barnacle Detachment by Active Surface Strain. Biofouling 2016, 32, 1017–1028. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J. Modern Approaches to Marine Antifouling Coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef] [Green Version]
- Chong, Y.B.; Zhang, H.; Yue, C.Y.; Yang, J.L. Fabrication and release behavior of microcapsules with double-layered shell containing clove oil for antibacterial applications. ACS Appl. Mater. Interfaces 2018, 10, 15532–15541. [Google Scholar] [CrossRef]
- Bergek, J.; Trojer, M.A.; Mok, A.; Nordstierna, L. Controlled release of microencapsulated 2-n-octyl-4-isothiazolin-3-one from coatings: Effect of microscopic and macroscopic pores. Colloids Surf. A 2014, 458, 155–167. [Google Scholar] [CrossRef]
- Nordstierna, L.; Abdalla, A.A.; Masuda, M.; Skarnemark, G.; Nyden, M. Molecular release from painted surfaces: Free and encapsulated biocides. Prog. Org. Coat. 2010, 69, 45–48. [Google Scholar] [CrossRef]
- Yang, M.; Gu, L.; Yang, B.; Wang, L.; Sun, Z.; Zheng, J.; Zhang, J.; Hou, J.; Lin, C. Antifouling composites with self-adaptive controlled release based on an active compound intercalated into layered double hydroxides. Appl. Surf. Sci. 2017, 426, 185–193. [Google Scholar] [CrossRef]
- Yuri, L.; Wang, W.; Zhang, L.; Rawil, F. Halloysite Clay Nanotubes for Loading and Sustained Release of Functional Compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar]
- Lvov, Y.; Shchukin, D.G.; Mohwald, H.; Price, R.R. Halloysite Clay Nanotubes for Controlled Release of Protective Agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Guo, B.; Jia, D. Newly Emerging Applications of Halloysite Nanotubes: A Review. Polym. Int. 2010, 59, 574–582. [Google Scholar] [CrossRef]
- Riela, S.; Massaro, M.; Colletti, C.G.; Bommarito, A.; Giordano, C.; Milioto, S.; Noto, R.; Poma, P.; Lazzara, G. Development and Characterization of Co-Loaded Curcumin/Triazole-Halloysite Systems and Evaluation of Their Potential Anticancer Activity. Int. J. Pharm. 2014, 475, 613–623. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Hitzky, E.; Darder, M.; Aranda, P.; Ariga, K. Advances in Biomimetic and Nanostructured Biohybrid Materials. Adv. Mater. 2010, 22, 323–336. [Google Scholar] [CrossRef]
- Kausar, A. Review on Polymer/Halloysite Nanotube Nanocomposite. Polym. Plast. Technol. Eng. 2018, 57, 548–564. [Google Scholar] [CrossRef]
- Yee, M.S.-L.; Khiew, P.S.; Tan, Y.F.; Kok, Y.-Y.; Cheong, K.W.; Chiu, W.S.; Leong, C.-O. Potent Antifouling Silver-Polymer Nanocomposite Microspheres Using Ion-Exchange Resin as Templating Matrix. Colloids Surf. A 2014, 457, 382–391. [Google Scholar] [CrossRef]
- Yee, M.S.-L.; Khiew, P.S.; Lim, S.S.; Chiu, W.S.; Tan, Y.F.; Kok, Y.-Y.; Leong, C.-O. Enhanced Marine Antifouling Performance of Silver-Titania Nanotube Composites from Hydrothermal Processing. Colloids Surf. A 2017, 520, 701–711. [Google Scholar] [CrossRef]
- Figueiredo, J.; Oliveira, T.; Ferreira, V.; Sushkova, A.; Silva, S.; Carneiro, D.; Cardoso, D.N.; Gonçalves, S.F.; Maia, F.; Rocha, C. Toxicity of Innovative Anti-Fouling Nano-Based Solutions to Marine Species. Environ. Sci. Nano 2019, 6, 1418–1429. [Google Scholar] [CrossRef]
- Vinokurov, V.A.; Stavitskaya, A.V.; Chudakov, Y.A.; Glotov, A.P.; Ivanov, E.V.; Gushchin, P.A.; Lvov, Y.M.; Maximov, A.L.; Muradov, A.V.; Karakhanov, E.A. Core-Shell Nanoarchitecture: Schiff-Base Assisted Synthesis of Ruthenium in Clay Nanotubes. Pure Appl. Chem. 2018, 90, 825. [Google Scholar] [CrossRef]
- Vinokurov, V.A.; Stavitskaya, A.V.; Glotov, A.P.; Novikov, A.A.; Zolotukhina, A.V.; Kotelev, M.S.; Gushchin, P.A.; Ivanov, E.V.; Darrat, Y.; Lvov, Y.M. Nanoparticles Formed onto/into Halloysite Clay Tubules: Architectural Synthesis and Applications. Chem. Rec. 2018, 18, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Vinokurov, V.; Glotov, A.; Chudakov, Y.; Stavitskaya, A.; Ivanov, E.; Gushchin, P.; Zolotukhina, A.; Maximov, A.; Karakhanov, E.; Lvov, Y. Core/Shell Ruthenium–Halloysite Nanocatalysts for Hydrogenation of Phenol. Ind. Eng. Chem. Res. 2017, 56, 14043–14052. [Google Scholar] [CrossRef]
- Vinokurov, V.A.; Stavitskaya, A.V.; Chudakov, Y.A.; Ivanov, E.V.; Shrestha, L.K.; Ariga, K.; Darrat, Y.A.; Lvov, Y.M. Formation of Metal Clusters in Halloysite Clay Nanotubes. Sci. Technol. Adv. Mater. 2017, 18, 147–151. [Google Scholar] [CrossRef]
- Luo, P.; Zhao, Y.; Zhang, B.; Liu, J.; Yang, Y.; Liu, J. Study on the adsorption of neutral red from aqueous solution onto halloysite nanotubes. Water Res. 2010, 44, 1489–1497. [Google Scholar] [CrossRef]
- ASTM D 3623-78a. Standard Test Method for Testing Antifouling Panels in Shallow Submergence; ASTM International: West Conshohocken, PA, USA, 2012.
- Abdullayev, E.; Sakakibara, K.; Okamoto, K.; Wei, W.; Ariga, K.; Lvov, Y. Natural Tubule Clay Template Synthesis of Silver Nanorods for Antibacterial Composite Coating. ACS Appl. Mater. Interfaces 2011, 3, 4040–4046. [Google Scholar] [CrossRef]
- Stavitskaya, A.; Batasheva, S.; Vinokurov, V.; Fakhrullina, G.; Sangarov, V.; Lvov, Y.; Fakhrullin, R. Antimicrobial Applications of Clay Nanotube-Based Composites. Nanomaterials 2019, 9, 708. [Google Scholar] [CrossRef] [Green Version]
- Jana, S.; Kondakova, A.V.; Shevchenko, S.N.; Sheval, E.V.; Gonchar, K.A.; Timoshenko, V.Y.; Vasiliev, A.N. Halloysite Nanotubes with Immobilized Silver Nanoparticles for Anti-Bacterial Application. Colloids Surf. B 2017, 151, 249–254. [Google Scholar] [CrossRef]
- Lazzara, G.; Cavallaro, G.; Panchal, A.; Fakhrullin, R.; Stavitskaya, A.; Vinokurov, V.; Lvov, Y. An Assembly of Organic-Inorganic Composites Using Halloysite Clay Nanotubes. Curr. Opin. Colloid Interface Sci. 2018, 35, 42–50. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Wang, W.; Zhang, L.; Vinokurov, V.; Stavitskaya, A.; Lvov, Y. Development of Marine Antifouling Epoxy Coating Enhanced with Clay Nanotubes. Materials 2019, 12, 4195. https://doi.org/10.3390/ma12244195
Fu Y, Wang W, Zhang L, Vinokurov V, Stavitskaya A, Lvov Y. Development of Marine Antifouling Epoxy Coating Enhanced with Clay Nanotubes. Materials. 2019; 12(24):4195. https://doi.org/10.3390/ma12244195
Chicago/Turabian StyleFu, Ye, Wencai Wang, Liqun Zhang, Vladimir Vinokurov, Anna Stavitskaya, and Yuri Lvov. 2019. "Development of Marine Antifouling Epoxy Coating Enhanced with Clay Nanotubes" Materials 12, no. 24: 4195. https://doi.org/10.3390/ma12244195





