An Overview of Parameters Controlling the Decomposition and Degradation of Ti-Based Mn+1AXn Phases
Abstract
:1. Introduction
2. Kinetics of Phase Decomposition
3. Controlling Parameters on Decomposition or Degradation
3.1. Role of Vacuum Annealing
3.2. Role of Argon Atmosphere
3.3. Role of Pore Microstructure
3.4. Role of High Pressure
3.5. Role of Ion-Irradiation
3.6. Role of Miscellaneous Factors
4. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Barsoum, M.W. The Mn+1AXn phases: A new class of solids: Thermodynamically stable nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Zhang, H.B.; Bao, Y.W.; Zhou, Y.C. Current status in layered ternary carbides: A review. J. Mater. Sci. Technol. 2009, 25, 1–38. [Google Scholar]
- Sun, Z.M. Progress in research and development on MAX phases. Int. Mater. Rev. 2011, 56, 1–43. [Google Scholar] [CrossRef]
- Barsoum, M.W. El-Raghy, The MAX phases: Unique new carbide and nitride materials. Am. Sci. 2001, 89, 334–343. [Google Scholar] [CrossRef]
- Low, I.M.; Lee, S.K.; Barsoum, M.W.; Lawn, B.R. Contact Hertzian response of Ti3SiC2 ceramics. J. Am. Ceram. Soc. 1998, 81, 225. [Google Scholar] [CrossRef]
- Low, I.M. Vickers contact damage of micro-layered Ti3SiC2. J. Eur. Ceram. Soc. 1998, 18, 709–713. [Google Scholar] [CrossRef]
- Tallman, D.J.; Hoffman, E.N.; Caspi, E.N.; Garcia-Diaz, B.L.; Kohse, G.; Sindelar, R.L.; Barsoum, M.W. Effect of neutron irradiation on select MAX phases. Acta Mater. 2015, 85, 132–143. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, Q.; Shi, L.Q.; O’Connor, D.J.; King, B.V.; Kisi, E.H.; Venkatachalam, D.K. Damage tolerance of Ti3SiC2 to high energy iodine irradiation. Appl. Surf. Sci. 2012, 258, 6281–6287. [Google Scholar] [CrossRef]
- Whittle, K.R.; Blackford, M.G.; Aughterson, R.D.; Moricca, S.; Lumpkin, G.R.; Riley, D.P.; Zaluzec, N.J. Radiation tolerance of Mn+1AXn phases, Ti3AlC2 and Ti3SiC2. Acta Mater. 2010, 58, 4362–4368. [Google Scholar] [CrossRef]
- Low, I.M. Thermal Decomposition of MAX Phases. Available online: https://www.azom.com/article.aspx?ArticleID=6711 (accessed on 1 February 2019).
- Low, I.M.; Wren, E.; Prince, K.E.; Anatacio, A. Characterisation of phase relations and properties in air-oxidised Ti3SiC2. Mater. Sci. Eng. A 2007, 466, 140–147. [Google Scholar] [CrossRef]
- Low, I.M.; Pang, W.K. Kinetics of decomposition in MAX phases at elevated temperature. Mater. Aust. Mag. 2011, 6, 33–35. [Google Scholar]
- Low, I.M.; Oo, Z.; Prince, K.E. Effect of vacuum annealing on the phase stability of Ti3SiC2. J. Am. Ceram. Soc. 2007, 90, 2610–2615. [Google Scholar] [CrossRef]
- Oo, Z.; Low, I.M.; O’Connor, B.H. Dynamic study of the thermal stability of impure Ti3SiC2 in argon and air by neutron diffraction. Physica B 2006, 385–386, 499–501. [Google Scholar] [CrossRef]
- Pang, W.K.; Low, I.M.; O’Connor, B.H.; Studer, A.J.; Peterson, V.K.; Palmquist, J.P. Diffraction study of high-temperature thermal dissociation of Maxthal Ti2AlC in vacuum. J. Alloys Compd. 2010, 509, 172–176. [Google Scholar] [CrossRef]
- Pang, W.K.; Low, I.M.; O’Connor, B.H.; Studer, A.J.; Peterson, V.K.; Sun, Z.M.; Palmquist, J.-P. Comparison of thermal stability in MAX 211 and 312 phases. J. Phys. Conf. Ser. 2010, 251, 012025. [Google Scholar] [CrossRef]
- Pang, W.K.; Low, I.M. Diffraction study of thermal dissociation in the ternary Ti-Al-C system. J. Aust. Ceram. Soc. 2009, 45, 39–43. [Google Scholar]
- Pang, W.K.; Low, I.M.; Sun, Z.M. In-situ high-temperature diffraction study of thermal dissociation of Ti3AlC2 in vacuum. J. Am. Ceram. Soc. 2010, 93, 2871–2876. [Google Scholar] [CrossRef]
- Low, I.M.; Pang, W.K.; Kennedy, S.J.; Smith, R.I. High-temperature thermal stability of Ti2AlN and Ti4AlN3: A comparative diffraction study. J. Eur. Ceram. Soc. 2011, 31, 159–166. [Google Scholar] [CrossRef]
- Pang, W.K.; Low, I.M.; Kennedy, S.J.; Smith, R.I. Diffraction study on the thermal stability of Ti2AlN at 1500–1800 °C in vacuum. Mater. Sci. Eng. A 2010, 528, 137–142. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.; Bao, Y.; Li, M. Titanium silicon carbide pest induced by nitridation. J. Am. Ceram. Soc. 2008, 91, 494–499. [Google Scholar] [CrossRef]
- Feng, A.; Orling, T.; Munir, Z.A. Field-activated pressure-assisted combustion synthesis of polycrystalline Ti3SiC2. J. Mater. Res. 1999, 14, 925–939. [Google Scholar] [CrossRef]
- Gao, N.F.; Miyamoto, Y.; Zhang, D. On physical and thermochemical properties of high-purity Ti3SiC2. Mater. Lett. 2002, 55, 61–66. [Google Scholar] [CrossRef]
- Emmerlich, J.; Music, D.; Eklund, P.; Wilhelmsson, O.; Jansson, U.; Schneider, J.M.; Högberg, H.; Hultman, L. Thermal stability of Ti3SiC2 thin films. Acta Mater. 2007, 55, 1479–1488. [Google Scholar] [CrossRef]
- Barsoum, M.W. MAX Phases: Properties of Machinable Ternary Carbides and Nitrides; John Wiley & Sons: New York, NY, USA, 2013. [Google Scholar]
- Barsoum, M.W.; El-Raghy, T. Synthesis and characterization of remarkable ceramic: Ti3SiC2. J. Am. Ceram. Soc. 1996, 79, 1953–1956. [Google Scholar] [CrossRef]
- Tzenov, N.; Barsoum, M.W.; El-Raghy, T. Influence of small amounts of Fe and V on the synthesis and stability of Ti3SiC2. J. Eur. Ceram. Soc. 2000, 20, 801–806. [Google Scholar] [CrossRef]
- Radakrishnan, R.; Williams, J.J.; Akinc, M. Synthesis and high-temperature stability of Ti3SiC2. J. Alloys Compd. 1999, 285, 85–88. [Google Scholar] [CrossRef]
- Callen, H.B. Thermodynamics and an Introduction to Thermostatistics; Wiley: New York, NY, USA, 1985. [Google Scholar]
- Zeng, J.; Ren, S.; Lu, J. Phase evolution of Ti3SiC2 annealing in vacuum at elevated temperatures. Int. J. Appl. Ceram. Technol. 2013, 10, 527–539. [Google Scholar] [CrossRef]
- Zhang, P.; Ngai, T.L.; Xie, H.; Li, Y. Erosion behaviour of a Ti3SiC2 cathode under low-current vacuum arc. J. Phys. D Appl. Phys. 2013, 46, 395202. [Google Scholar] [CrossRef]
- Chen, J.X.; Zhou, Y.C.; Zhang, H.B.; Wan, D.T.; Liu, M.Y. Thermal stability of Ti3AlC2/Al2O3 Composites in High Vacuum. Mater. Chem. Phys. 2007, 104, 109–122. [Google Scholar] [CrossRef]
- Eklund, P.; Beckers, M.; Jansson, U.; Högberg, H.; Hultman, L. The Mn+1AXn phases: Materials science and thin-film processing. Thin Solid Films 2010, 518, 1851–1878. [Google Scholar] [CrossRef]
- Beckers, M.; Schell, N.; Martins, R.M.S.; Mucklich, A.; Moller, W. Phase stability of epitaxially grown Ti2AlN thin films. Appl. Phys. Lett. 2006, 89, 074101. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, H.; Chai, J.; Shen, L.; Seng, H.L.; Pan, J.; Wong, L.M.; Sullivan, M.B.; Wang, S.J. Desorption of Al and phase transformation of Ti2AlN MAX thin film upon annealing in ultra-high-vacuum. J. Phys. Chem. C 2014, 118, 20927–20939. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Wang, L.; Li, X.; Ke, P.; Wang, A. Dense and high-stability Ti2AlN MAX phase coatings prepared by the combined cathodic arc/sputter technique. App. Surf. Sci. 2017, 396, 1435–1442. [Google Scholar] [CrossRef]
- Xiao, L.-O.; Li, S.-B.; Song, G.; Sloof, W.G. Synthesis and thermal stability of Cr2AlC. J. Eur. Ceram. Soc. 2011, 31, 1497–1502. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.-B.; Yao, B.; Zhang, J.; Lu, X.; Zhou, Y. Thermal stability and thermal shock resistance of Fe2AlB2. Ceram. Int. 2018, 44, 16035–16039. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, G.; Pei, Y.; Fang, D. Thermal stability of bulk Zr2Al4C5 ceramic at elevated temperatures. Int. J. Refract. Met. Hard Mater. 2012, 30, 102–106. [Google Scholar] [CrossRef]
- Gai, J.; Chen, J.; Li, M. Thermal stability of Ti3AlC2 in Ar-10vol.%CO atmosphere. J. Eur. Ceram. Soc. 2016, 36, 2837–2841. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, G.; Qian, Y.; Xu, J.; Li, M. Deposition of phase-pure Cr2AlC coating by DC magnetron sputtering and post annealing using Cr-Al-C targets with controlled elemental composition but different phase compositions. J. Mater. Sci. Technol. 2018, 34, 466–471. [Google Scholar] [CrossRef]
- Smialek, J.L.; Garg, A. Interfacial reactions of a MAX phase/superalloy hybrid. Surf. Interface Anal. 2015, 47, 844–853. [Google Scholar] [CrossRef]
- Chen, C.; Li, F.Z.; Xu, C.H.; Zhang, H.-B.; Peng, S.M. High-temperature hydrogenation behaviour of bulk titanium silicon carbide. Adv. Appl. Ceram. 2016, 115, 288–293. [Google Scholar] [CrossRef]
- Qin, J.; He, D. Phase stability of Ti3SiC2 at high pressure and high temperature. Ceram. Int. 2013, 39, 9361–9367. [Google Scholar] [CrossRef]
- Qin, J.; He, D.; Lei, L.; An, P.; Fang, L.; Li, Y.; Wang, F.; Kou, Z. Differential thermal analysis study of phase segregation of Ti2AlC under high pressure and high temperature. J. Alloys Compd. 2009, 476, L8–L10. [Google Scholar] [CrossRef]
- Qin, J.; He, D.; Chen, C.; Wang, J.; Hu, J.; Yang, B. Phase segregation of titanium-aluminium carbide (Ti2AlC) at high pressure and high temperature. J. Alloys Compd. 2008, 462, L24–L27. [Google Scholar] [CrossRef]
- An, P.; He, Z.; Qin, J.; Li, Z.; Li, Y.; Kou, Z.; He, D. Stability of titanium-aluminium nitride (Ti2AlN) at high pressure and high temperatures. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2011, 26, 914–919. [Google Scholar] [CrossRef]
- Tallman, D.J.; He, L.; Garcia-Diaz, B.L.; Hoffman, E.N.; Kohse, G.; Sindelar, R.L.; Barsoum, M.W. Effect of neutron irradiation on defect evolution in Ti3SiC2 and Ti2AlC. J. Nucl. Mater. 2016, 468, 194–206. [Google Scholar] [CrossRef]
- Tallman, D.J.; He, L.; Gan, J.; Caspi, E.N.; Hoffman, E.N.; Barsoum, M.W. Effects of neutron irradiation of Ti3SiC2 and Ti3AlC2 in the 121–1085 °C temperature range. J. Nucl. Mater. 2017, 484, 120–134. [Google Scholar] [CrossRef]
- Ang, C.; Silva, C.; Shih, C.; Koyanagi, T.; Katoh, Y.; Zinkle, S.J. Anisotropic swelling and microcracking of neutron irradiated Ti3AlC2–Ti5Al2C3 materials. Scr. Mater. 2016, 114, 74–78. [Google Scholar] [CrossRef]
- Ang, C.; Parish, C.M.; Shih, C.; Silva, C.; Katoh, Y. Microstructure and mechanical properties of titanium aluminum carbides neutron irradiated at 400–700 °C. J. Eur. Ceram. Soc. 2017, 37, 2353–2363. [Google Scholar] [CrossRef]
- Qi, Q.; Cheng, G.J.; Shi, L.Q.; O’Connor, D.G.; King, B.V.; Kisi, E.H. Damage accumulation and recovery in C+-irradiated Ti3SiC2. Acta Mater. 2014, 66, 317–325. [Google Scholar] [CrossRef]
- Liu, C.; Shi, L.; Qi, Q.; O’Connor, D.G.; King, B.V.; Kisi, E.H.; Qing, X.B.; Wang, B.Y. Surface damage of Ti3SiC2 by MeV iodine bombardment. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2013, 307, 536–540. [Google Scholar] [CrossRef]
- Bentzel, G.W.; Ghidiu, M.; Anasori, B.; Barsoum, M.W. On the interactions of Ti2AlC, Ti3AlC2, Ti3SiC2 and Cr2AlC with silicon carbide and pyrolytic carbon at 1300 °C. J. Eur. Ceram. Soc. 2015, 35, 4107–4114. [Google Scholar] [CrossRef]
- Chiker, N.; Haddad, A.; Hadji, Y.; Benammar, M.E.A.; Azzaz, M.; Yahi, M.; Sahraoui, T.; Hadji, M.; Barsoum, M.W. Infiltration behavior of Cu and Ti fillers into Ti2AlC/Ti3AlC2 composites during tungsten inert gas (TIG) brazing. Ceram. Int. 2018, 44, 3282–3290. [Google Scholar] [CrossRef]
- Liu, M.; Chen, J.; Cui, H.; Sun, X.; Liu, S.; Xie, M. Ag/Ti3AlC2 composites with high hardness, high strength and high conductivity. Mater. Lett. 2018, 213, 269–273. [Google Scholar] [CrossRef]
- Septiadi, A.; Fitriani, P.; Sharma, A.S.; Yoon, D.H. Low pressure joining of SiCf/SiC composites using Ti3AlC2 or Ti3SiC2 MAX phase tape. J. Korean Ceram. Soc. 2017, 54, 340–348. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional, ordered, double transition metals carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef] [PubMed]
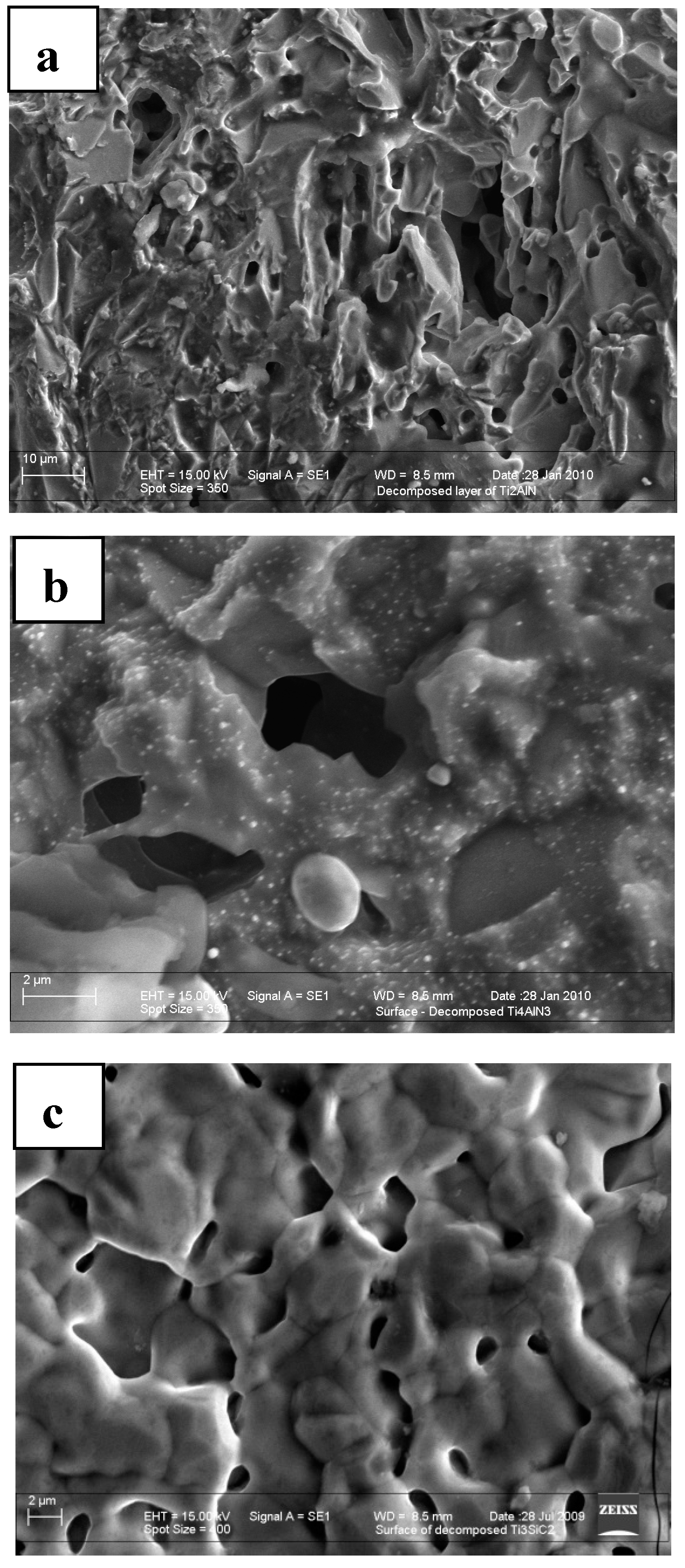

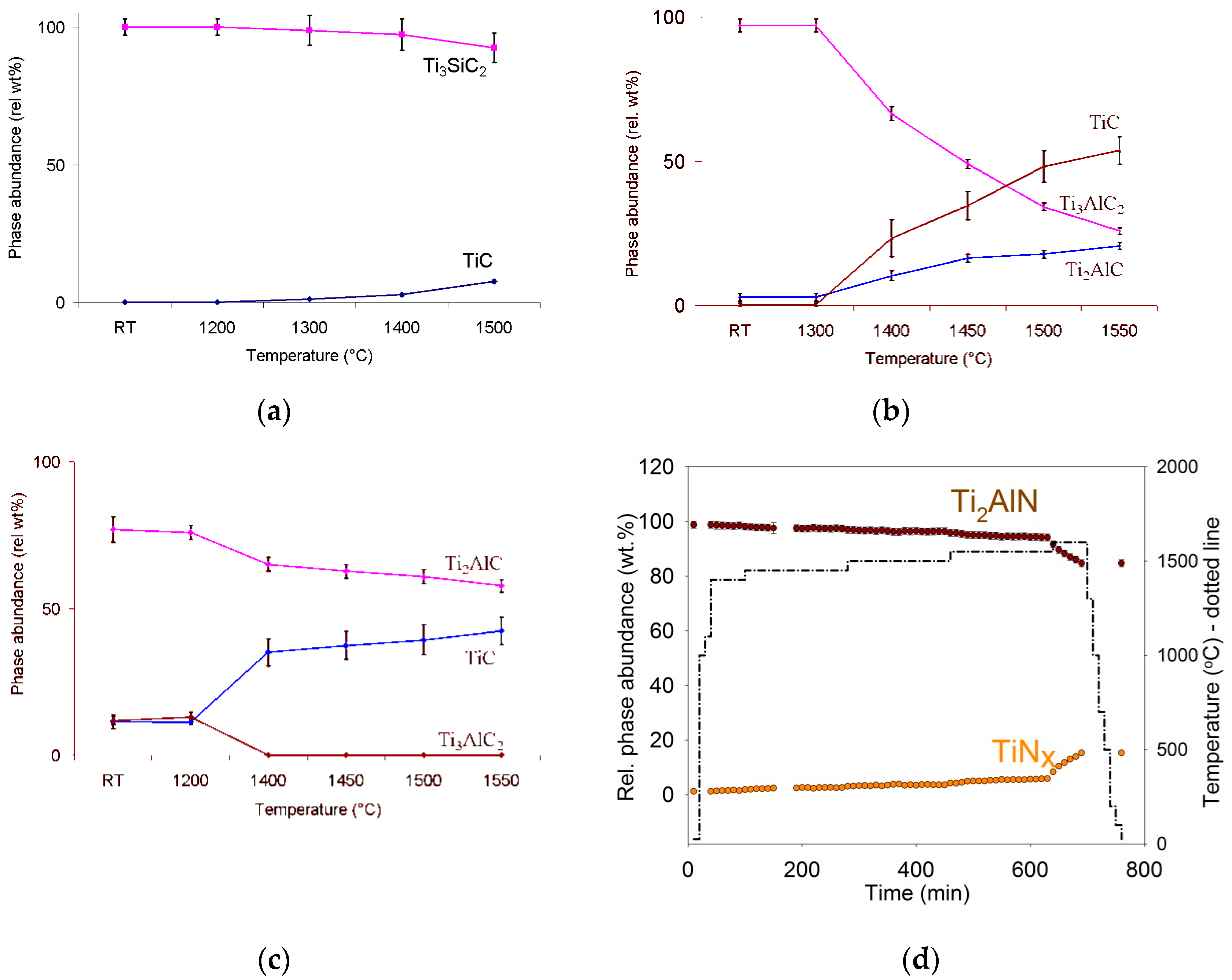
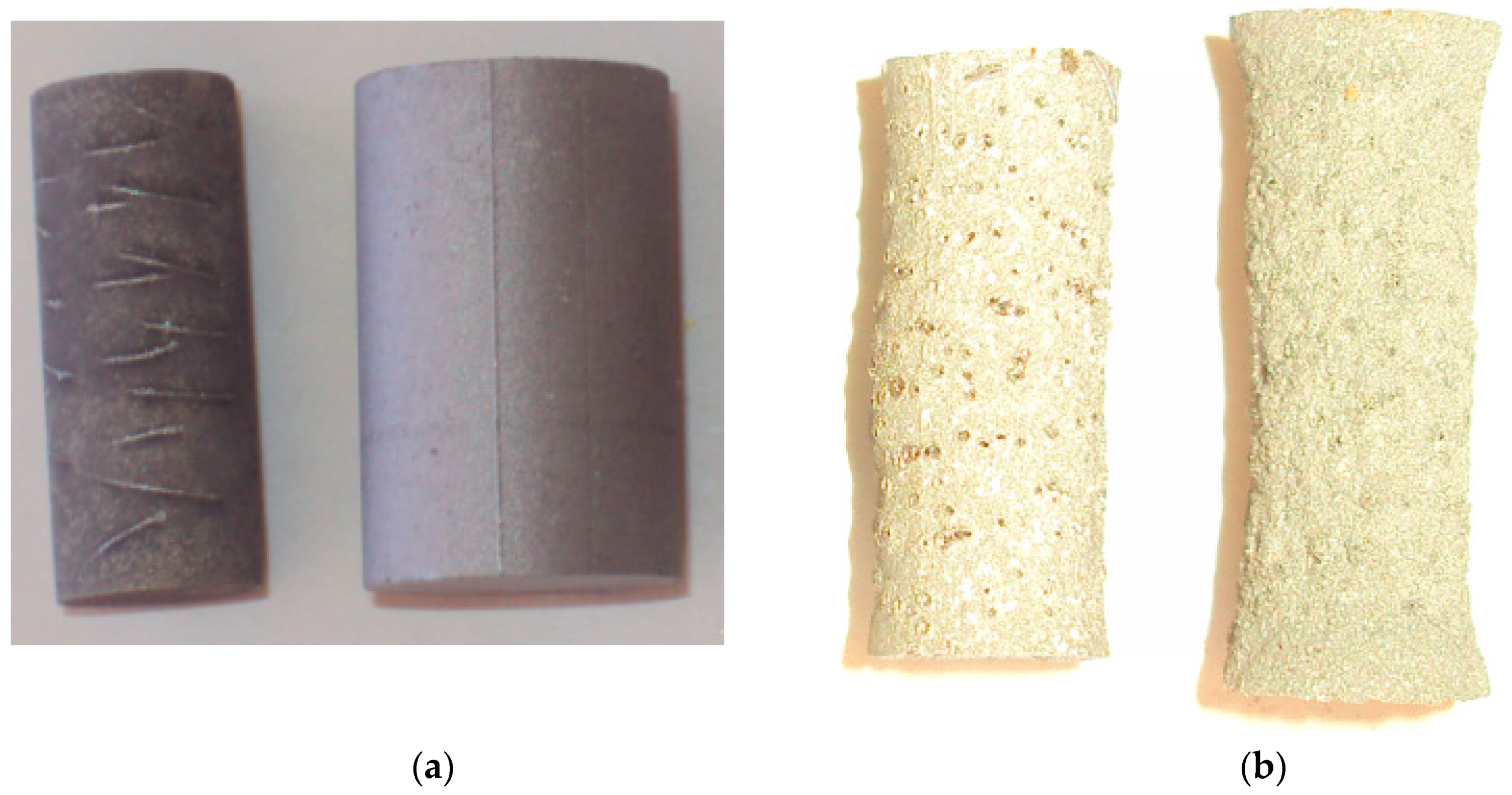
| MAX Phase | Activation Energy (kJ mol−1) | Pore Size (µm) | Proposed Reactions |
|---|---|---|---|
| Ti3SiC2 | 169.6 | 1.0–3.0 | |
| Ti3AlC2 (bulk) | −71.9 | 0.5–0.8 | |
| Ti3AlC2 (powder) | 71.9 | >1.0 | |
| Ti2AlC | 85.7 | 2.0–10.0 | |
| Ti2AlN | 573.8 | 2.0–8.0 | |
| Ti4AlN3 | 410.8 | 1.8–3.0 | Ti4AlN3 → 4TiN0.75 + Al(g) |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Low, I.-M. An Overview of Parameters Controlling the Decomposition and Degradation of Ti-Based Mn+1AXn Phases. Materials 2019, 12, 473. https://doi.org/10.3390/ma12030473
Low I-M. An Overview of Parameters Controlling the Decomposition and Degradation of Ti-Based Mn+1AXn Phases. Materials. 2019; 12(3):473. https://doi.org/10.3390/ma12030473
Chicago/Turabian StyleLow, It-Meng. 2019. "An Overview of Parameters Controlling the Decomposition and Degradation of Ti-Based Mn+1AXn Phases" Materials 12, no. 3: 473. https://doi.org/10.3390/ma12030473





