Crystallization Behavior of Al70Fe12.5V12.5Nb5 Amorphous Alloy Formed by Mechanical Alloying
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maurya, R.S.; Sahu, A.; Laha, T. Effect of sintering temperature on phase transformation during consolidation ofmechanically alloyed Al86Ni6Y6Co2 amorphous powders by spark plasma sintering. J. Non-Cryst. Solids 2016, 453, 1–7. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Leng, J.; Wang, T.; Wang, Y. Alloying Behavior and Properties of Al-Based Composites Reinforced with Al85Fe15 Metallic Glass Particles Fabricated by Mechanical Alloying and Hot Pressing Consolidation. JOM 2017, 69, 748–755. [Google Scholar] [CrossRef]
- Gao, M.; Lu, W.; Yang, B.; Zhang, S.; Wang, J. High corrosion and wear resistance of Al-based amorphous metallic coating synthesized by HVAF spraying. J. Alloys Compd. 2018, 735, 1363–1373. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.; Xing, W.; Ouyang, D.; Liu, L. 3D printing of Fe-based bulk metallic glass composites with combined high strength and fracture toughness. Mater. Des. 2018, 143, 285–296. [Google Scholar] [CrossRef]
- Liao, J.P.; Yang, B.J.; Zhang, Y.; Lu, W.Y.; Gu, X.J.; Wang, J.Q. Evaluation of glass formation and critical casting diameter in Al-based metallic glasses. Mater. Des. 2015, 88, 222–226. [Google Scholar] [CrossRef]
- Yin, J.; Cai, H.; Cheng, X.; Zhang, X. Al-based bulk metallic glass with large plasticity and ultrahigh strength. J. Alloys Compd. 2015, 648, 276–279. [Google Scholar] [CrossRef]
- Wu, N.C.; Zuo, L.; Wang, J.Q.; Ma, E. Designing aluminum-rich bulk metallic glasses via electronic-structure-guided microalloying. Acta Mater. 2016, 108, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.J.; Lu, W.Y.; Zhang, J.L.; Wang, J.Q.; Ma, E. Melt fluxing to elevate the forming ability of Al-based bulk metallic glasses. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.C. Materials Synthesis by Mechanical Alloying. Annu. Rev. Mater. Res. 1989, 19, 121–143. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Mula, S.; Mondal, K.; Ghosh, S.; Pabi, S.K. Structure and mechanical properties of Al–Ni–Ti amorphous powder consolidated by pressure-less, pressure-assisted and spark plasma sintering. Mater. Sci. Eng. A 2010, 527, 3757–3763. [Google Scholar] [CrossRef]
- Tan, Z.; Wang, L.; Xue, Y.; Cheng, X.; Zhang, L. structural modification of Al65Cu16.5Ti18.5 amorphous powder through annealing and post milling, improving thermal stability. J. Mater. Sci. Technol. 2016, 32, 1326–1331. [Google Scholar] [CrossRef]
- Maurya, R.S.; Sahu, A.; Laha, T. Quantitative phase analysis in Al86Ni8Y6 bulk glassy alloy synthesized by consolidating mechanically alloyed amorphous powder via spark plasma sintering. Mater. Des. 2016, 93, 96–103. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Nguyen, O.T.; Dudina, D.V.; Le, V.V.; Kim, J.S. Crystallization Kinetics of Al-Fe and Al-Fe-Y Amorphous Alloys Produced by Mechanical Milling. J. Nanomater. 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Tan, Z.; Xue, Y.F.; Cheng, X.W.; Zhang, L.; Chen, W.W.; Wang, L.; Zhang, H.F.; Fu, H.M. Effect of element fitting on composition optimization of Al-Cu-Ti amorphous alloy by mechanical alloying. Trans. Nonferrous Met. Soc. China 2015, 25, 3348–3353. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, W.; Dong, C.; Qiang, J.B.; Xie, G.Q.; Fujita, K.; Inoue, A. Enhancement of glass-forming ability and corrosion resistance of Zr-based Zr-Ni-Al bulk metallic glasses with minor addition of Nb. J. Appl. Phys. 2011, 110, 023513. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.F.; Han, F.S. Preparation of Al72Ni8Ti8Zr6Nb3Y3 amorphous powders and bulk materials. Int. J. Min. Met. Mater. 2016, 23, 1187–1195. [Google Scholar] [CrossRef]
- Mostaan, H.; Karimzadeh, F.; Abbasi, M.H. Thermodynamic analysis of nanocrystalline and amorphous phase formation in Nb–Al system during mechanical alloying. Powder Metall. 2012, 55, 142–147. [Google Scholar] [CrossRef]
- Tavoosi, M.; Karimzadeh, F.; Enayati, M.H. Formation and characterization of amorphous–nanocrystalline Al80Fe10M10 [M = Fe, Nb, Ti, Ni, (Ni0.5Ti0.5)] alloys. J. Alloys Compd. 2013, 551, 584–590. [Google Scholar] [CrossRef]
- Peng, Z.; Suryanarayana, C.; Froes, F.S. Mechanical alloying of Nb-Al powders. Metall. Mater. Trans. A 1996, 27, 41–48. [Google Scholar] [CrossRef]
- Miracle, D.B. The efficient cluster packing model—An atomic structural model for metallic glasses. Acta Mater. 2006, 54, 4317–4336. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, X.; Li, X.; Wang, D.; Qin, Y.; Han, F. Bulk amorphous Al75V12.5Fe12.5−xCux alloys fabricated by consolidation of mechanically alloyed amorphous powders. J. Alloys Compd. 2014, 586, 645–649. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-Solution Phase Formation Rules for Multi-component Alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Lu, Z.P.; Liu, C.T. A new glass-forming ability criterion for bulk metallic glasses. Acta Mater. 2002, 50, 3501–3512. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Zhu, Z.; Wang, H.; Li, Y.; Kato, H.; Zhang, H. Effect of substituting elements on thermal stability and glass-forming ability of an Al-based AlNiEr metallic glass. J. Alloys Compd. 2017, 707, 97–101. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Ozawa, T.A. New method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]


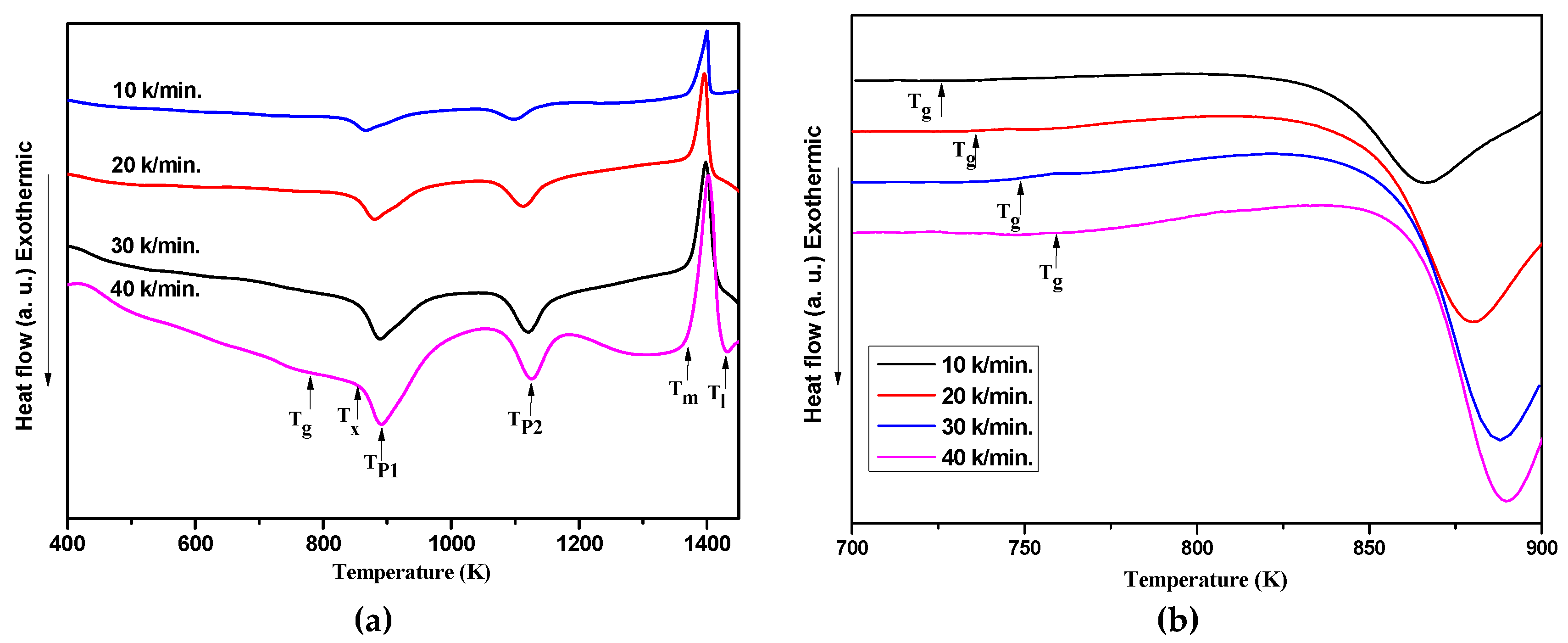


| β(K/min) | Tg(K) | Tx (K) | Tp1 (K) | Tp2 (K) | Tm(K) | Tl (K) | ΔTx(K) | Trg | γ |
|---|---|---|---|---|---|---|---|---|---|
| 10 | 726.7 | 844.2 | 866.2 | 1097.7 | 1375.2 | 1408.7 | 117.5 | 0.516 | 0.395 |
| 20 | 736.9 | 854.1 | 881.0 | 1112.4 | 1377.9 | 1407.9 | 117.2 | 0.522 | 0.398 |
| 30 | 749.8 | 862.5 | 888.8 | 1120.1 | 1377.5 | 1416.9 | 112.7 | 0.529 | 0.398 |
| 40 | 759.0 | 865.5 | 890.9 | 1125.7 | 1376.5 | 1427.3 | 106.5 | 0.532 | 0.396 |
| Equations | Ex (KJ/mol) | Ep1 (KJ/mol) | Ep2 (KJ/mol) |
|---|---|---|---|
| Kissinger | 366.3 ± 23.9 | 326.2 ± 35.0 | 489.4 ± 12.2 |
| Ozawa | 380.5 ± 23.9 | 340.8 ± 35.0 | 507.8 ± 12.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, X.; Si, Y.; Zhong, X.; Han, F. Crystallization Behavior of Al70Fe12.5V12.5Nb5 Amorphous Alloy Formed by Mechanical Alloying. Materials 2019, 12, 383. https://doi.org/10.3390/ma12030383
Liu X, Wang X, Si Y, Zhong X, Han F. Crystallization Behavior of Al70Fe12.5V12.5Nb5 Amorphous Alloy Formed by Mechanical Alloying. Materials. 2019; 12(3):383. https://doi.org/10.3390/ma12030383
Chicago/Turabian StyleLiu, Xuan, Xingfu Wang, Yongli Si, Xiaokang Zhong, and Fusheng Han. 2019. "Crystallization Behavior of Al70Fe12.5V12.5Nb5 Amorphous Alloy Formed by Mechanical Alloying" Materials 12, no. 3: 383. https://doi.org/10.3390/ma12030383




