Enhanced Electrochemical Performance of Li1.27Cr0.2Mn0.53O2 Layered Cathode Materials via a Nanomilling-Assisted Solid-state Process
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Li1.27Cr0.2Mn0.53O2 Using the Solid-State Reaction Method
2.2. Instrumentation
3. Result and Discussion
3.1. Phase Purity of Synthesized Cathode Powders
3.2. Valence States of Mn and Cr Ions within the Cathode Powder
3.3. SEM Observation of the Prepared Powder
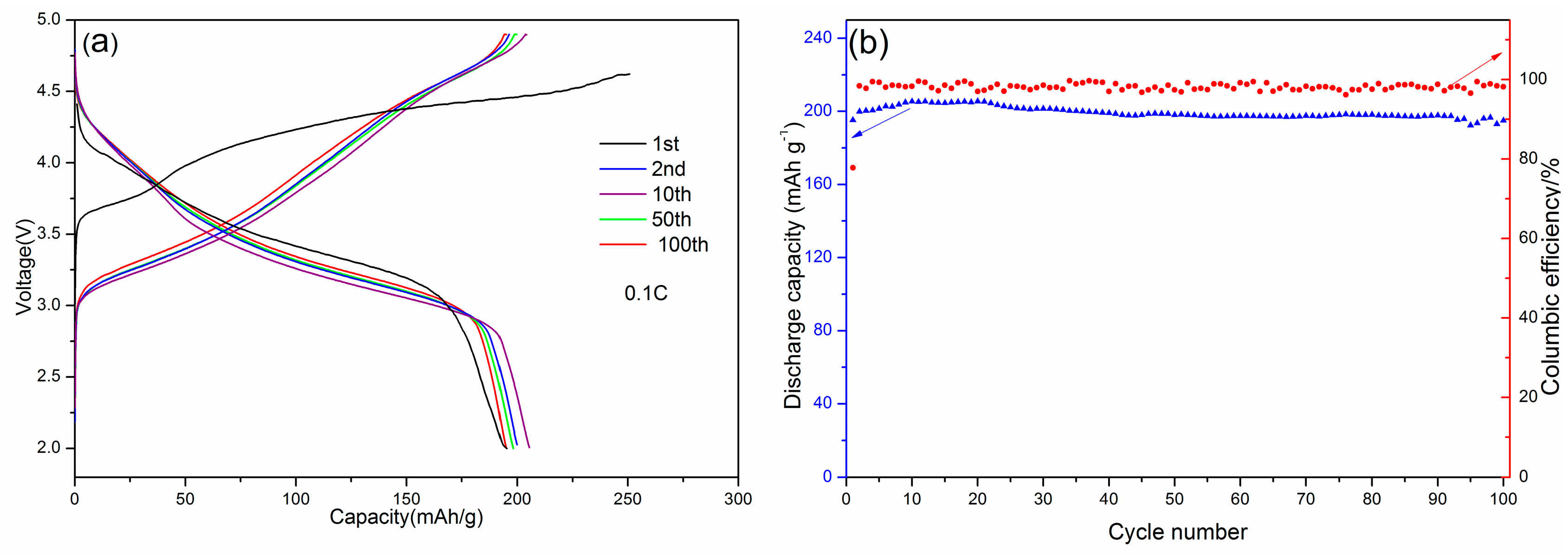
3.4. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, Z.M.; Zhao, L.C. Structure and electrochemical properties of LiMn2O4. Trans. Nonferrous Met. Soc. China 2007, 17, 659–664. [Google Scholar] [CrossRef]
- Aziz, N.A.A.; Abdullah, T.K.; Mohamad, A.A. Synthesis ofLiCoO2 Prepared by Sol-gel Method. Procedia Chem. 2016, 19, 861–864. [Google Scholar] [CrossRef]
- Cheng, E.J.; Taylor, N.J.; Wolfenstine, J.; Sakamoto, J. Elastic properties of lithium cobalt oxide (LiCoO2). J. Asian Ceram. Soc. 2017, 5, 113–117. [Google Scholar] [CrossRef]
- Zheng, Y.; Hao, X.; Niu, J.; Pan, B. Layered o-LiMnO2 prepared for lithium ion batteries by mechanical alloying. Mater. Lett. 2016, 163, 98–101. [Google Scholar] [CrossRef]
- Wen, J.G.; Bareño, J.; Lei, C.H.; Kang, S.H.; Balasubramanian, M.; Petrov, I.; Abraham, D.P. Analytical electron microscopy of Li1.2Co0.4Mn0.4O2 for lithium-ion batteries. Solid State Ionics 2011, 182, 98–107. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, X.; Elam, J.W.; Belharouak, I. Electrochemical characterization of voltage fade of Li1.2Ni0.2Mn0.6O2 cathode. Solid State Ionics 2014, 268, 231–235. [Google Scholar] [CrossRef]
- Li, J.; Jeong, S.; Kloepsch, R.; Winter, M.; Passerini, S. Improved electrochemical performance of LiMO2 (M = Mn, Ni, Co)–Li2MnO3 cathode materials in ionic liquid-based electrolyte. J. Power Sources 2013, 239, 490–495. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, S.W.; Moon, H.S.; Kim, H.J.; Cho, B.W.; Cho, W.I.; Choi, J.B.; Park, J.W. Electrochemical properties of Li–Cr–Mn–O cathode materials for lithium secondary batteries. J. Power Sources 2004, 129, 319–323. [Google Scholar] [CrossRef]
- Zhang, B.; Li, L.; Zheng, J. Characterization of multiple metals (Cr, Mg) substituted LiNi0.8Co0.1Mn0.1O2 cathode materials for lithium ion battery. Int. J. Min. Met. Mater. 2012, 520, 190–194. [Google Scholar] [CrossRef]
- Kim, G.T.; Kim, J.U.; Sim, Y.J.; Kim, K.W. Electrochemical properties of LiCrxNi0.5−xMn0.5O2 prepared by co-precipitation method for lithium secondary batteries. J. Power Sources 2006, 158, 1414–1418. [Google Scholar] [CrossRef]
- Ko, Y.N.; Kim, J.H.; Lee, J.K.; Kang, Y.C.; Lee, J.H. Electrochemical properties of nanosized LiCrO2·Li2MnO3 composite powders prepared by a new concept spray pyrolysis. Electrochim. Acta 2012, 69, 345–350. [Google Scholar] [CrossRef]
- Wu, X.; Ryu, K.S.; Hong, Y.S.; Park, Y.J.; Chang, S.H. Properties of Li[CrxLi(1−x)/3Mn2(1−x)/3]O2 (0.1 ≤ x ≤ 0.2) material prepared by quenching. J. Power Sources 2004, 132, 219–224. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, S.H.; Nahm, K.S.; Chung, H.T.; Lee, Y.S.; Lee, J.H.; Boo, S.; Kim, J. Structural and electrochemical study of Li[CrxLi(1−x)/3Mn2(1−x)/3]O2 (0 ≤ x ≤ 0.328) cathode materials. J. Alloys Compod. 2008, 449, 343–348. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, S.H.; Mangani, I.R.; Lee, J.H.; Boo, S.; Kim, J. Synthesis and materials characterization of Li2MnO3–LiCrO2 system nanocomposite electrode materials. Mater. Res. Bull. 2007, 42, 1374–1383. [Google Scholar] [CrossRef]
- Yang, J.; Guo, B.; He, H.; Li, Y.; Song, C.; Liu, G. LiNi0.5Mn0.5O2 hierarchical nanorods as high-capacity cathode materials for Li-ion batteries. J. Alloys Compod. 2017, 698, 714–718. [Google Scholar] [CrossRef]
- Sun, Y.; Shiosaki, Y.; Xia, Y.; Noguchi, H. The preparation and electrochemical performance of solid solutions LiCoO2–Li2MnO3 as cathode materials for lithium ion batteries. J. Power Sources 2006, 159, 1353–1359. [Google Scholar] [CrossRef]
- Liao, D.Q.; Xia, C.Y.; Xi, X.M.; Zhou, C.X.; Xiao, K.S.; Chen, X.Q.; Qin, S.B. Li Rich Layered Cathode Material Li[Li0.157Ni0.138Co0.134Mn0.571]O2 Synthesized with Solid-State Coordination Method. J. Electron. Mater. 2016, 45, 2981–2986. [Google Scholar] [CrossRef]
- Wu, X.; Chang, S.H.; Park, Y.J.; Ryu, K.S. Studies on capacity increase of Li1.27Cr0.2Mn0.53O2-based lithium batteries. J. Power Sources 2004, 137, 105–110. [Google Scholar] [CrossRef]
- Kim, S.; Noh, J.K.; Yu, S.; Chang, W.; Chung, K.Y.; Cho, B.W. Effects of transition metal doping and surface treatment to improve the electrochemical performance of Li2MnO3. J. Electroceram. 2013, 30, 159–165. [Google Scholar] [CrossRef]
- Pang, W.K.; Lee, J.Y.; Wei, Y.S.; Wu, S.H. Preparation and characterization of Cr-doped LiMnO2 cathode materials by Pechini’s method for lithium ion batteries. Mater. Chem. Phys. 2013, 139, 241–246. [Google Scholar] [CrossRef]
- Ko, Y.N.; Choi, S.H.; Kang, Y.C.; Park, S.B. Characteristics of Li2TiO3–LiCrO2 composite cathode powders prepared by ultrasonic spray pyrolysis. J. Power Sources 2013, 244, 336–343. [Google Scholar] [CrossRef]
- Xia, L.; Lee, S.; Jiang, Y.; Xia, Y.; Chen, G.Z.; Liu, Z. Fluorinated electrolytes for li-ion batteries: The lithium difluoro (oxalato) borate additive for stabilizing the solid electrolyte interphase. ACS Omega 2017, 2, 8741–8750. [Google Scholar] [CrossRef]
- Kim, J.S.; Johnson, C.S.; Vaughey, J.T.; Thackeray, M.M.; Hackney, S.A.; Yoon, W.; Grey, C.P. Electrochemical and Structural Properties of x Li2M’O3·(1 − x) LiMn0.5Ni0.5O2 Electrodes for Lithium Batteries (M’ = Ti, Mn, Zr; 0 ≤ x ≤ 0.3). Chem. Mater. 2004, 16, 1996–2006. [Google Scholar] [CrossRef]
- Li, D.; Sasaki, Y.; Kobayakawa, K.; Sato, Y. Morphological, structural, and electrochemical characteristics of LiNi0.5Mn0.4M0.1O2 (M = Li, Mg, Co, Al). J. Power Sources 2006, 157, 488–493. [Google Scholar] [CrossRef]
- Ganter, M.J.; DiLeo, R.A.; Schauerman, C.M.; Rogers, R.E.; Raffaelle, R.P.; Landi, B.J. Differential scanning calorimetry analysis of an enhanced LiNi0.8Co0.2O2 cathode with single wall carbon nanotube conductive additives. Electrochim. Acta 2011, 56, 7272–7277. [Google Scholar] [CrossRef]
- Ding, J.J.; Zhou, Y.N.; Sun, Q.; Fu, Z.W. Cycle performance improvement of NaCrO2 cathode by carbon coating for sodium ion batteries. Electrochem. Commun. 2012, 22, 85–88. [Google Scholar] [CrossRef]
- Wu, X.; Kim, S.B. Improvement of electrochemical properties of LiNi0.5Mn1.5O4 spinel. J. Power Sources 2002, 109, 53–57. [Google Scholar] [CrossRef]
- Shaju, K.M.; Subba Rao, G.V.; Chowdari, B.V.R. Electrochemical Kinetic Studies of Li-Ion in O2-Structured Li2/3(Ni1/3Mn2/3)O2 and Li(2/3)+x(Ni1/3Mn2/3)O2 by EIS and GITT. J. Electrochem. Soc. 2003, 150, A1–A13. [Google Scholar] [CrossRef]
- Levi, M.D. Solid-State Electrochemical Kinetics of Li-Ion Intercalation into Li1−xCoO2: Simultaneous Application of Electroanalytical Techniques SSCV, PITT, and EIS. J. Electrochem. Soc. 1999, 146, 1279. [Google Scholar] [CrossRef]
- Li, X.; Xin, H.; Liu, Y.; Li, D.; Yuan, X.; Qin, X. Effect of niobium doping on the microstructure and electrochemical properties of lithium-rich layered Li[Li0.2Ni0.2Mn0.6]O2 as cathode materials for lithium ion batteries. RSC Adv. 2015, 5, 45351–45358. [Google Scholar] [CrossRef]
- Luo, D.; Fang, S.; Yang, L.; Hirano, S. Improving the electrochemical performance of layered Li-rich transition-metal oxides by alleviating the blockade effect of surface lithium. J. Mater. Chem. A 2016, 4, 5184–5190. [Google Scholar] [CrossRef]

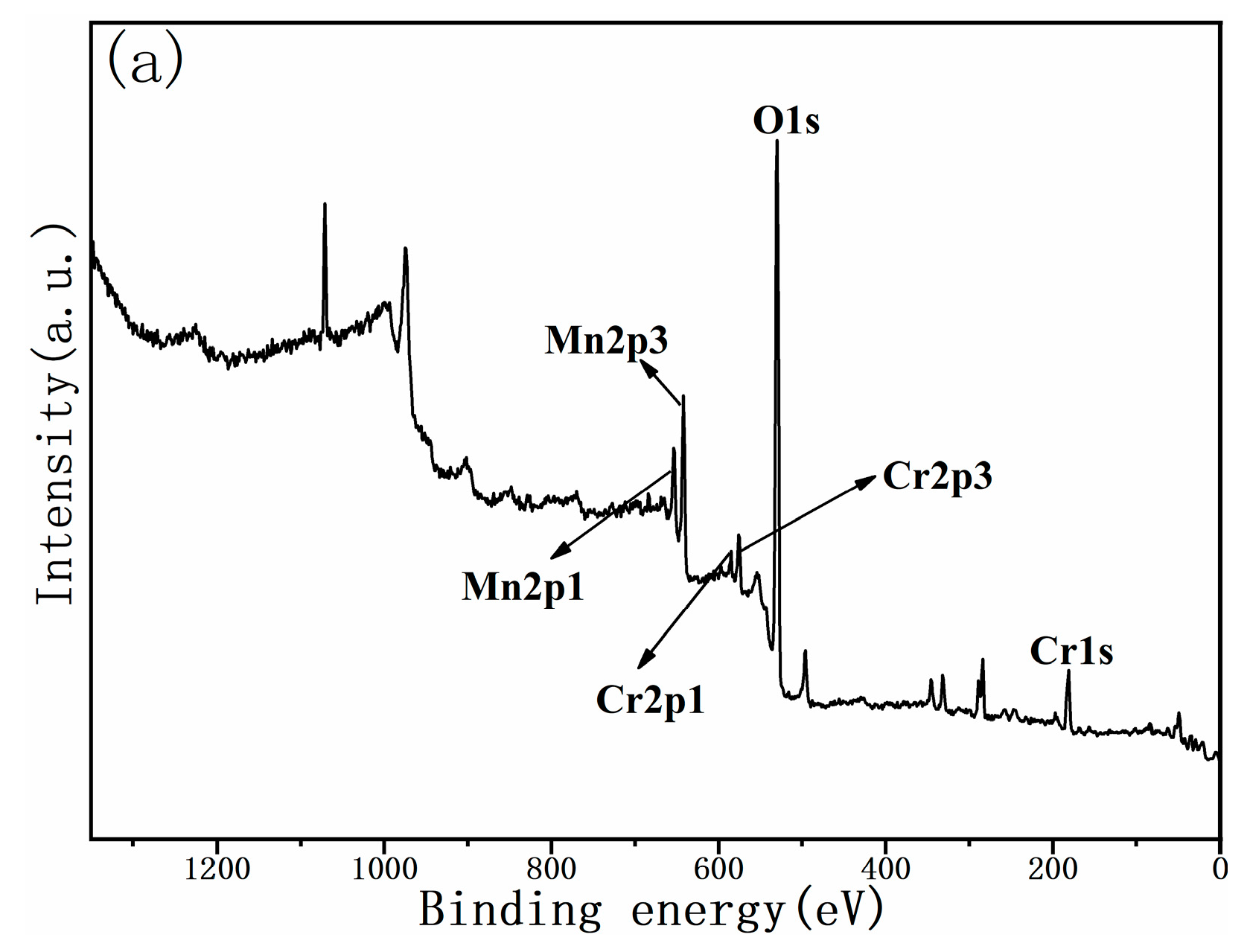
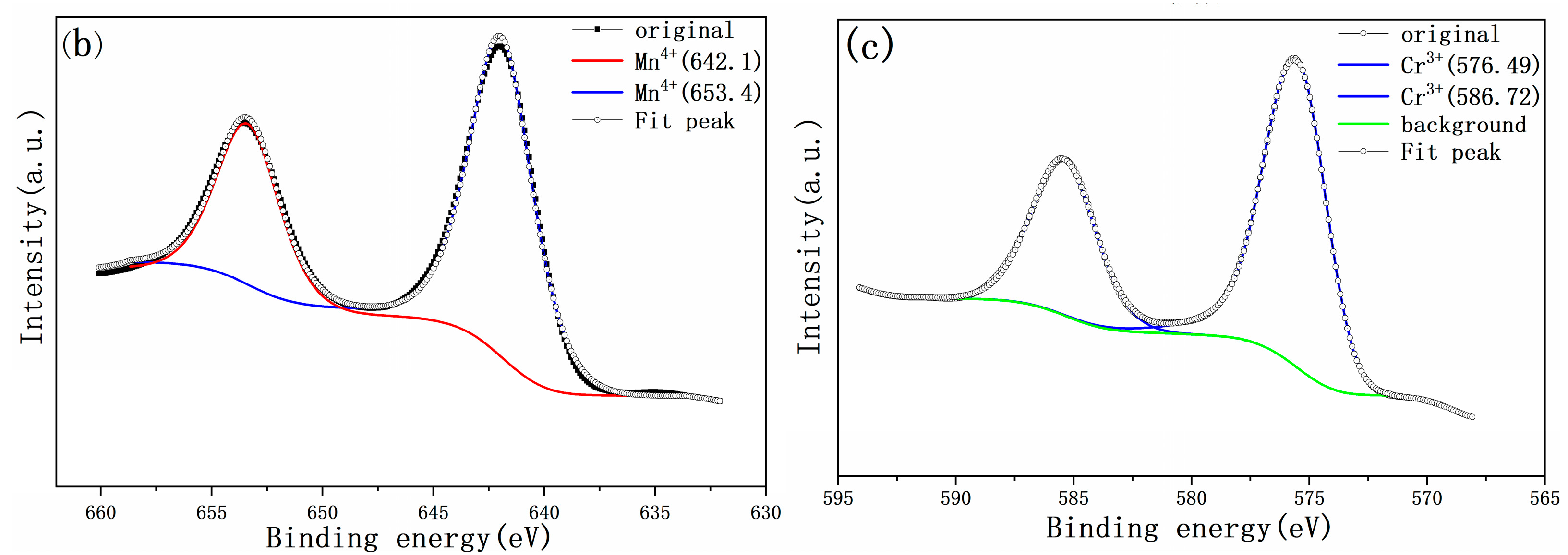
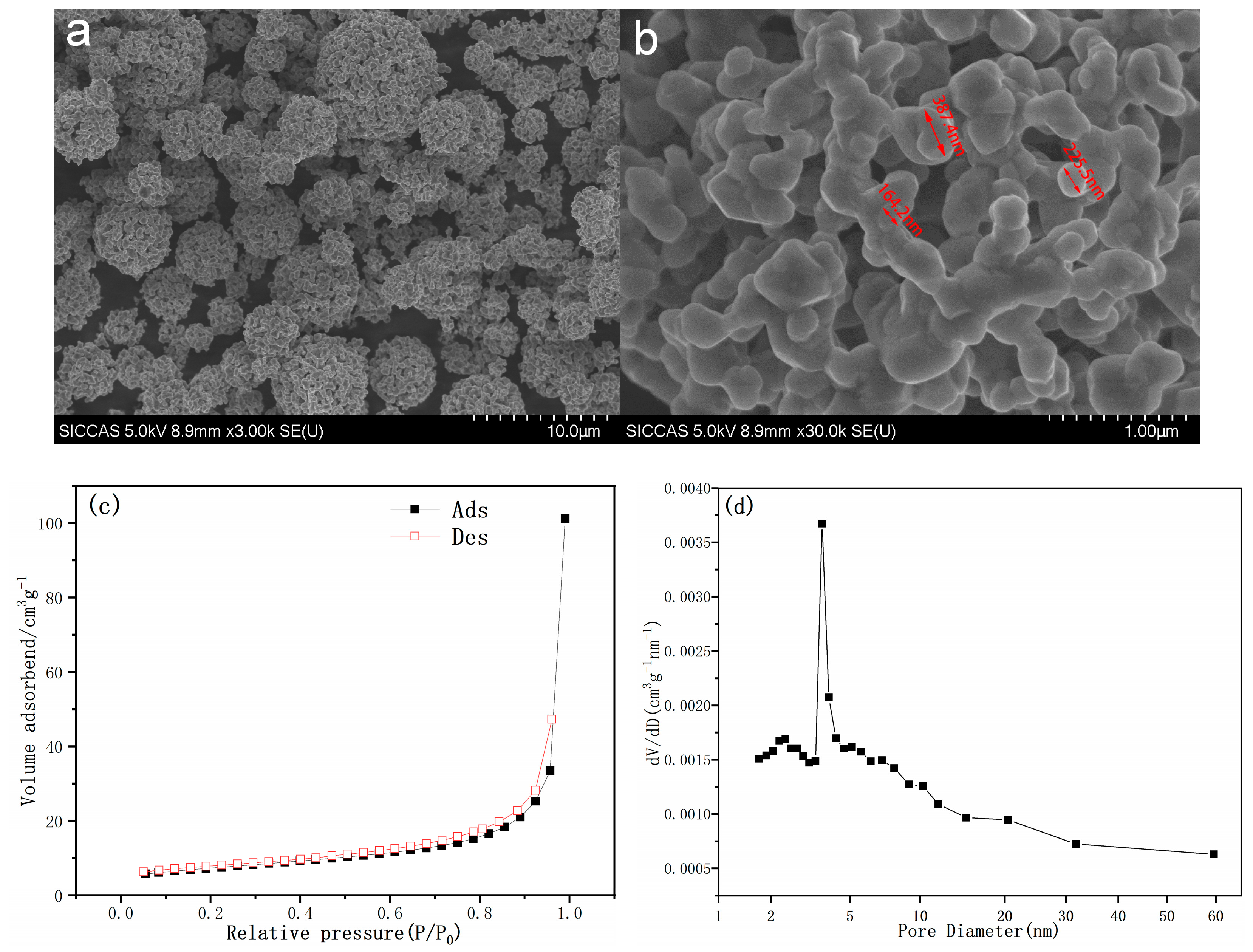

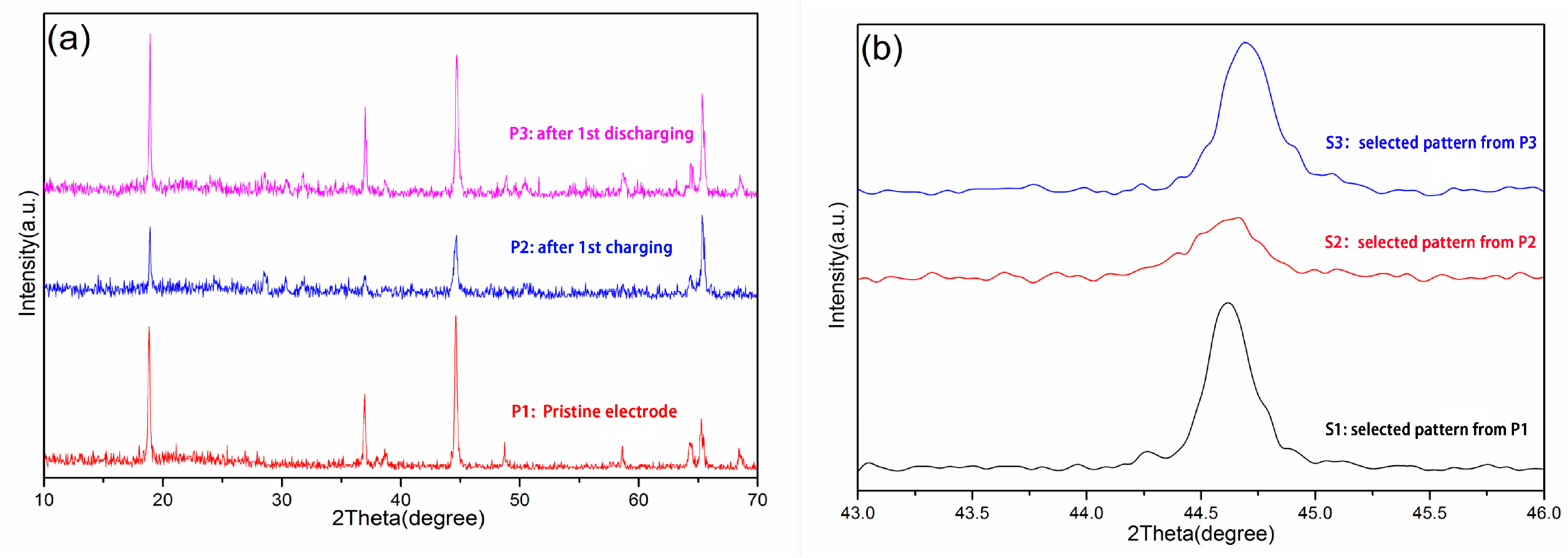


| Sample | Atom | Wyckoff Position | x | y | z | Occupancy | R (%) | Rwp (%) |
|---|---|---|---|---|---|---|---|---|
| S950 | Li (1) | 3b | 0 | 0 | 0.5 | 0.9998 | 9.49 | 10.44 |
| Li (2) | 3a | 0 | 0 | 0 | 0.2686 | |||
| Mn (1) | 3a | 0 | 0 | 0 | 0.5274 | |||
| Cr (1) | 3a | 0 | 0 | 0 | 0.1984 | |||
| O (1) | 6c | 0 | 0 | 0.2416 | 1 |
| Sample | a(Å) | c(Å) | V(Å3) | I003/I104 |
|---|---|---|---|---|
| S950 | 2.8643 | 14.2645 | 101.35 | 1.625 |
| Source | Component | Valance State | BE/eV | FWHM/eV | Relative Area/% |
|---|---|---|---|---|---|
| Figure 2b | 642.5 | Mn4+ | 642.1 | 1.9 | 100 |
| Mn3+ | - | - | 0 | ||
| Figure 2b | 653.4 | Mn4+ | 653.37 | 2.1 | 100 |
| Mn3+ | - | - | 0 | ||
| Figure 2c | 576.5 | Cr3+ | 576.49 | 1.96 | 100 |
| Cr6+ | - | - | 0 | ||
| Figure 2c | 586.8 | Cr3+ | 586.72 | 2 | 100 |
| Cr6+ | - | - | 0 |
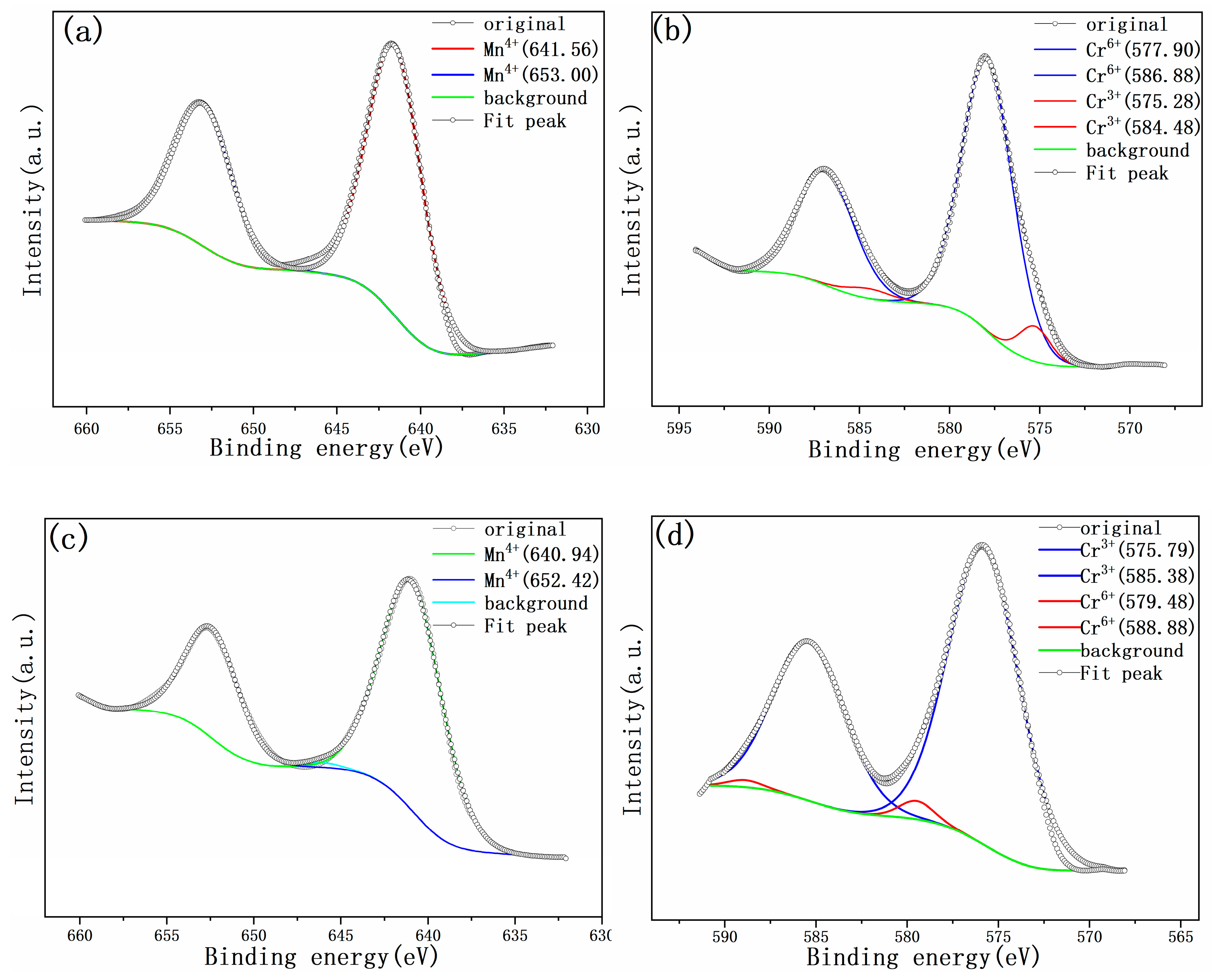
| Source | Component/eV | Valance State | BE/eV | FWHM/eV | Relative Area/% |
|---|---|---|---|---|---|
| Figure 6a Charged electrode | 641.55 | Mn4+ | 641.56 | 1.71 | 100 |
| Mn3+ | - | - | 0 | ||
| Figure 6a Charged electrode | 653.00 | Mn4+ | 653.03 | 1.55 | 100 |
| Mn3+ | - | - | 0 | ||
| Figure 6b Charged electrode | 578.01 | Cr6+ | 577.85 | 1.73 | 95.8 |
| Cr3+ | 575.19 | 1.08 | 4.2 | ||
| Figure 6b Charged electrode | 587.43 | Cr6+ | 586.97 | 1.93 | 96.2 |
| Cr3+ | 584.68 | 1.49 | 3.8 | ||
| Figure 6c Discharged electrode | 641.01 | Mn4+ | 640.94 | 1.34 | 100 |
| Mn3+ | - | - | 0 | ||
| Figure 6c Discharged electrode | 652.42 | Mn4+ | 652.45 | 1.76 | 100 |
| Mn3+ | - | - | 0 | ||
| Figure 6d Discharged electrode | 576.21 | Cr6+ | 579.48 | 0.58 | 2.5 |
| Cr3+ | 575.79 | 1.75 | 97.5 | ||
| Figure 6d Discharged electrode | 585.7 | Cr6+ | 588.88 | 0.53 | 2.1 |
| Cr3+ | 585.38 | 1.79 | 97.9 |
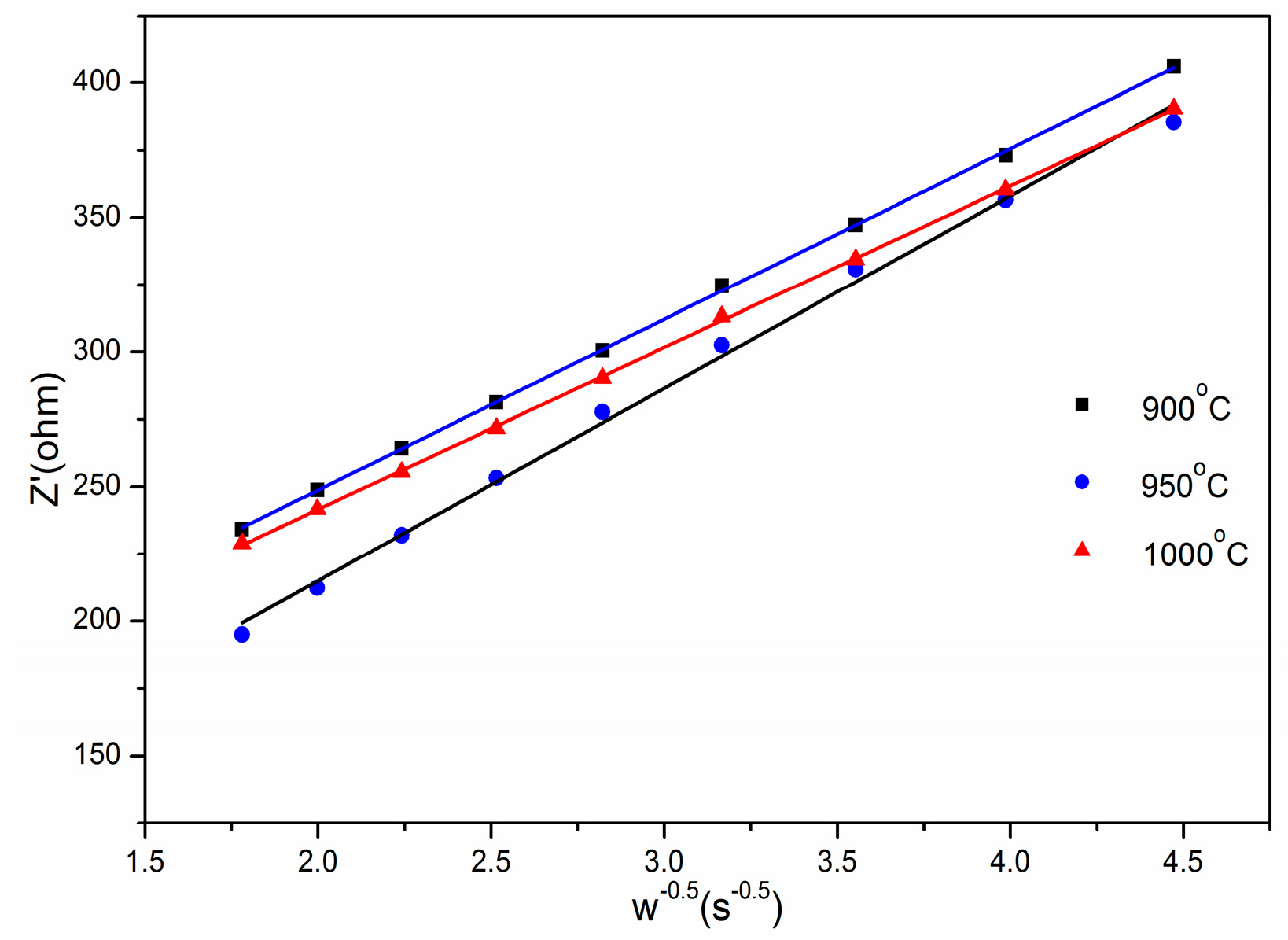
| Cycles | Rs(Ω) | Rct(Ω) |
|---|---|---|
| 1th cycle | 3.50 | 233.88 |
| 30th cycle | 2.64 | 132.88 |
| 100th cycle | 3.34 | 91.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.; Dong, J.; Guan, L.; Zhang, D. Enhanced Electrochemical Performance of Li1.27Cr0.2Mn0.53O2 Layered Cathode Materials via a Nanomilling-Assisted Solid-state Process. Materials 2019, 12, 468. https://doi.org/10.3390/ma12030468
Chang C, Dong J, Guan L, Zhang D. Enhanced Electrochemical Performance of Li1.27Cr0.2Mn0.53O2 Layered Cathode Materials via a Nanomilling-Assisted Solid-state Process. Materials. 2019; 12(3):468. https://doi.org/10.3390/ma12030468
Chicago/Turabian StyleChang, Chengkang, Jian Dong, Li Guan, and Dongyun Zhang. 2019. "Enhanced Electrochemical Performance of Li1.27Cr0.2Mn0.53O2 Layered Cathode Materials via a Nanomilling-Assisted Solid-state Process" Materials 12, no. 3: 468. https://doi.org/10.3390/ma12030468





