Pozzolanic Activity of Zeolites: The Role of Si/Al Ratio
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Estimation of Pozzolanic Activity
3. Results and Discussion
3.1. Materials Characterization
3.2. Preliminary Evaluation of Pozzolanicity
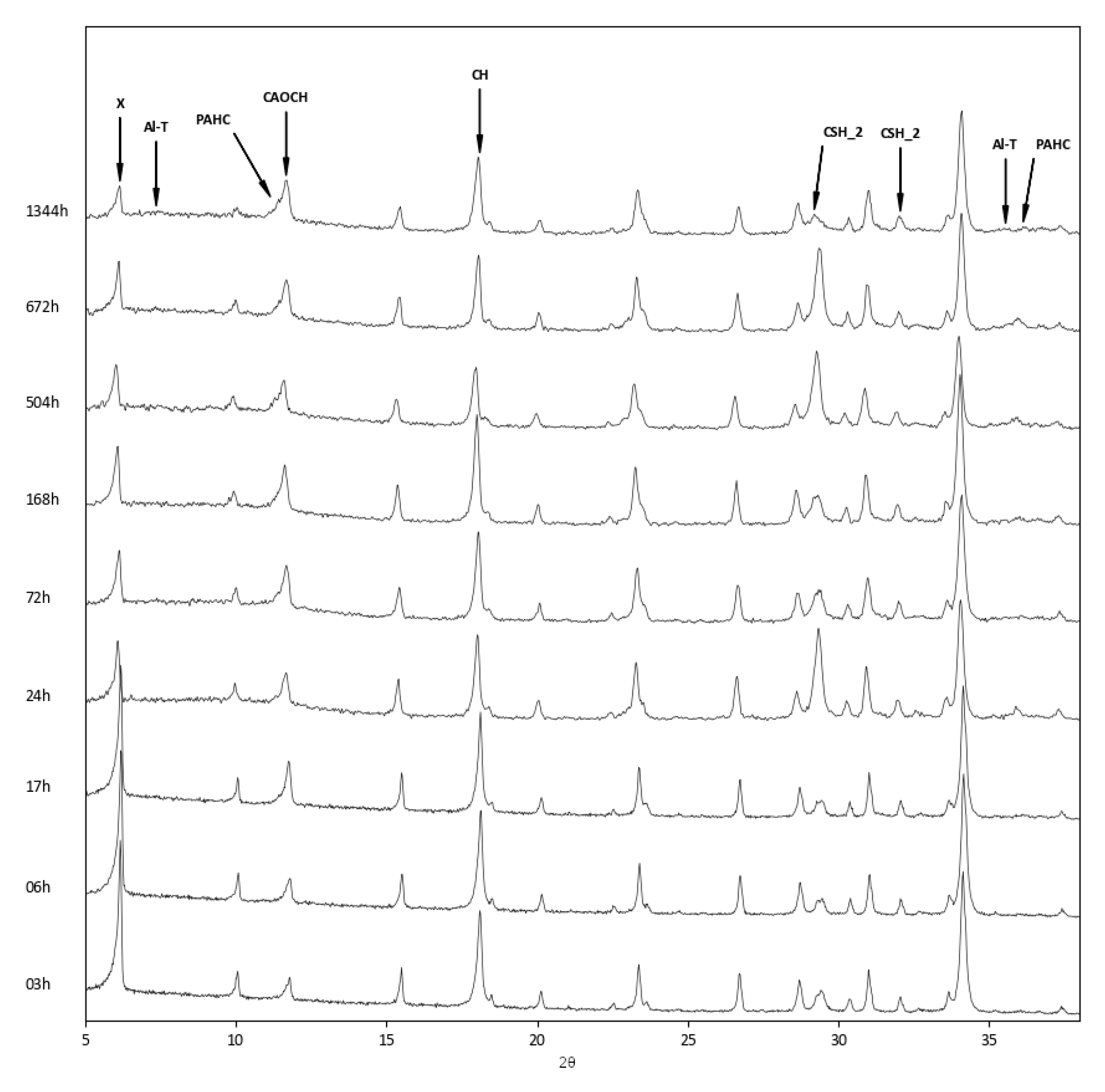
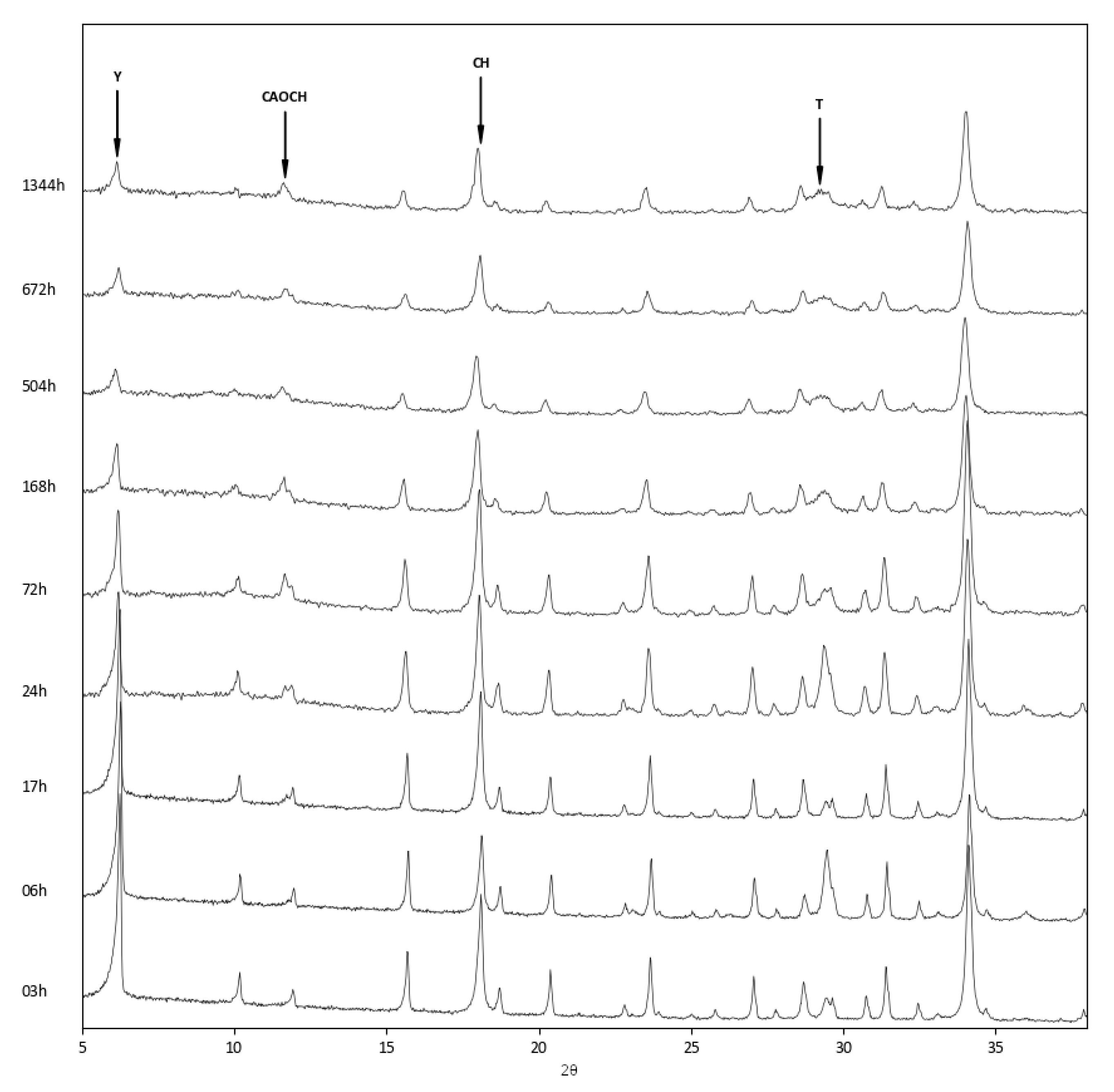
3.3. Reactions in Zeolite–Lime–Water System
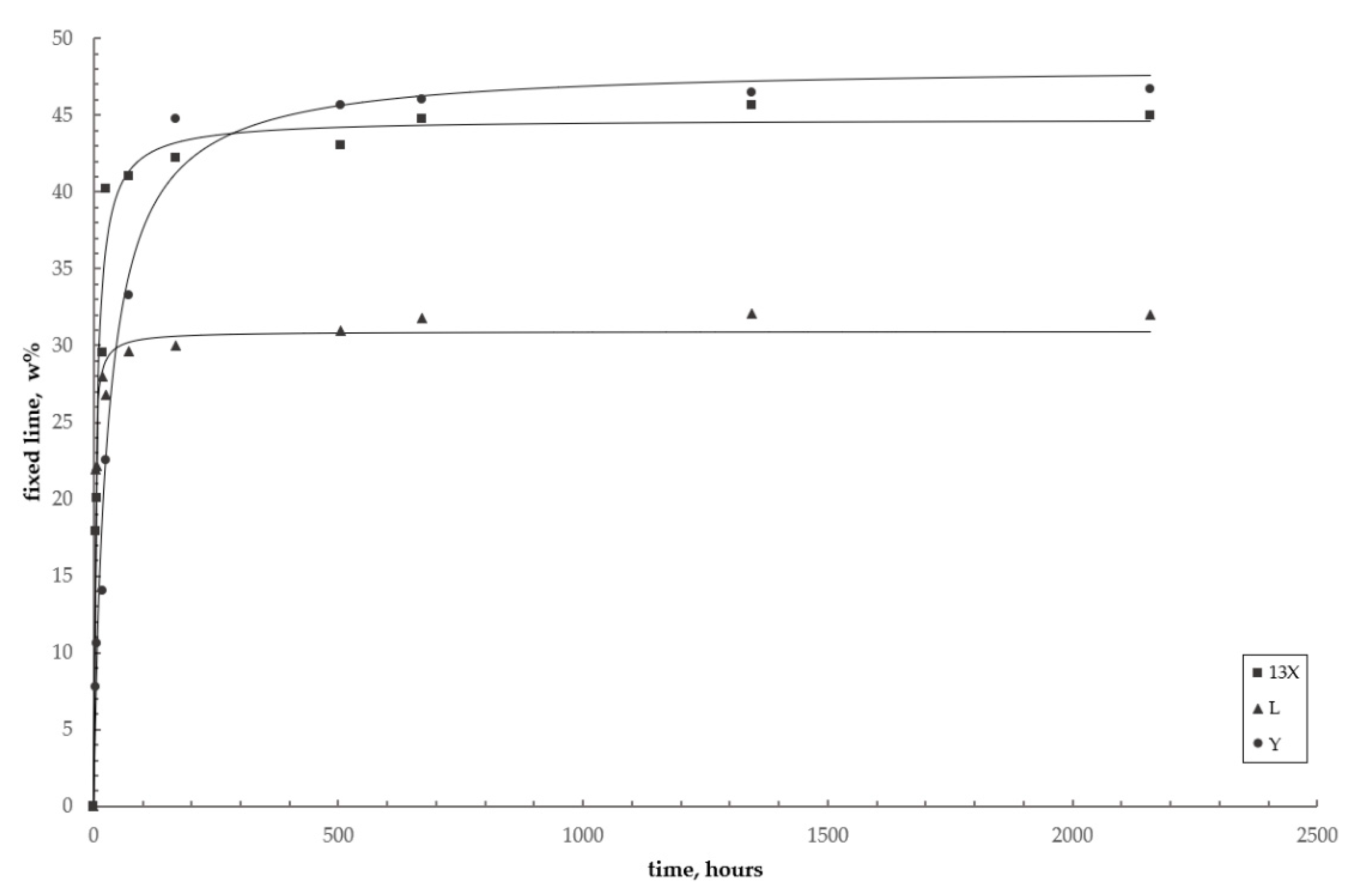
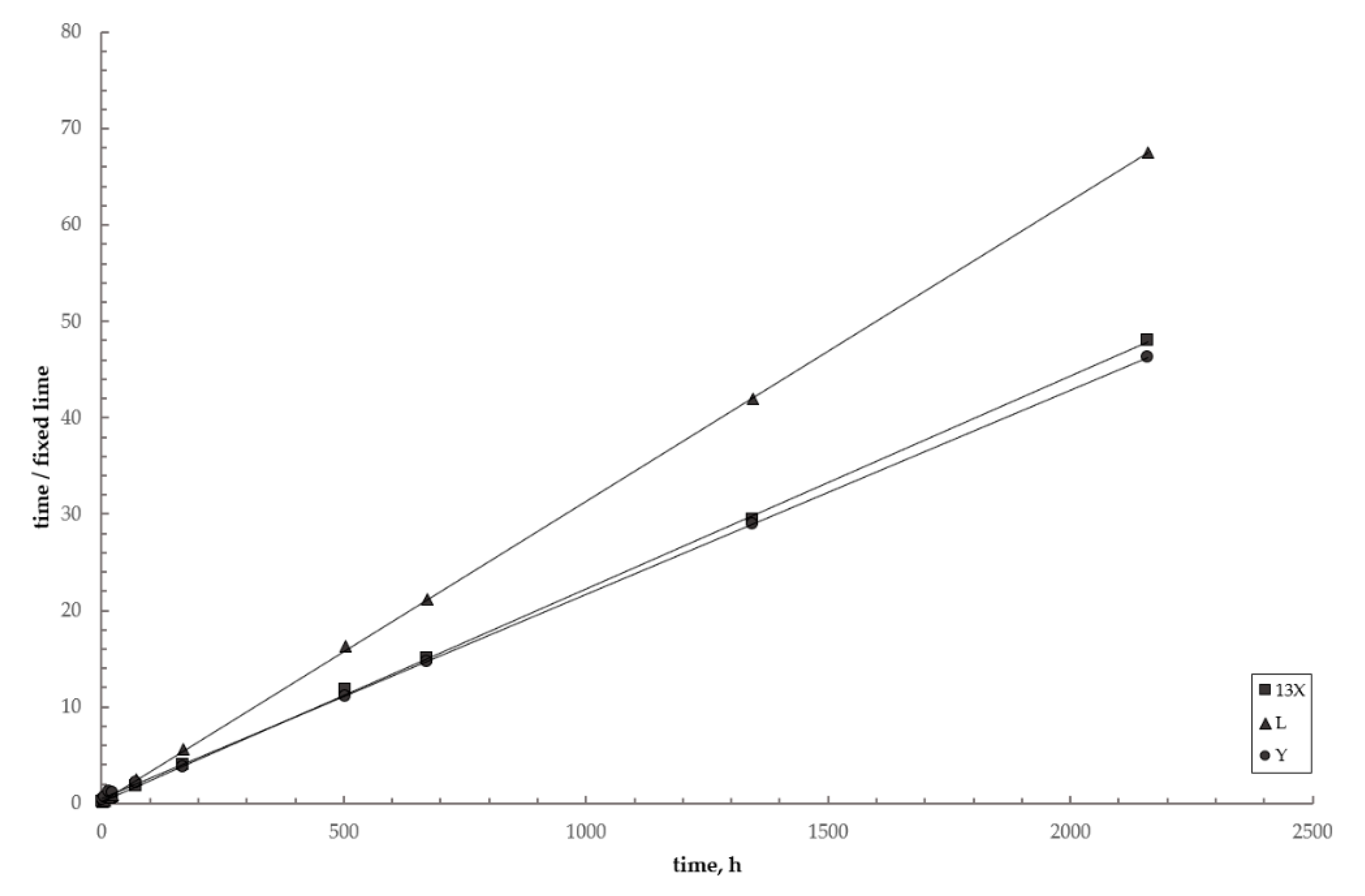
3.4. Reaction Kinetics
4. Conclusions
- During the first few hours, the main active factors are the Si/Al ratio (more precisely, the aluminium content) and the ion exchange capacity of the participating zeolite: both factors, which are in fact correlated, increase the amount of calcium removed from the contacting solution. Accordingly, zeolites L and X were able to subtract more calcium than Y zeolite, even if the concurrent effect of their smaller particle size could also have played a role.
- As the reaction proceeds, the specific surface and the silicon content of the zeolite become the dominant factor governing the reaction kinetics: the L zeolite fixed the lower lime amount (30%) in the shortest time (3 days), while the Y zeolite fixed about 47% of the lime in 90 days.
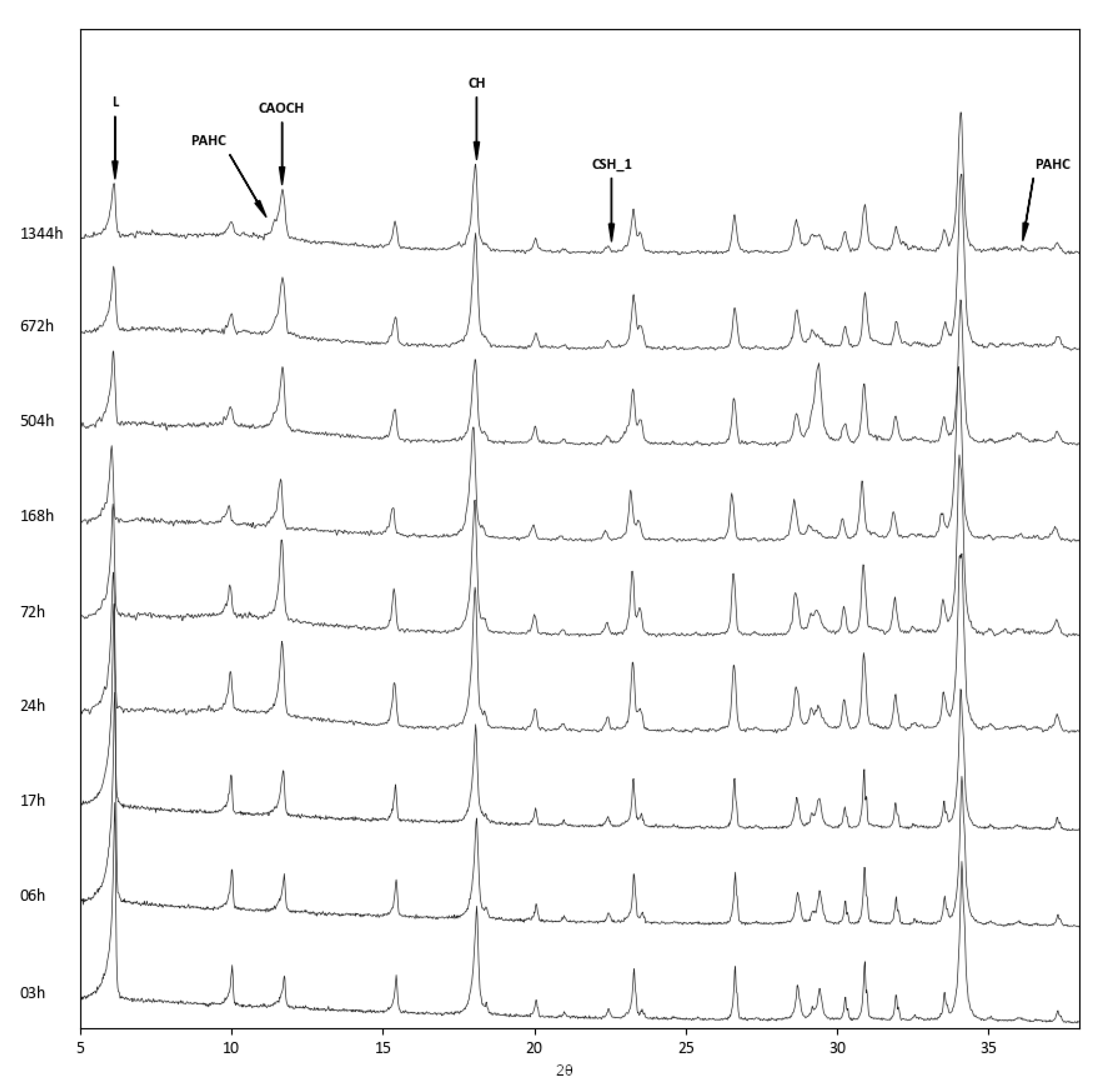
- Concerning the reaction products, while all three systems developed calcium aluminium oxide carbonate hydrate, which formed at first as a reaction product between zeolite and lime, at longer reaction times, the more aluminium-rich zeolites showed the presence of paraalumohydrocalcite, while the silicon-rich Y zeolite produced tobermorite as a reaction product.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; Krieger: Malabar, FL, USA, 1984. [Google Scholar]
- Aprea, P.; Caputo, D.; Gargiulo, N.; de Gennaro, B.; Iucolano, F.; Liguori, B.; Colella, C. Ion exchange kinetics and thermodynamics of hydrosodalite, a narrow pore zeolite. J. Porous Mater. 2014, 21, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Liguori, B.; Cassese, A.; Colella, C. Safe immobilization of Cr(III) in heat-treated zeolite tuff compacts. J. Hazard. Mater. 2006, 137, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Liguori, B.; Ferone, C.; Anaclerio, S.; Colella, C. Monoclinic Sr-celsian by thermal treatment of Sr-exchanged zeolite A, LTA-type framework. Solid State Ion. 2008, 179, 2358–2364. [Google Scholar] [CrossRef]
- Ahmadi, B.; Shekarchi, M. Use of natural zeolite as a supplementary cementitious material. Cem. Concr. Compos. 2010, 32, 134–141. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A.; Kazemian, A.; Sarvari, M.; Ahmadi, B. Use of natural zeolite to produce self-consolidating concrete with low portland cement content and high durability. J. Mater. Civ. Eng. 2012, 25, 589–596. [Google Scholar] [CrossRef]
- Yilmaz, B.; Uçar, A.; Öteyaka, B.; Uz, V. Properties of zeolitic tuff (clinoptilolite) blended portland cement. Build. Environ. 2007, 42, 3808–3815. [Google Scholar] [CrossRef]
- Özen, S.; Göncüoğlu, M.C.; Liguori, B.; De Gennaro, B.; Cappelletti, P.; Gatta, G.D.; Iucolano, F.; Colella, C. A comprehensive evaluation of sedimentary zeolites from Turkey as pozzolanic addition of cement-and lime-based binders. Constr. Build. Mater. 2016, 105, 46–61. [Google Scholar] [CrossRef]
- Liguori, B.; Iucolano, F.; De Gennaro, B.; Marroccoli, M.; Caputo, D. Zeolitized tuff in environmental friendly production of cementitious material: Chemical and mechanical characterization. Constr. Build. Mater. 2015, 99, 272–278. [Google Scholar] [CrossRef]
- Liguori, B.; Caputo, D.; Iucolano, F. Fiber-reinforced lime-based mortars: Effect of zeolite addition. Constr. Build. Mater. 2015, 77, 455–460. [Google Scholar] [CrossRef]
- Caputo, D.; Liguori, B.; Colella, C. Some advances in understanding the pozzolanic activity of zeolites: The effect of zeolite structure. Cem. Concr. Compos. 2008, 30, 455–462. [Google Scholar] [CrossRef]
- Liguori, B.; Iucolano, F.; Caputo, D.; Colella, C. LTA zeolite as pozzolanic addition for hydraulic mortars: An effective, promising use. Adv. Porous Mater. 2013, 1, 129–135. [Google Scholar] [CrossRef]
- Chen, J.J.; Ng, P.L.; Li, L.G.; Kwan, A.K.H. Use of superfine zeolite in conjunction with silica fume—Effects on rheology and strength of cementitious paste. Powder Technol. 2018, 328, 75–83. [Google Scholar] [CrossRef]
- Coppola, L.; Bellezze, T.; Belli, A.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Cabrini, M.; Candamano, S.; Cappai, M.; Caputo, D.; et al. Binders alternative to Portland cement and waste management for sustainable construction—Part 2. J. Appl. Biomater. Funct. Mater. 2018, 16, 207–221. [Google Scholar] [PubMed] [Green Version]
- Coppola, L.; Bellezze, T.; Belli, A.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Cabrini, M.; Candamano, S.; Cappai, M.; Caputo, D.; et al. Binders alternative to Portland cement and waste management for sustainable construction—part 1. J. Appl. Biomater. Funct. Mater. 2018, 16, 186–202. [Google Scholar]
- Senderov, E.E.; Khitarov, N.I. Synthesis of thermodynamically stable zeolites in Na2O-Al2O3-SiO2-H2O system. Adv. Chem. Ser. 1971, 101, 149–154. [Google Scholar]
- Molinari, C.; Zanelli, C.; Dondi, M. Zeolites and modified clays in environmentally sustainable building materials. In Modified Clay and Zeolite Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 289–307. [Google Scholar]
- Andrejkovičová, S.; Velosa, A.L.; Ferraz, E.; Rocha, F. Influence of clay minerals addition on mechanical properties of air lime-metakaolin mortars. Constr. Build. Mater. 2014, 65, 132–139. [Google Scholar] [CrossRef]
- Ferraz, E.; Andrejkovičová, S.; Velosa, A.L.; Silva, A.S.; Rocha, F. Synthetic zeolite pellets incorporated to air lime-metakaolin mortars: Mechanical properties. Constr. Build. Mater. 2014, 69, 243–252. [Google Scholar] [CrossRef]
- Barrer, R.M.; Denny, P.J. 202. Hydrothermal chemistry of the silicates. Part X. A partial study of the field CaO–Al2O3–SiO2–H2O. J. Chem. Soc. 1961, 983–1000. [Google Scholar] [CrossRef]
- Mertens, G.; Snellings, R.; Van Balen, K.; Bicer-Simsir, B.; Verlooy, P.; Elsen, J. Pozzolanic reactions of common natural zeolites with lime and parameters affecting their reactivity. Cem. Concr. Res. 2009, 39, 233–240. [Google Scholar] [CrossRef]
- Perraki, T.; Kakali, G.; Kontoleon, F. The effect of natural zeolites on the early hydration of Portland cement. Microporous Mesoporous Mater. 2003, 61, 205–212. [Google Scholar] [CrossRef]
- Uzal, B.; Turanli, L.; Yücel, H.; Göncüoǧlu, M.C.; Çulfaz, A. Pozzolanic activity of clinoptilolite: A comparative study with silica fume, fly ash and a non-zeolitic natural pozzolan. Cem. Concr. Res. 2010, 40, 398–404. [Google Scholar] [CrossRef]
- Vigil de la Villa, R.; Fernández, R.; García, R.; Villar-Cociña, E.; Frías, M. Pozzolanic activity and alkaline reactivity of a mordenite-rich tuff. Microporous Mesoporous Mater. 2009, 126, 125–132. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.L.; Olson, D.H.D. Atlas of Zeolite Framework Types; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Kühl, G.H. Crystallization of low-solica faujasite (SiO2Al2O3∼ 2.0). Zeolites 1987, 7, 451–457. [Google Scholar] [CrossRef]
- Methods of Testing Cement–Part 5: Pozzolanicity Test for Pozzolanic Cements; European Committee For Standardization (CEN): Brussels, Belguim, 2011; p. 196-5.
- Mendoza, O.; Tobón, J.I. An alternative thermal method for identification of pozzolanic activity in Ca(OH)2/pozzolan pastes. J. Therm. Anal. Calorim. 2013, 114, 589–596. [Google Scholar] [CrossRef]
- Frías, M.; Villar-Cociña, E.; De Rojas, M.I.S.; Valencia-Morales, E. The effect that different pozzolanic activity methods has on the kinetic constants of the pozzolanic reaction in sugar cane straw-clay ash/lime systems: Application of a kinetic–diffusive model. Cem. Concr. Res. 2005, 35, 2137–2142. [Google Scholar] [CrossRef]
- Colella, C. Use of thermal analysis in zeolite research and application. In Characterization Techniques of Glasses and Ceramics; Springer: Berlin, Germany, 1999; pp. 112–137. [Google Scholar]
- Galarneau, A.; Di Renzo, F.; Fajula, F.; Vedrine, J. (Eds.) Zeolites and mesoporous materials at the dawn of the 21st century: Proceedings of the 13th International Zeolite Conference, Montpellier, France, 8–13 July 2001; Elsevier: Amsterdam, The Netherlands, 2001; Volume 135. [Google Scholar]



| Oxides | Samples | |||
|---|---|---|---|---|
| Parameters | LSX 1 | L | X | Y |
| SiO2 | 41.73 | 40.54 | 48.36 | 66.59 |
| Al2O3 | 35.11 | 36.84 | 32.49 | 20.88 |
| Na2O | 15.67 | 21.36 | 19.15 | 12.52 |
| K2O | 7.48 | 1.25 | – | – |
| Si/Al (mole) | 1.01 | 0.93 | 1.25 | 2.71 |
| (Na + K)/Al (mole) | 0.97 | 0.97 | 0.97 | 0.98 |
| Cell parameter, Å | 25.011 | 25.057 | 24.948 | 24.651 |
| BET (m2/g) | 488 | n.a. 2 | 408 | 669 |
| Sample | Grain Size Range (μm) | Average (μm) | Median (μm) |
|---|---|---|---|
| LSX | 0.70–64.37 | 18.73 | 3.37 |
| L | 0.31–1.25 | 0.63 | 0.60 |
| X | 0.43–3.40 | 1.55 | 1.62 |
| Y | 0.38–70.85 | 26.91 | 25.17 |
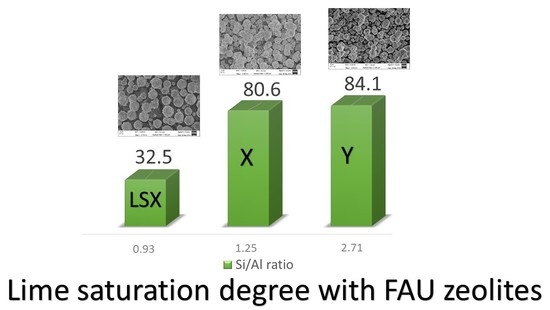
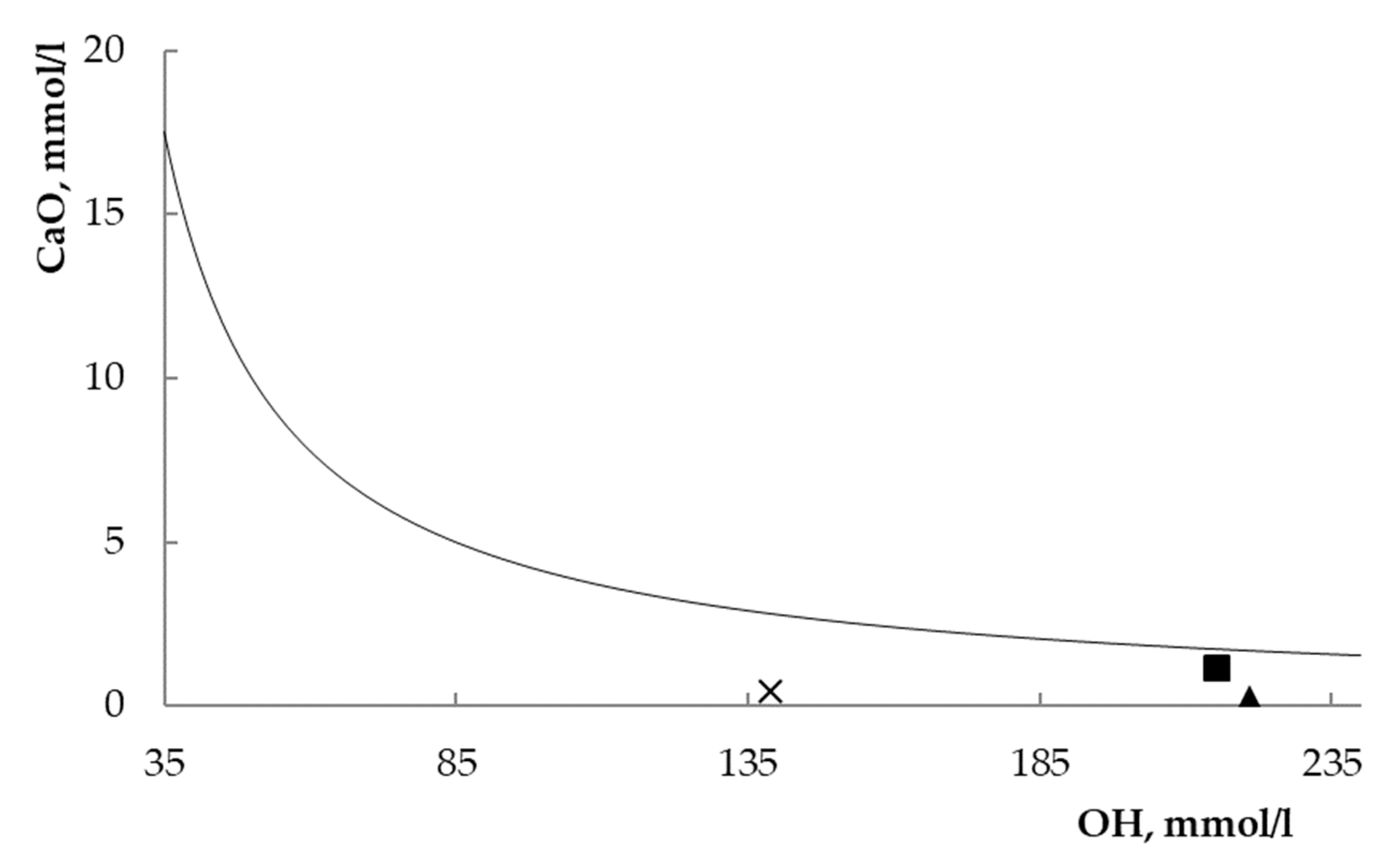
| Zeolite | [OH] mmol/l | [CaO] mmol/l | [CaO]eq mmol/l | Undersaturation Degree, % * |
|---|---|---|---|---|
| L | 215.3 | 1.18 | 1.75 | 32.5 |
| X | 220.9 | 0.33 | 1.70 | 80.6 |
| Y | 138.8 | 0.45 | 2.83 | 84.1 |
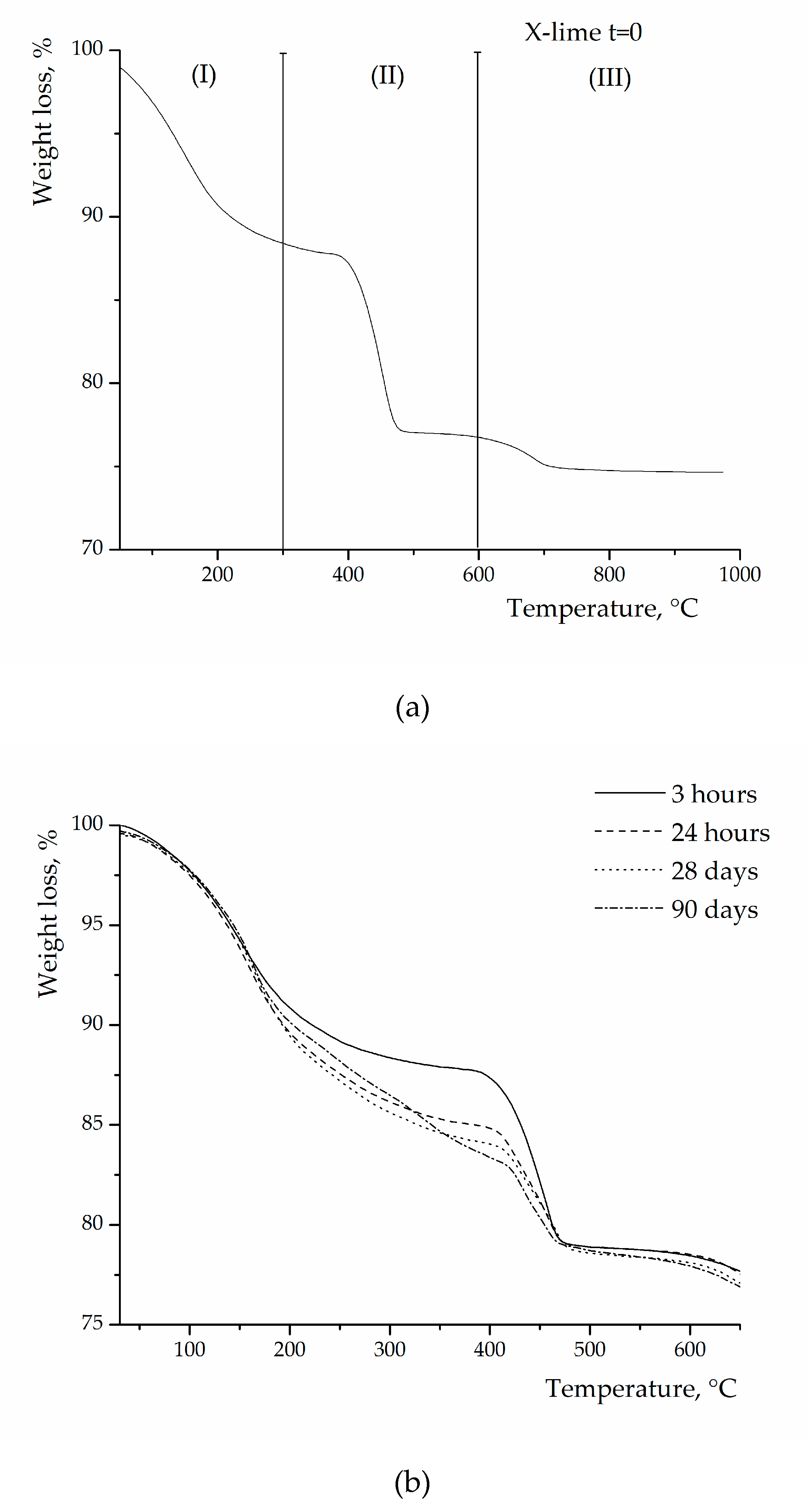
| Hours. | 3 | 6 | 17 | 24 | 72 | 168 | 504 | 672 | 1344 | 2160 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zeo | |||||||||||
| L | 21.92 | 22.19 | 27.99 | 26.81 | 29.62 | 30.00 | 31.00 | 31.79 | 32.07 | 32.00 | |
| X | 17.93 | 20.06 | 29.56 | 40.18 | 41.00 | 42.19 | 43.01 | 44.75 | 45.66 | 45.00 | |
| Y | 7.76 | 10.59 | 14.06 | 22.56 | 33.24 | 44.75 | 45.66 | 46.00 | 46.50 | 46.67 | |
| System | leq, w% * | k, h−1 | R2 |
|---|---|---|---|
| Zeolite L–lime–water | 30.9 | 1.92 × 10–2 | 0.981 |
| Zeolite X–lime–water | 44.7 | 3.75 × 10–3 | 0.978 |
| Zeolite Y–lime–water | 48.2 | 7.24 × 10–4 | 0.986 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liguori, B.; Aprea, P.; Gennaro, B.d.; Iucolano, F.; Colella, A.; Caputo, D. Pozzolanic Activity of Zeolites: The Role of Si/Al Ratio. Materials 2019, 12, 4231. https://doi.org/10.3390/ma12244231
Liguori B, Aprea P, Gennaro Bd, Iucolano F, Colella A, Caputo D. Pozzolanic Activity of Zeolites: The Role of Si/Al Ratio. Materials. 2019; 12(24):4231. https://doi.org/10.3390/ma12244231
Chicago/Turabian StyleLiguori, Barbara, Paolo Aprea, Bruno de Gennaro, Fabio Iucolano, Abner Colella, and Domenico Caputo. 2019. "Pozzolanic Activity of Zeolites: The Role of Si/Al Ratio" Materials 12, no. 24: 4231. https://doi.org/10.3390/ma12244231