Pressure-Dependent Stability of Imidazolium-Based Ionic Liquid/DNA Materials Investigated by High-Pressure Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
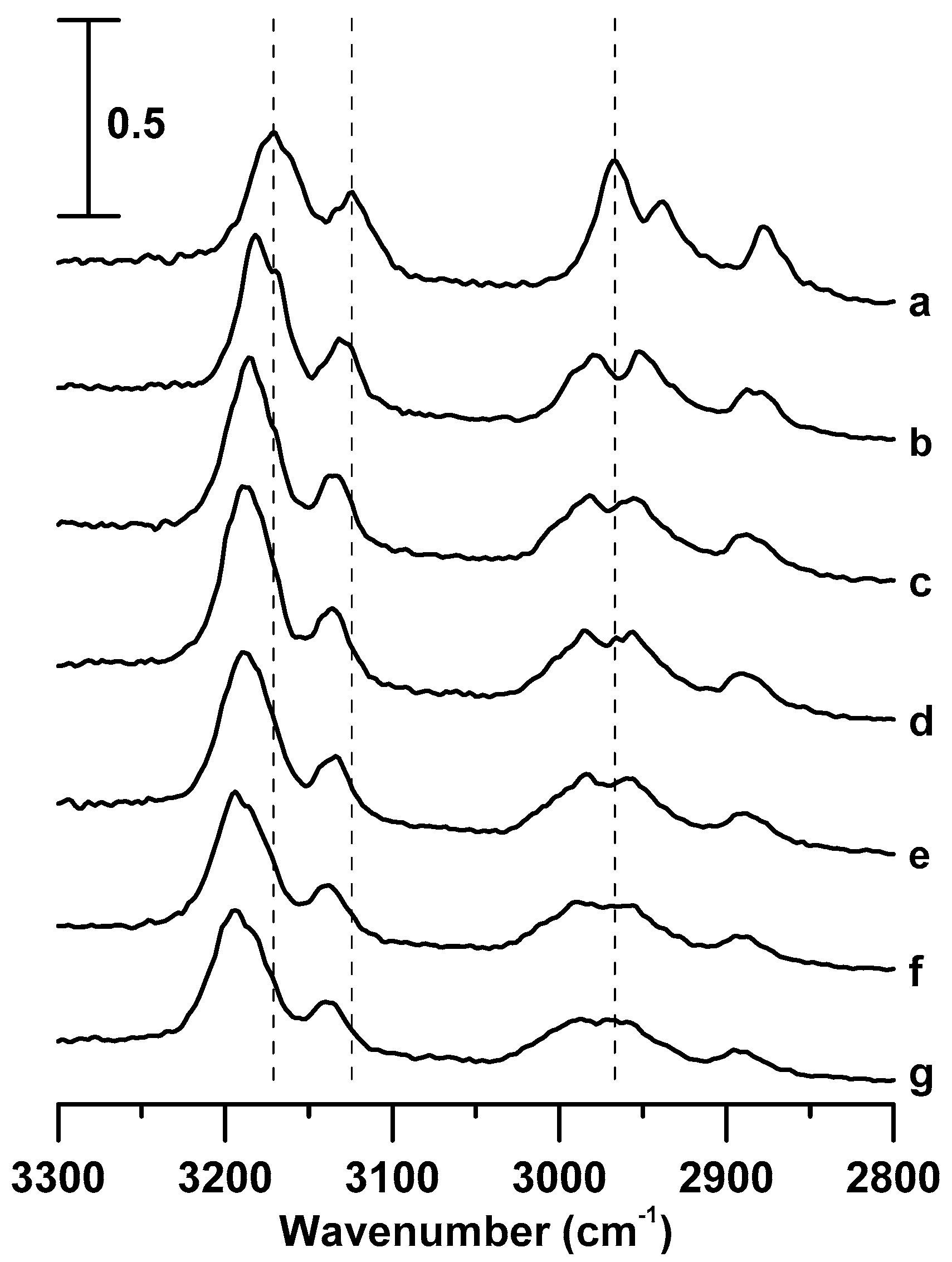
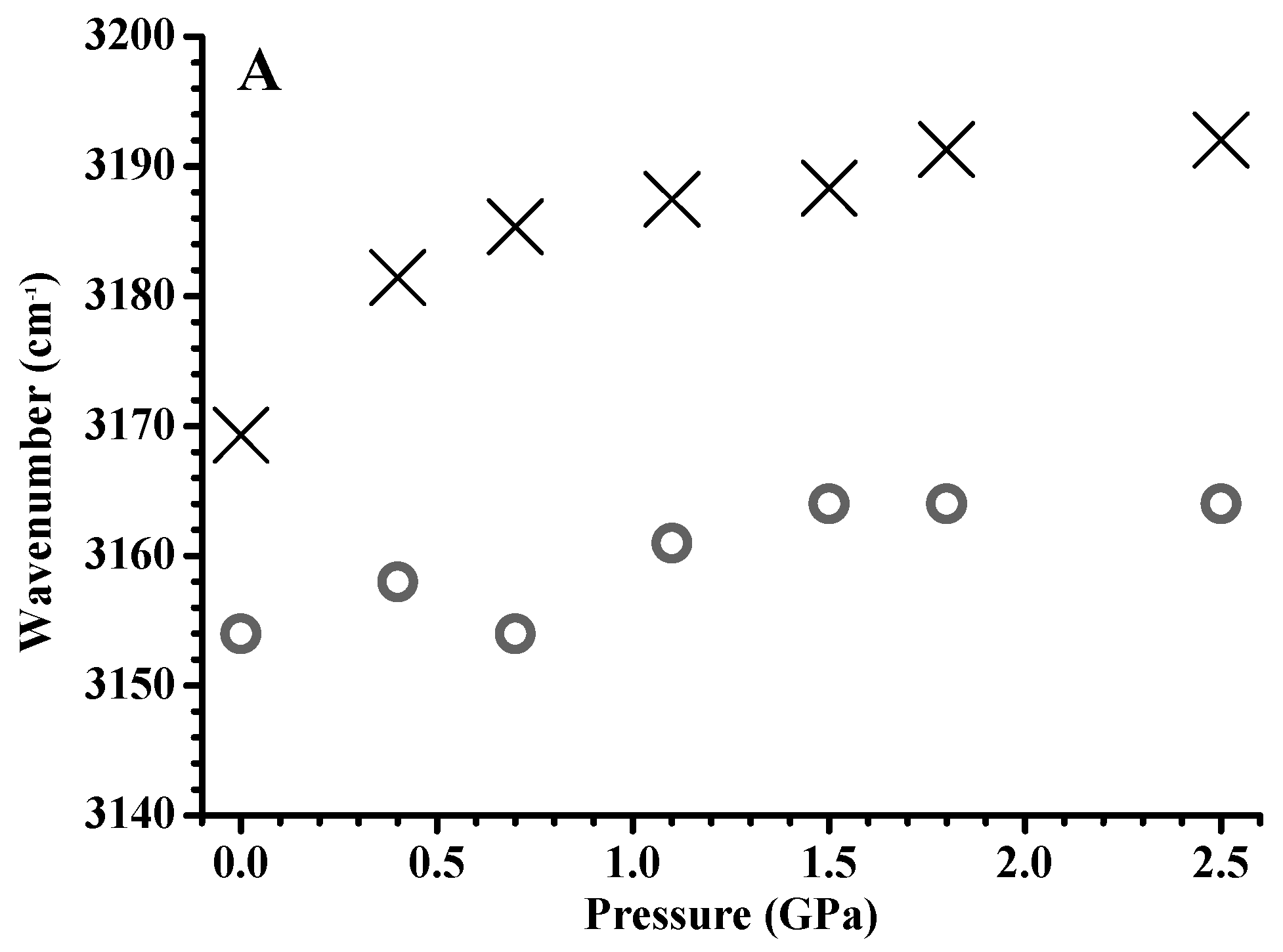
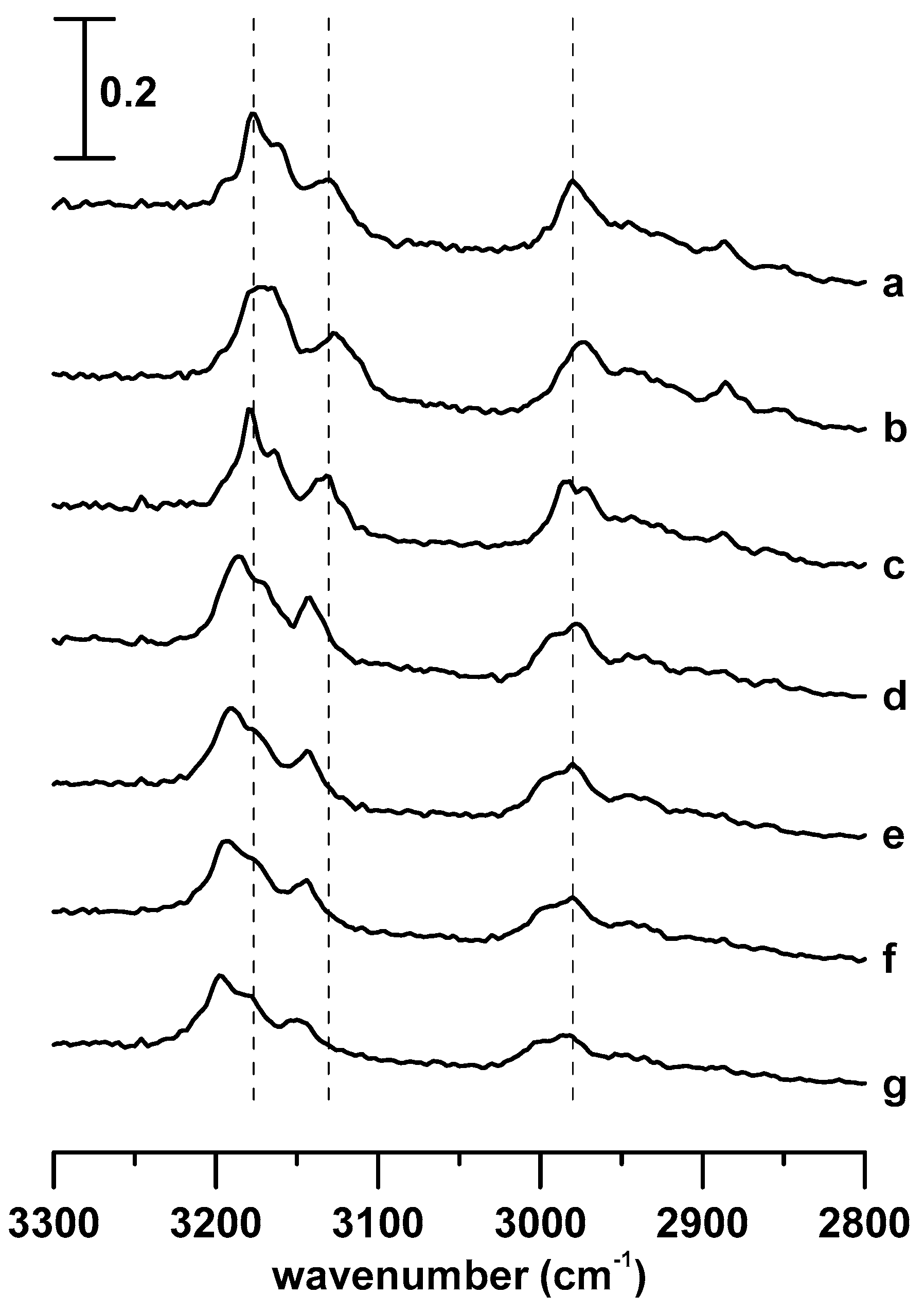
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saenger, W. Principles of Nucleic Acid Structure; Springer Science & Business Media: New York, NY, USA, 2013; ISBN 1461251907. [Google Scholar]
- Nowak, E.; Wisła-Świder, A.; Khachatryan, G.; Fiedorowicz, M.; Danel, K. Possible sensor applications of selected DNA–surfactant complexes. Eur. Biophys. J. 2019, 48, 371–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H. Nucleic acid nanotechnology. Science 2004, 306, 2048–2049. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.K.; Rawat, K.; Aswal, V.K.; Kohlbrecher, J.; Bohidar, H.B. Imidazolium based ionic liquid induced DNA gelation at remarkably low concentration. Colloids Surf. A 2018, 538, 184–191. [Google Scholar] [CrossRef]
- Chworos, A.; Severcan, I.; Koyfman, A.Y.; Weinkam, P.; Oroudjev, E.; Hansma, H.G.; Jaeger, L. Building programmable jigsaw puzzles with RNA. Science 2004, 306, 2068–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, W.M.; Quispe, J.D.; Joyce, G.F. A 1.7-kilobase single-stranded DNA that folds into a nanoscale octahedron. Nature 2004, 427, 618–621. [Google Scholar]
- Liao, S.; Seeman, N.C. Translation of DNA signals into polymer assembly instructions. Science 2004, 306, 2072–2074. [Google Scholar] [CrossRef] [Green Version]
- Wengel, J. Nucleic acid nanotechnology—towards Ångström-scale engineering. Org. Biomol. Chem. 2004, 2, 277–280. [Google Scholar] [CrossRef]
- Jangjian, P.-C.; Liu, T.-F.; Li, M.-Y.; Tsai, M.-S.; Chang, C.-C. Room temperature negative differential resistance in DNA-based molecular devices. Appl. Phys. Lett. 2009, 94, 043105. [Google Scholar] [CrossRef]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and nanostructure in ionic liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Cruz, H.; Fanselow, M.; Holbrey, J.D.; Seddon, K.R. Determining relative rates of cellulose dissolution in ionic liquids through in situ viscosity measurement. Chem. Commun. 2012, 48, 5620–5622. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, J.; Obert, K.; Fries, J.; Bösmann, A.; Wasserscheid, P.; Leipertz, A. Determination of glucose and cellobiose dissolved in the ionic liquid 1-ethyl-3-methylimidazolium acetate using Fourier transform infrared spectroscopy. Appl. Spectrosc. 2009, 63, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Fumino, K.; Peppel, T.; Geppert-Rybczyńska, M.; Zaitsau, D.H.; Lehmann, J.K.; Verevkin, S.P.; Köckerling, M.; Ludwig, R. The influence of hydrogen bonding on the physical properties of ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 14064–14075. [Google Scholar] [CrossRef] [PubMed]
- Triolo, A.; Russina, O.; Bleif, H.-J.; Di Cola, E. Nanoscale segregation in room temperature ionic liquids. J. Phys. Chem. B 2007, 111, 4641–4644. [Google Scholar] [CrossRef]
- Tateishi-Karimata, H.; Sugimoto, N. Biological and nanotechnological applications using interactions between ionic liquids and nucleic acids. Biophys. Rev. 2018, 10, 931–940. [Google Scholar] [CrossRef]
- Teng, Y.; Tateishi-Karimata, H.; Tsuruoka, T.; Sugimoto, N. A Turn-On Detection of DNA Sequences by Means of Fluorescence of DNA-Templated Silver Nanoclusters via Unique Interactions of a Hydrated Ionic Liquid. Molecules 2018, 23, 2889. [Google Scholar] [CrossRef] [Green Version]
- Vlassi, E.; Pispas, S. Imidazolium Quaternized Polymers Based on Poly (Chloromethyl Styrene) and their Complexes with FBS Proteins and DNA. Macromol. Chem. Phys. 2015, 216, 1718–1728. [Google Scholar] [CrossRef]
- Manojkumar, K.; Prabhu Charan, K.T.; Sivaramakrishna, A.; Jha, P.C.; Khedkar, V.M.; Siva, R.; Jayaraman, G.; Vijayakrishna, K. Biophysical characterization and molecular docking studies of imidazolium based polyelectrolytes–DNA complexes: Role of hydrophobicity. Biomacromolecules 2015, 16, 894–903. [Google Scholar] [CrossRef]
- Garai, A.; Ghoshdastidar, D.; Senapati, S.; Maiti, P.K. Ionic liquids make DNA rigid. J. Chem. Phys. 2018, 149, 045104. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Yi, L.; Mou, Y.; Cao, J.; Wang, C. Effect of a molecule of imidazolium bromide ionic liquid on the structure and properties of cytosine by density functional theory. Chem. Phys. Lett. 2018, 708, 109–116. [Google Scholar] [CrossRef]
- Wang, X.; Cui, F. Binding characteristics of imidazolium-based ionic liquids with calf thymus DNA: Spectroscopy studies. J. Fluor. Chem. 2018, 213, 68–73. [Google Scholar] [CrossRef]
- Taboury, J.A.; Liquier, J.; Taillandier, E. Characterization of DNA structures by infrared spectroscopy: Double helical forms of poly (dG-dC) poly (dG-dC), poly (dD8G-dC) poly (dD8G-dC), and poly (dG-dm5C) poly (dG-dm5C). Can. J. Chem. 1985, 63, 1904–1909. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Jena, S.; Dutta, J.; Chakrabarty, S.; Biswal, H.S. Critical Assessment of the Interaction between DNA and Choline Amino Acid Ionic Liquids: Evidences of Multimodal Binding and Stability Enhancement. ACS Cent. Sci. 2018, 4, 1642–1651. [Google Scholar] [CrossRef]
- Ghoshdastidar, D.; Senapati, S. Dehydrated DNA in B-form: Ionic liquids in rescue. Nucleic Acids Res. 2018, 46, 4344–4353. [Google Scholar] [CrossRef]
- Wang, T.-H.; Hong, S.-Y.; Chang, H.-C. The validity of high pressure IR for detecting the interactions between β-cyclodextrin and imidazolium based ionic liquids. AIP Adv. 2019, 9, 075007. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.-H.; Lin, E.-Y.; Chang, H.-C. Pressure-Dependent Confinement Effect of Ionic Liquids in Porous Silica. Nanomaterials 2019, 9, 620. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-H.; Chang, H.-C.; Fu, Y.-P. Utilizing Infrared Spectroscopy to Analyze the Interfacial Structures of Ionic Liquids/Al2O3 and Ionic Liquids/Mica Mixtures under High Pressures. Nanomaterials 2019, 9, 373. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Lin, W.; Li, L.-H.; Malliakas, C.D.; Zhang, R.; Groesbeck, M.; Bao, J.-K.; Zhang, D.; Sterer, E.; Kanatzidis, M.G. Pressure-Induced Superconductivity and Flattened Se6 Rings in the Wide Band Gap Semiconductor Cu2I2Se6. J. Am. Chem. Soc. 2019, 141, 15174–15182. [Google Scholar] [CrossRef]
- Zhang, R.; Cai, W.; Bi, T.; Zarifi, N.; Terpstra, T.; Zhang, C.; Verdeny, Z.V.; Zurek, E.; Deemyad, S. Effects of nonhydrostatic stress on structural and optoelectronic properties of methylammonium lead bromide perovskite. J. Phys. Chem. Lett. 2017, 8, 3457–3465. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Zhang, R.; Yao, Y.; Deemyad, S. Piezochromism and structural and electronic properties of benz [a] anthracene under pressure. Phys. Chem. Chem. Phys. 2017, 19, 6216–6223. [Google Scholar] [CrossRef]
- Wong, P.T.T.; Moffatt, D.J.; Baudais, F.L. Crystalline quartz as an internal pressure calibrant for high-pressure infrared spectroscopy. Appl. Spectrosc. 1985, 39, 733–735. [Google Scholar] [CrossRef]
- Wong, P.T.T.; Moffatt, D.J. The uncoupled OH or OD stretch in water as an internal pressure gauge for high-pressure infrared spectroscopy of aqueous systems. Appl. Spectrosc. 1987, 41, 1070–1072. [Google Scholar] [CrossRef]
- Jonas, J.; Jonas, A. High pressure NMR spectroscopy of proteina and membranes. Annu. Rev. Biophys. Biomol. Struct. 1994, 23, 287–318. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Matsumoto, K.; Hagiwara, R. Effects of Alkyl Chain Length on Properties of 1-Alkyl-3-methylimidazolium Fluorohydrogenate Ionic Liquid Crystals. Chem. Eur. J. 2010, 16, 12970–12976. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.-H.; Shen, M.-H.; Chang, H.-C. Pressure-Dependent Stability of Imidazolium-Based Ionic Liquid/DNA Materials Investigated by High-Pressure Infrared Spectroscopy. Materials 2019, 12, 4202. https://doi.org/10.3390/ma12244202
Wang T-H, Shen M-H, Chang H-C. Pressure-Dependent Stability of Imidazolium-Based Ionic Liquid/DNA Materials Investigated by High-Pressure Infrared Spectroscopy. Materials. 2019; 12(24):4202. https://doi.org/10.3390/ma12244202
Chicago/Turabian StyleWang, Teng-Hui, Min-Hsiu Shen, and Hai-Chou Chang. 2019. "Pressure-Dependent Stability of Imidazolium-Based Ionic Liquid/DNA Materials Investigated by High-Pressure Infrared Spectroscopy" Materials 12, no. 24: 4202. https://doi.org/10.3390/ma12244202




