Fabrication and Microstructure of Laminated HAP–45S5 Bioglass Ceramics by Spark Plasma Sintering
Abstract
:1. Introduction
2. Materials and Methods
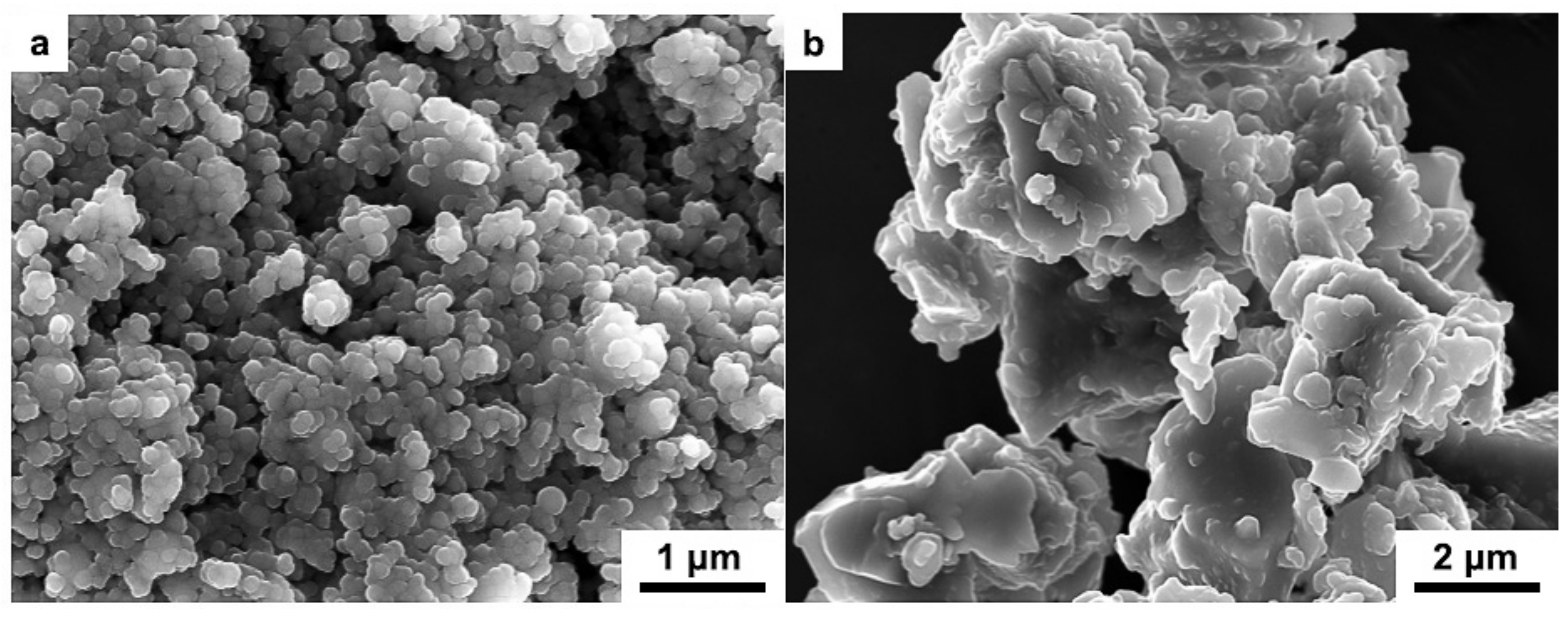
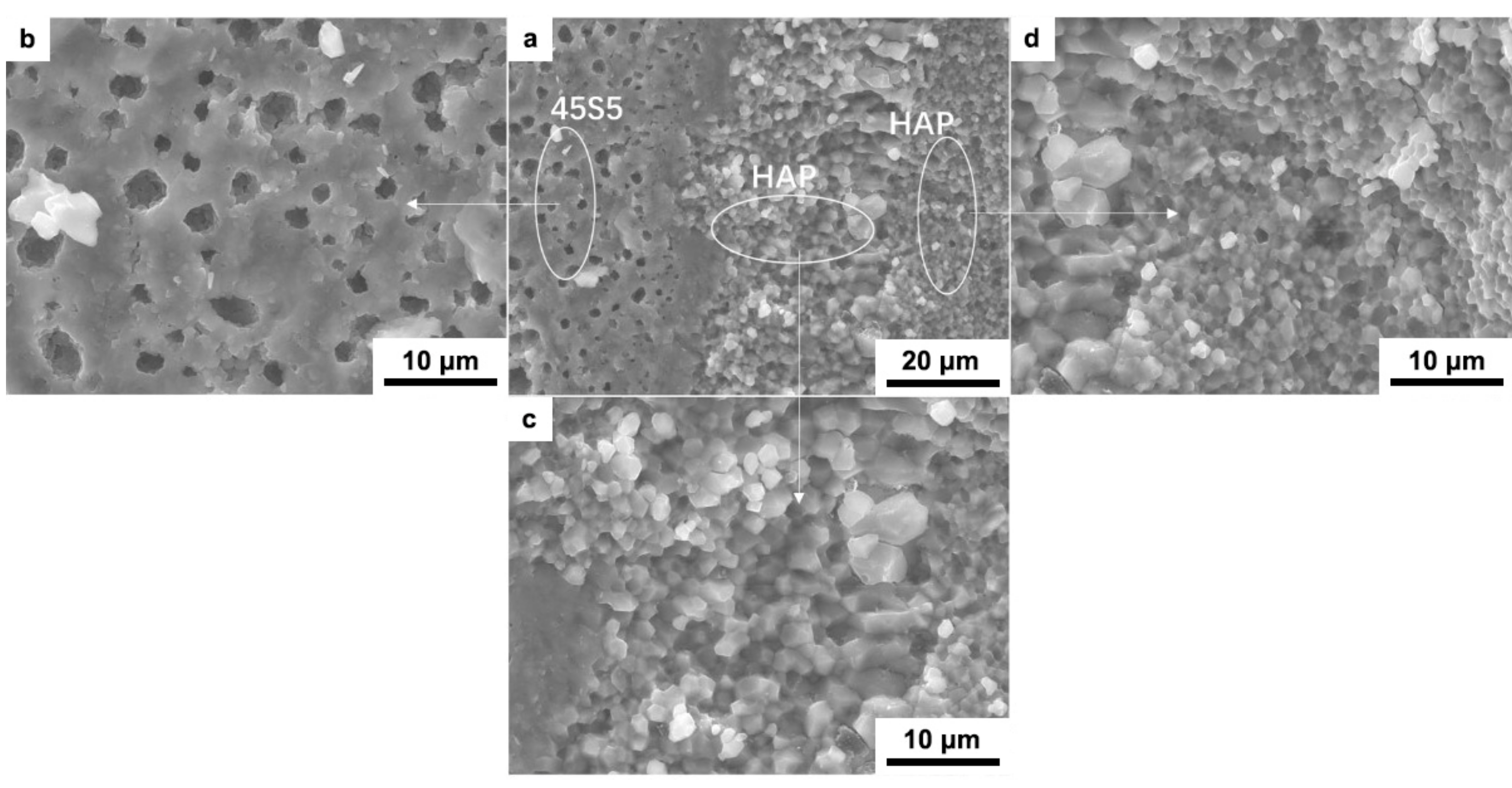
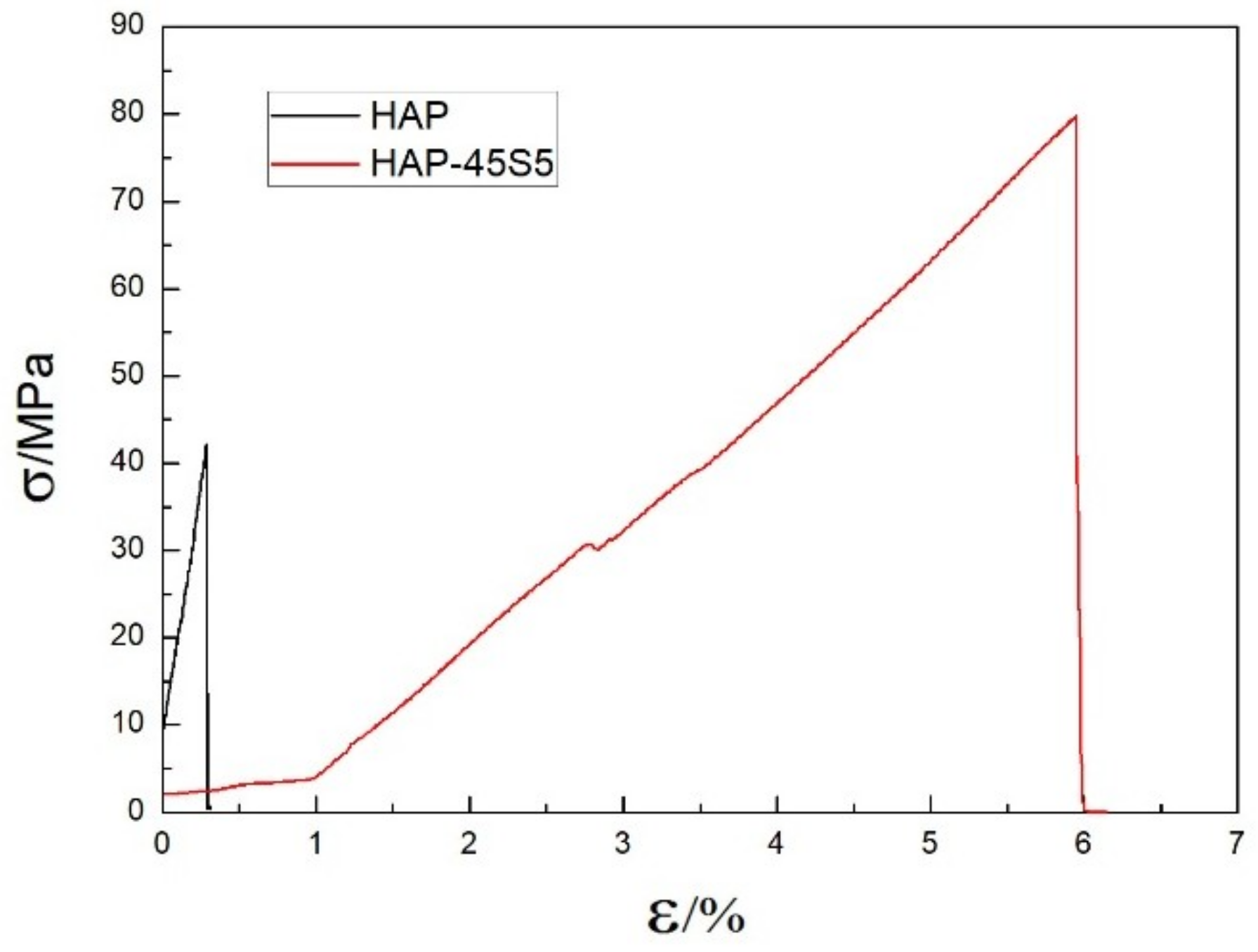
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xie, Y.; Cheng, L.; Li, L.; Mei, H.; Zhang, L. Strengthening/toughening of laminated SiCw/SiC ceramic composites by heat treatment. Ceram. Int. 2015, 41, 10024–10029. [Google Scholar] [CrossRef]
- Xiang, L.; Cheng, L.; Hou, Y.; Wang, F.; Li, L.; Zhang, L. Fabrication and mechanical properties of laminated HfC–SiC/BN ceramics. J. Eur. Ceram. Soc. 2014, 34, 3635–3640. [Google Scholar] [CrossRef]
- Shi, G.; Wu, Z.; Wang, Z.; Liang, J. The mechanical properties and microstructure of the bionic alloy–ceramic laminated composite. Mater. Des. 2012, 33, 300–305. [Google Scholar] [CrossRef]
- Wang, C.; Huang, Y.; Zan, Q.; Guo, H.; Cai, S. Biomimetic structure design—A possible approach to change the brittleness of ceramics in nature. Mater. Sci. Eng. C 2000, 11, 9–12. [Google Scholar] [CrossRef]
- Vecchio, K.S.; Jiang, F. Fracture toughness of Ceramic-Fiber-Reinforced Metallic-Intermetallic-Laminate (CFR-MIL) composites. Mater. Sci. Eng. A 2016, 649, 407–416. [Google Scholar] [CrossRef]
- Clegg, W.J.; Kendall, K.; Alford, N.M.; Button, T.W.; Birchall, J.D. A simple way to make tough ceramics. Nature 1990, 347, 455–457. [Google Scholar] [CrossRef]
- Xie, Y.; Cheng, L.; Li, L.; Mei, H.; Zhang, L. Fabrication of laminated SiCw/SiC ceramic composites by CVI. J. Eur. Ceram. Soc. 2013, 33, 1701–1706. [Google Scholar] [CrossRef]
- Lü, Z.; Jiang, D.; Zhang, J.; Lin, Q.; Huang, Z. ZrB2–SiC laminated ceramic composites. J Eur. Ceram. Soc. 2012, 32, 1435–1439. [Google Scholar] [CrossRef]
- Wang, H.; Fan, B.; Feng, L.; Chen, D.; Lu, H.; Xu, H.; Wang, C.; Zhang, R. The fabrication and mechanical properties of SiC/ZrB2 laminated ceramic composite prepared by spark plasma sintering. Ceram. Int. 2012, 38, 5015–5022. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Y.; Qiao, G.; Yang, J.; Wang, H. Preparation and R-curve properties of laminated Si/SiC ceramics from paper. Mater. Sci. Eng. A 2008, 492, 327–332. [Google Scholar] [CrossRef]
- Hadad, M.; Blugan, G.; Kübler, J.; Rosset, E.; Rohr, L.; Michler, J. Tribological behaviour of Si3N4 and Si3N4–%TiN based composites and multi-layer laminates. Wear 2006, 260, 634–641. [Google Scholar] [CrossRef]
- Blugan, G.; Dobedoe, R.; Lugovy, M.; Koebel, S.; Kuebler, J. Si3N4–TiN based micro-laminates with rising R-curve behaviour. Compos. Part B Eng. 2006, 37, 459–465. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kundu, B.; Chanda, A.; Sen, S. Effect of functionalisation of CNT in the preparation of HAp–CNT biocomposites. Ceram. Int. 2015, 41, 3766–3774. [Google Scholar] [CrossRef]
- White, A.A.; Best, S.M.; Kinloch, I.A. Hydroxyapatite? Carbon Nanotube Composites for Biomedical Applications: A Review. Int. J. Appl. Ceram. Technol. 2007, 4, 1–13. [Google Scholar] [CrossRef]
- Khanal, S.P.; Mahfuz, H.; Rondinone, A.J.; Leventouri, T. Improvement of the fracture toughness of hydroxyapatite (HAp) by incorporation of carboxyl functionalized single walled carbon nanotubes (CfSWCNTs) and nylon. Mater. Sci. Eng. C 2016, 60, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Htun, Z.L.; Ahmad, N.; Thant, A.A.; Noor, A.M. Characterization of CaO-ZrO2 Reinforced Hap Biocomposite for Strength and Toughness Improvement. Procedia Chem. 2016, 19, 510–516. [Google Scholar] [CrossRef]
- Ankur, G.; Garima, T.; Debrupa, L.; Kantesh, B. Compression Molded Ultra High Molecular Weight Polyethylene-Hydroxyapatite-Aluminum Oxide-Carbon Nanotube Hybrid Composites for Hard Tissue Replacement. J. Mater. Sci. Technol. 2013, 29, 514–522. [Google Scholar]
- Lee, B.; Lee, C.; Youn, M.; Song, H. Relationship between microstructure and mechanical properties of fibrous HAp-(t-ZrO2)/Al2O3-(m-ZrO2) composites. Mater. Sci. Eng. A 2007, 458, 11–16. [Google Scholar] [CrossRef]
- Deng, M.; Wen, H.L.; Dong, X.L.; Li, F.; Xu, X.; Li, H.; Li, J.Y.; Zhou, X.D. Effects of 45S5 bioglass on surface properties of dental enamel subjected to 35% hydrogen peroxide. Int. J. Oral Sci. 2013, 5, 103–110. [Google Scholar] [CrossRef]
- Kashyap, S.; Griep, K.; Nychka, J.A. Crystallization kinetics, mineralization and crack propagation in partially crystallized bioactive glass 45S5. Mater. Sci. Eng. C 2011, 31, 762–769. [Google Scholar] [CrossRef]
- Cacciotti, I.; Lombardi, M.; Bianco, A.; Ravaglioli, A.; Montanaro, L. Sol-gel derived 45S5 bioglass: Synthesis, microstructural evolution and thermal behaviour. J. Mater. Sci. Mater. Med. 2012, 23, 1849–1866. [Google Scholar] [CrossRef] [PubMed]
- Touri, R.; Moztarzadeh, F.; Sadeghian, Z.; Bizari, D.; Tahriri, M.; Mozafari, M. The use of carbon nanotubes to reinforce 45S5 bioglass-based scaffolds for tissue engineering applications. Biomed. Res. Int. 2013, 2013, 465086. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass (R). J. Mater. Sci.-Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Porwal, H.; Grasso, S.; Cordero-Arias, L.; Li, C.; Boccaccini, A.R.; Reece, M.J. Processing and bioactivity of 45S5 Bioglass((R))-graphene nanoplatelets composites. J. Mater. Sci. Mater. Med. 2014, 25, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Plewinski, M.; Schickle, K.; Lindner, M.; Kirsten, A.; Weber, M.; Fischer, H. The effect of crystallization of bioactive bioglass 45S5 on apatite formation and degradation. Dent. Mater. 2013, 29, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhang, J.; Jia, C.; Nie, J.; Chu, K. Preparation and characterization of mechanical properties of carbon nanotube/45S5Bioglass composites for biologic applications. Mater. Sci. Eng. A 2011, 528, 1553–1557. [Google Scholar] [CrossRef]
- Mondal, S.; Hoang, G.; Manivasagan, P.; Moorthy, M.S.; Nguyen, T.P.; Vy Phan, T.T.; Kim, H.H.; Kim, M.H.; Nam, S.Y.; Oh, J. Nano-hydroxyapatite bioactive glass composite scaffold with enhanced mechanical and biological performance for tissue engineering application. Ceram. Int. 2018, 44, 15735–15746. [Google Scholar] [CrossRef]
- Sebdani, M.M.; Fathi, M.H. Novel hydroxyapatite–forsterite–bioglass nanocomposite coatings with improved mechanical properties. J. Alloy Compd. 2011, 509, 2273–2276. [Google Scholar] [CrossRef]
- Melinte, G.; Baia, L.; Simon, V.; Simon, S. Hydrogen peroxide versus water synthesis of bioglass–nanocrystalline hydroxyapatite composites. J. Mater. Sci. 2011, 46, 7393–7400. [Google Scholar] [CrossRef]
- Ravarian, R.; Moztarzadeh, F.; Hashjin, M.S.; Rabiee, S.M.; Khoshakhlagh, P.; Tahriri, M. Synthesis, characterization and bioactivity investigation of bioglass/hydroxyapatite composite. Ceram. Int. 2010, 36, 291–297. [Google Scholar] [CrossRef]
- Meng, Y.H.; Tang, C.Y.; Tsui, C.P. Fabrication and Characterization of Bioglass-Modified HA–ZrO2 Biocomposites. Compos. Interface 2012, 17, 551–558. [Google Scholar] [CrossRef]
- Demirkiran, H.; Hu, Y.; Zuin, L.; Appathurai, N.; Aswath, P.B. XANES analysis of calcium and sodium phosphates and silicates and hydroxyapatite–Bioglass®45S5 co-sintered bioceramics. Mater. Sci. Eng. C 2011, 31, 134–143. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Anesi, A.; Salvatori, R.; Chiarini, L.; Cannillo, V. Bioactive glass/hydroxyapatite composites: Mechanical properties and biological evaluation. Mater. Sci. Eng. C 2015, 51, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Thamma, U.; Kowal, T.; Falk, M.; Jain, H. Influence of nanoporosity on the nature of hydroxyapatite formed on bioactive calcium silicate model glass. J. Biomed. Mater. Res. B Appl. Biomater. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Hamdi, M.; Basirun, W.J.; Kondoh, K.; Umeda, J. Low pressure spark plasma sintered hydroxyapatite and Bioglass® composite scaffolds for bone tissue repair. Ceram. Int. 2018, 44, 23052–23062. [Google Scholar] [CrossRef]
- Bellucci, D.; Desogus, L.; Montinaro, S.; Orrù, R.; Cao, G.; Cannillo, V. Innovative hydroxyapatite/bioactive glass composites processed by spark plasma sintering for bone tissue repair. J. Eur. Ceram. Soc. 2017, 37, 1723–1733. [Google Scholar] [CrossRef]
- Kim, B.; Prajatelistia, E.; Han, Y.; Son, H.; Sakka, Y.; Kim, S. Transparent hydroxyapatite ceramics consolidated by spark plasma sintering. Scr. Mater. 2013, 69, 366–369. [Google Scholar] [CrossRef]
- Akin, I.; Goller, G. Processing Technologies for Bioceramic Based Composites. Handb. Bioceram. Biocompos. 2015, 639–666. [Google Scholar] [CrossRef]
- Bertolla, L.; Dlouhý, I.; Tatarko, P.; Viani, A.; Mahajan, A.; Chlup, Z.; Reece, M.J.; Boccaccini, A.R. Pressureless spark plasma–sintered Bioglass® 45S5 with enhanced mechanical properties and stress–induced new phase formation. J. Eur. Ceram. Soc. 2017, 37, 2727–2736. [Google Scholar] [CrossRef]
- Grasso, S.; Chinnam, R.K.; Porwal, H.; Boccaccini, A.R.; Reece, M.J. Low temperature spark plasma sintering of 45S5 Bioglass®. J. Non-Cryst. Solids 2013, 362, 25–29. [Google Scholar] [CrossRef]
- Gergely, G.; Sahin, F.C.; Göller, G.; Yücel, O.; Balázsi, C. Microstructural and mechanical investigation of hydroxyapatite–zirconia nanocomposites prepared by spark plasma sintering. J. Eur. Ceram. Soc. 2013, 33, 2313–2319. [Google Scholar] [CrossRef]
- Dubey, A.K.; Sitesh, G.; Nath, S.; Basu, B. Spark plasma sintering to restrict sintering reactions and enhance properties of hydroxyapatite–mullite biocomposites. Ceram. Int. 2011, 37, 2755–2761. [Google Scholar] [CrossRef]
- Figueiredo, A.; Coimbra, P.; Cabrita, A.; Guerra, F.; Figueiredo, M. Comparison of a xenogeneic and an alloplastic material used in dental implants in terms of physico-chemical characteristics and in vivo inflammatory response. Mater. Sci. Eng. C 2013, 33, 3506–3513. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M.; Trampuz, A.; Borens, O.; Bohner, M.; Ilchmann, T. Biofilm formation on bone grafts and bone graft substitutes: Comparison of different materials by a standard in vitro test and microcalorimetry. Acta Biomater. 2010, 6, 3791–3797. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Sola, A.; Cannillo, V. Hydroxyapatite and tricalcium phosphate composites with bioactive glass as second phase: State of the art and current applications. J. Biomed. Mater. Res. A 2016, 104, 1030–1056. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.L.; Montfort, M.J.; McLoughlin, S.W. Differential healing response of bone adjacent to porous implants coated with hydroxyapatite and 45S5 bioactive glass. J. Biomed. Mater. Res. 2001, 55, 603–612. [Google Scholar] [CrossRef]
- Tkachenko, S.; Cizek, J.; Mušálek, R.; Dvořák, K.; Spotz, Z.; Montufar, E.B.; Chráska, T.; Křupka, I.; Čelko, L. Metal matrix to ceramic matrix transition via feedstock processing of SPS titanium composites alloyed with high silicone content. J. Alloy Compd. 2018, 764, 776–788. [Google Scholar] [CrossRef]
- Montufar, E.B.; Casas-Luna, M.; Horynová, M.; Tkachenko, S.; Fohlerová, Z.; Diaz-de-la-Torre, S.; Dvořák, K.; Čelko, L.; Kaiser, J. High strength, biodegradable and cytocompatible alpha tricalcium phosphate-iron composites for temporal reduction of bone fractures. Acta Biomater. 2018, 70, 293–303. [Google Scholar] [CrossRef]
- Yang, H.; Qu, X.; Lin, W.; Wang, C.; Zhu, D.; Dai, K.; Zheng, Y. In vitro and in vivo studies on zinc-hydroxyapatite composites as novel biodegradable metal matrix composite for orthopedic applications. Acta Biomater. 2018, 71, 200–214. [Google Scholar] [CrossRef]
- Tkachenko, S.; Horynová, M.; Casas-Luna, M.; Diaz-de-la-Torre, S.; Dvořák, K.; Celko, L.; Kaiser, J.; Montufar, E.B. Strength and fracture mechanism of iron reinforced tricalcium phosphate cermet fabricated by spark plasma sintering. J. Mech. Behav. Biomed. 2018, 81, 16–25. [Google Scholar] [CrossRef]
- Jia, L.; Li, S.; Imai, H.; Chen, B.; Kondoh, K. Size effect of B4C powders on metallurgical reaction and resulting tensile properties of Ti matrix composites by in-situ reaction from Ti–B4C system under a relatively low temperature. Mater. Sci. Eng. A 2014, 614, 129–135. [Google Scholar] [CrossRef]
- Meng, Y.; Qiang, W.; Pang, J. Preparation and characterization of mechanical properties of carbon nanotube reinforced hydroxyapatite composites consolidated by spark plasma sintering. IOP Conf. Ser. Mater. Sci. Eng. 2017, 231, 12164. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Xu, J.L.; Yu, L.G.; Fang, X.Y.; Khor, K.A. Spark plasma sintering of sol–gel derived 45S5 Bioglass®-ceramics: Mechanical properties and biocompatibility evaluation. Mater. Sci. Eng. C 2012, 32, 494–502. [Google Scholar] [CrossRef]
- Kim, Y.G.; Seo, D.S.; Lee, J.K. Dissolution of synthetic and bovine bone-derived hydroxyapatites fabricated by hot-pressing. Appl. Surf. Sci. 2008, 255, 589–592. [Google Scholar] [CrossRef]
- Miranda, G.; Araújo, A.; Bartolomeu, F.; Buciumeanu, M.; Carvalho, O.; Souza, J.C.M.; Silva, F.S.; Henriques, B. Design of Ti6Al4V-HA composites produced by hot pressing for biomedical applications. Mater. Des. 2016, 108, 488–493. [Google Scholar] [CrossRef]
- Rapacz-Kmita, A.; Paluszkiewicz, C.; Ślósarczyk, A.; Paszkiewicz, Z. FTIR and XRD investigations on the thermal stability of hydroxyapatite during hot pressing and pressureless sintering processes. J. Mol. Struct. 2005, 744–747, 653–656. [Google Scholar] [CrossRef]
- Xihua, Z.; Changxia, L.; Musen, L.; Yunqiang, B.; Junlong, S. Fabrication of hydroxyapatite/diopside/alumina composites by hot-press sintering process. Ceram. Int. 2009, 35, 1969–1973. [Google Scholar] [CrossRef]
- Brzezińska-Miecznik, J.; Macherzyńska, B.; Lach, R.; Nowak, B. The effect of calcination and zirconia addition on HAp hot pressed materials. Ceram. Int. 2014, 40, 15815–15819. [Google Scholar] [CrossRef]
- Tancred, D.C.; McCormack, B.A.O.; Carr, A.J. A quantitative study of the sintering and mechanical properties of hydroxyapatite/phosphate glass composites. Biomaterials 1998, 19, 1735–1743. [Google Scholar] [CrossRef]
- Filho, H.N.; Pinto, T.F.; de Freitas, C.P.; Ribeiro-Junior, P.D.; Dos Santos, P.L.; Matsumoto, M.A. Autogenous Bone Grafts Contamination After Exposure to the Oral Cavity. J. Craniofac. Surg. 2014, 25, 412–414. [Google Scholar] [CrossRef]
- Vignoles, M.; Bonel, G.; Young, R.A. Occurrence of nitrogenous species in precipitated B-type carbonated hydroxyapatites. Calcif. Tissue Int. 1987, 40, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Feki, H.E.; Savariault, J.M.; Salah, A.B. Structure refinements by the Rietveld method of partially substituted hydroxyapatite: Ca9Na0.5(PO4)4.5(CO3)1.5(OH)2. J. Alloy Compd. 1999, 287, 114–120. [Google Scholar] [CrossRef]
- Desogus, L.; Cuccu, A.; Montinaro, S.; Orrù, R.; Cao, G.; Bellucci, D.; Sola, A.; Cannillo, V. Classical Bioglass® and innovative CaO-rich bioglass powders processed by Spark Plasma Sintering: A comparative study. J. Eur. Ceram. Soc. 2015, 35, 4277–4285. [Google Scholar] [CrossRef]
- Cerruti, M.; Morterra, C. Carbonate Formation on Bioactive Glasses. Langmuir 2004, 20, 6382–6388. [Google Scholar] [CrossRef] [PubMed]
- Oktar, F.N.; Göller, G. Sintering effects on mechanical properties of glass-reinforced hydroxyapatite composites. Ceram. Int. 2002, 28, 617–621. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Xynos, I.D.; Hukkanen, M.V.J.; Batten, J.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Bioglass ®45S5 Stimulates Osteoblast Turnover and Enhances Bone Formation In Vitro: Implications and Applications for Bone Tissue Engineering. Calcif. Tissue Int. 2000, 67, 321–329. [Google Scholar] [CrossRef]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology—Is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant Dent. 2017, 3, 23. [Google Scholar] [CrossRef]
- Cicciù, M.; Cervino, G.; Herford, A.; Famà, F.; Bramanti, E.; Fiorillo, L.; Lauritano, F.; Sambataro, S.; Troiano, G.; Laino, L. Facial Bone Reconstruction Using both Marine or Non-Marine Bone Substitutes: Evaluation of Current Outcomes in a Systematic Literature Review. Mar. Drugs 2018, 16, 27. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Cannillo, V. Bioactive glass-based composites for the production of dense sintered bodies and porous scaffolds. Mater. Sci. Eng. C 2013, 33, 2138–2151. [Google Scholar] [CrossRef]
- Shue, L.; Yufeng, Z.; Mony, U. Biomaterials for periodontal regeneration. Biomatter 2012, 4, 271–277. [Google Scholar] [CrossRef] [PubMed]





| Sample | Atomic Weight Percentage/% | ||||
|---|---|---|---|---|---|
| Si | Na | Ca | P | ||
| Raw powder | 45S5 bioglass | 35.31 | 30.93 | 29.59 | 4.17 |
| HAP | 0 | 0 | 68.26 | 31.74 | |
| Sintered specimen | 45S5 bioglass layer | 36.52 | 11.69 | 71.69 | 6.21 |
| coarse grain HAP layer | 0.97 | 52.44 | 24.00 | 22.60 | |
| fine grain HAP layer | 0 | 7.21 | 60.5 | 32.29 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Qiang, W.; Pang, J. Fabrication and Microstructure of Laminated HAP–45S5 Bioglass Ceramics by Spark Plasma Sintering. Materials 2019, 12, 484. https://doi.org/10.3390/ma12030484
Meng Y, Qiang W, Pang J. Fabrication and Microstructure of Laminated HAP–45S5 Bioglass Ceramics by Spark Plasma Sintering. Materials. 2019; 12(3):484. https://doi.org/10.3390/ma12030484
Chicago/Turabian StyleMeng, Ye, Wenjiang Qiang, and Jingqin Pang. 2019. "Fabrication and Microstructure of Laminated HAP–45S5 Bioglass Ceramics by Spark Plasma Sintering" Materials 12, no. 3: 484. https://doi.org/10.3390/ma12030484




