Microstructural Tuning of a Laser-Cladding Layer by Means of a Mix of Commercial Inconel 625 and AISI H13 Powders
Abstract
:1. Introduction
2. Materials and Methods
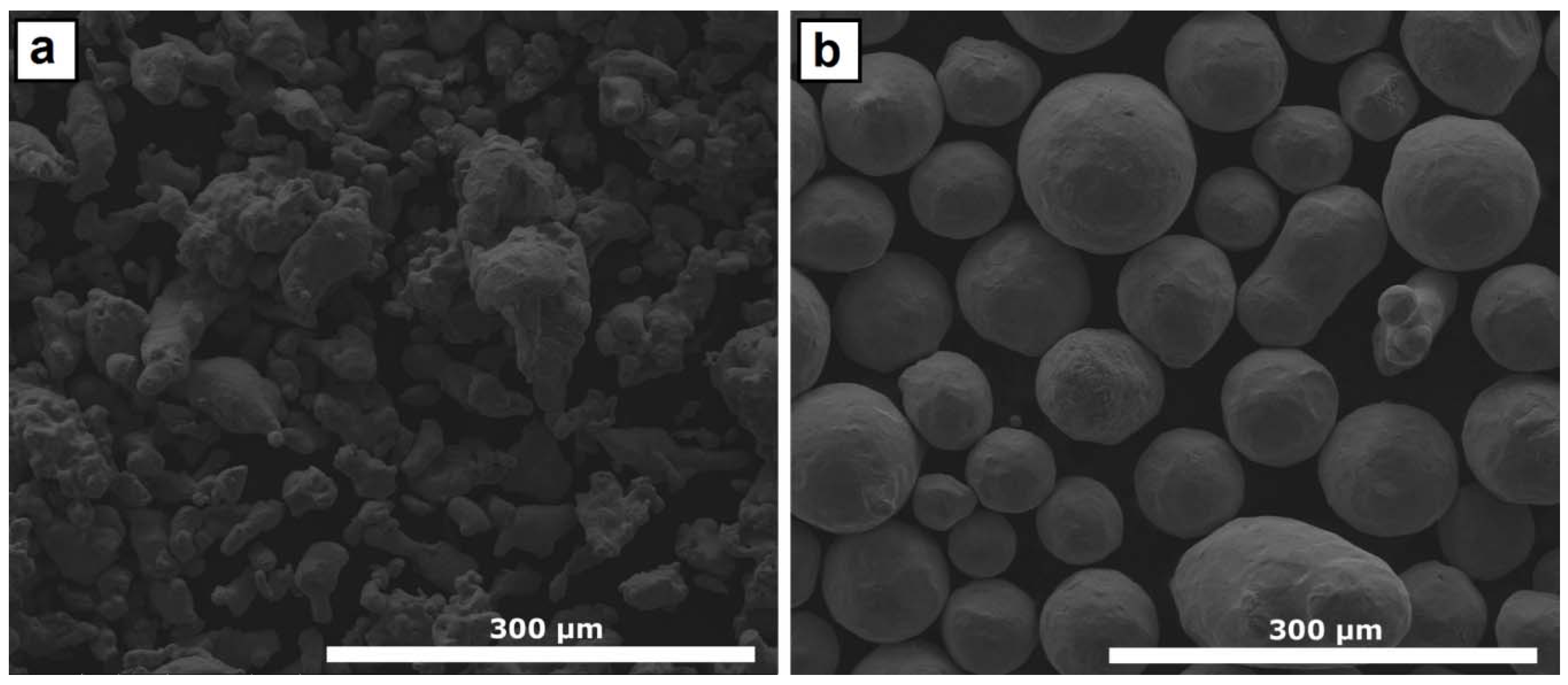
2.1. Materials
2.2. Laser Cladding
2.3. Characterization
3. Results
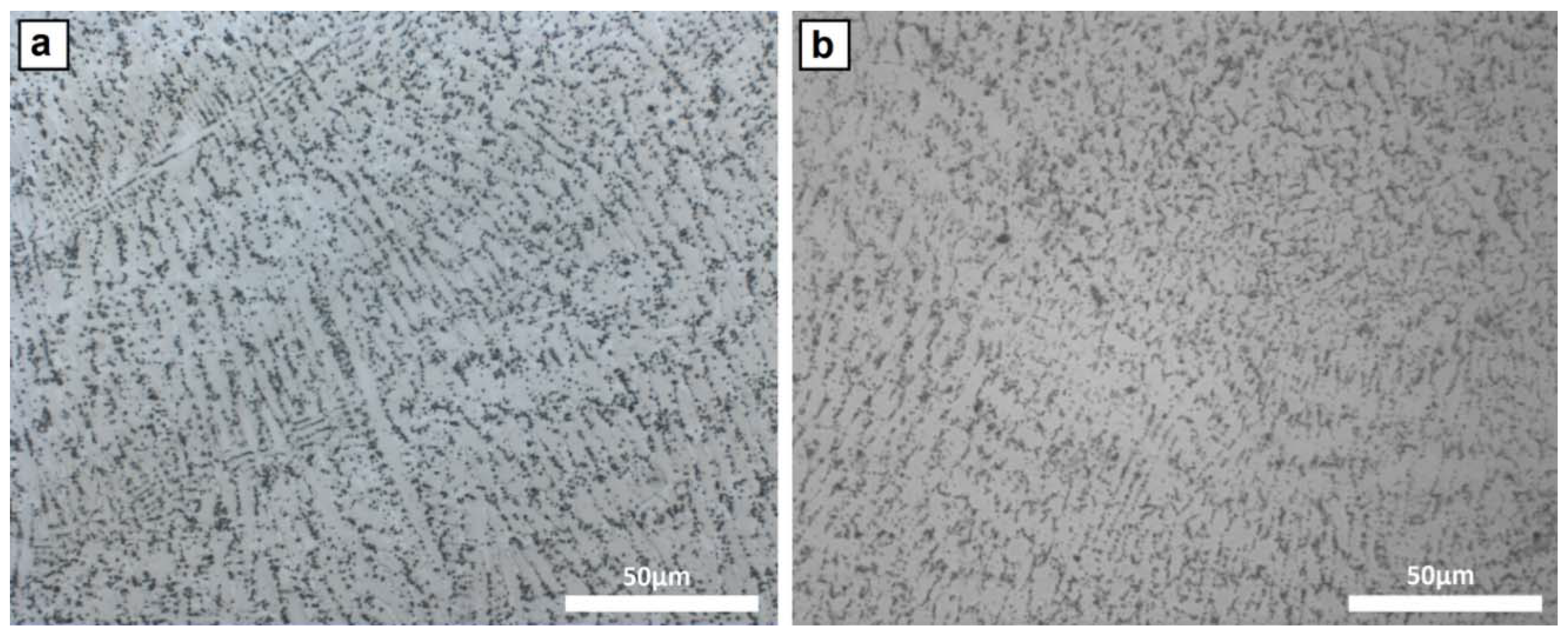
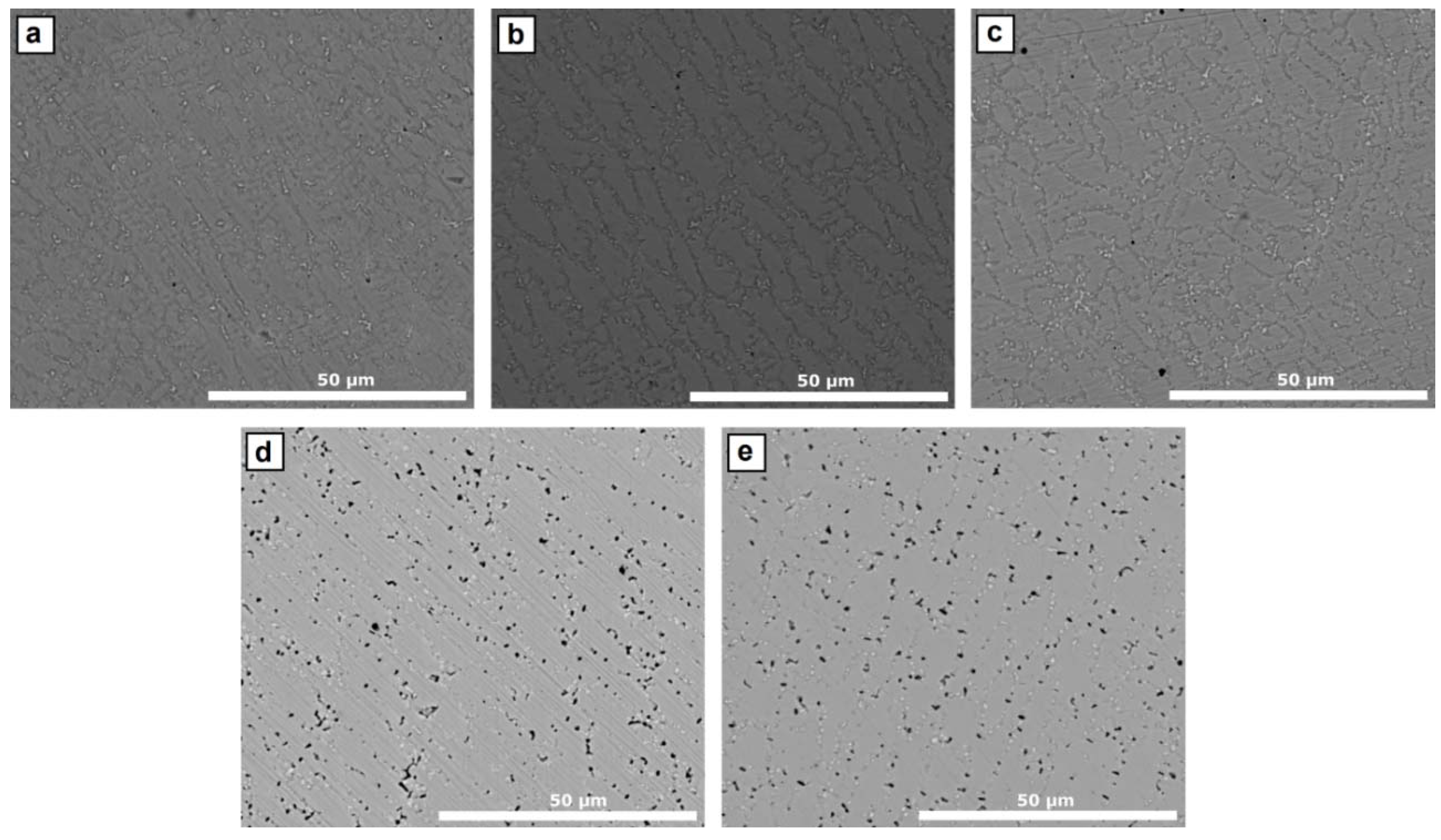
3.1. Microstructure
3.2. Hardness
4. Discussion
4.1. Microstructure
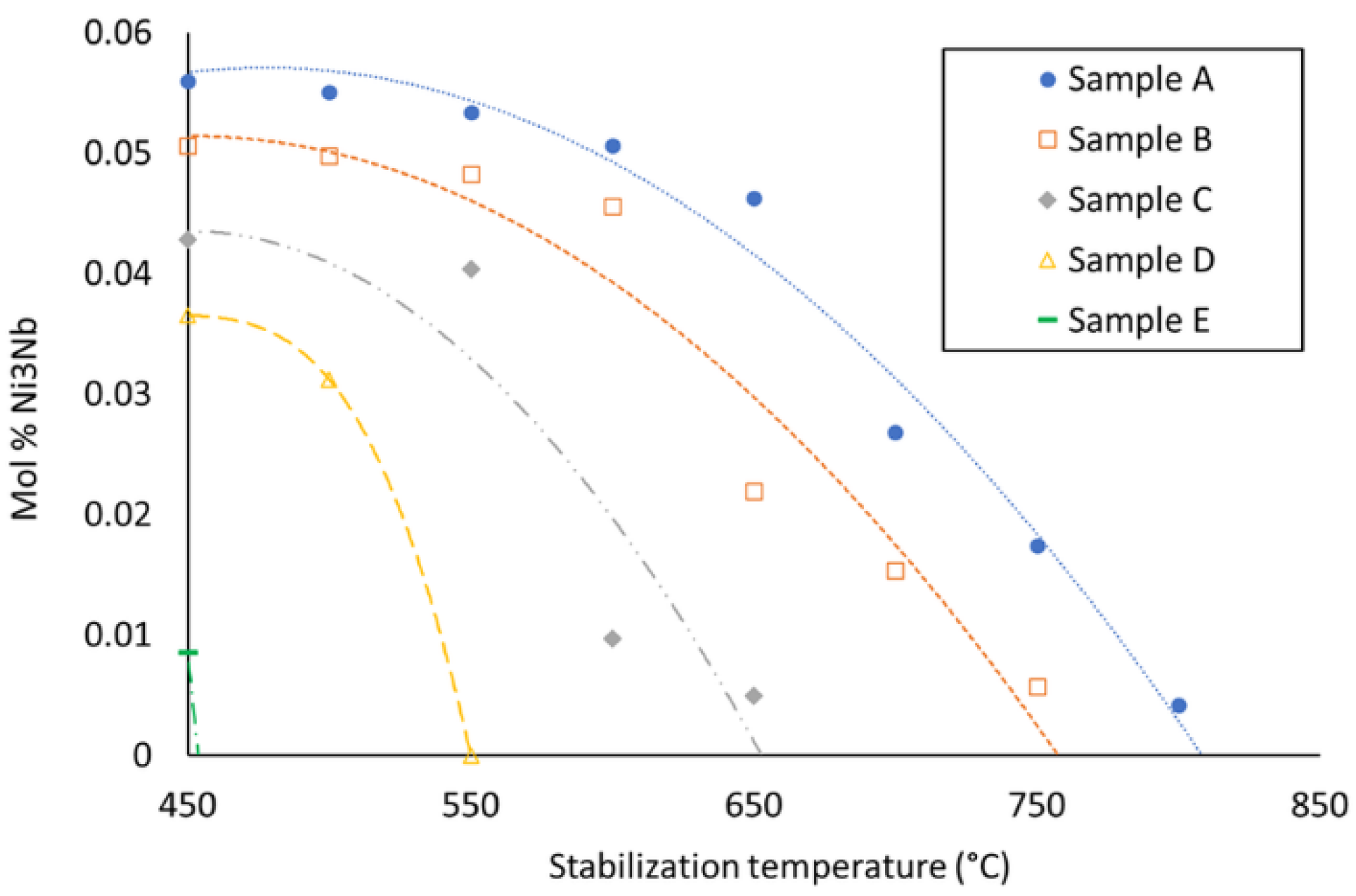
- According to the thermodynamic calculations by Thermo-Calc software (Solna, Sweden) using TCNI8: Ni-alloy v8.1 and MOBNI4: Ni-alloys mobility v4.0 databases/Nickel-based superalloys (TCNI8, MOBNI4) package (Figure 9) part of the Nb is combined with Ni in the submicrometric γ″ phase (Ni3Nb) and the rest precipitates as Laves phases together with Mo.
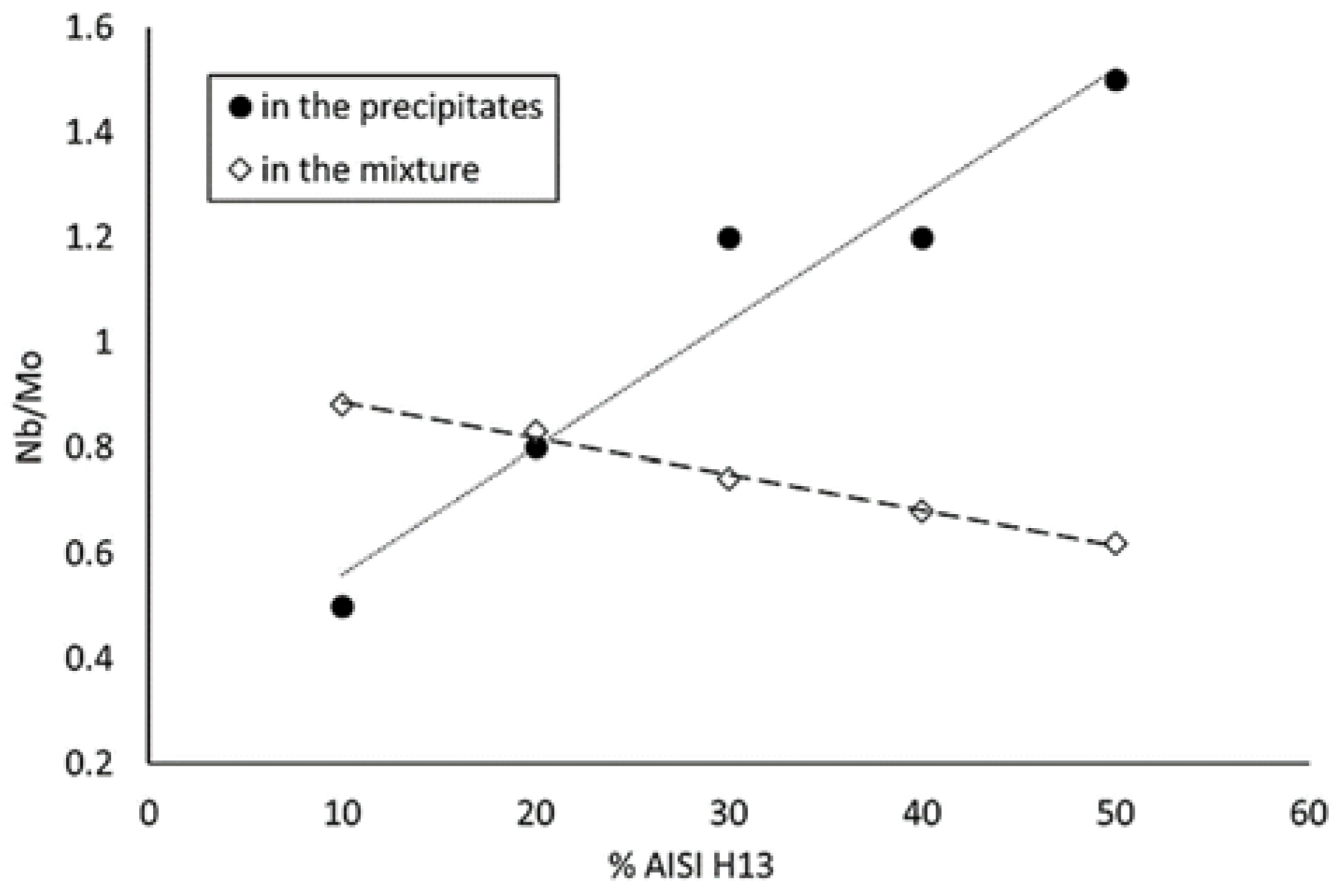
- As the Inconel 625 is enriched in AISI H13, the total amount of Nb + Mo in the mixture decreases.
- On the other hand, the stability of the γ″ phase from the nickel-based alloy is also reduced, thus liberating Nb, which tends to compensate the overall reduction of Nb and Mo in the precipitates.
- The amount of Nb and Mo that is actually available for forming Laves phases is thus approximately equivalent in the samples up to 30% AISI H13, but they become richer in Nb as the amount of AISI H13 increases.
- The mixture with 40 and 50% of AISI H13 steel contain less Laves precipitates, as the Nb liberated from the γ″ phase is no longer enough to compensate the overall decrease of Nb and Mo.
4.2. Hardness
5. Conclusions
- Additions over 10% of AISI H13 steel stabilize the formation of Laves phases that are detrimental for corrosion resistance and mechanical response.
- The volume fraction of the Laves phase remains constant in the range of mixtures from 10% to 30% of AISI H13 steel.
- A mechanism to explain this behavior has been proposed: the Nb and Mo available for Laves phase formation remains nearly constant due to the reduction of the stability of γ″ precipitates.
- The increase in C and Fe caused by the addition of AISI H13 steel in the studied range reduces the hardness of the mixture, meaning that an FCG involving an AISI H13 to Inconel 625 can show a soft transition layer.
- The hardness of the mixtures is reduced by the inhibition of γ″ hardening precipitates produced by steel addition and the inhibition of the alloying strategy of the H13 steel (carbide formation in a martensitic matrix).
Author Contributions
Funding
Conflicts of Interest
References
- Toyserkani, E.; Khajepour, A.; Corbin, S. Laser Cladding; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Leunda, J.; Sanz, C.; Soriano, C. Laser cladding strategies for producing WC reinforced NiCr coatings inside twin barrels. Surf. Coat. Technol. 2016, 307, 720–727. [Google Scholar] [CrossRef]
- Bartkowski, D.; Bartkowska, A. Wear resistance in the soil of Stellite-6/WC coatings produced using laser cladding method. Int. J. Refract. Met. Hard Mater. 2017, 64, 20–26. [Google Scholar] [CrossRef]
- Yakovlev, A.; Trunova, E.; Grevey, D.; Pilloz, M.; Smurov, I. Laser-assisted direct manufacturing of functionally graded 3D objects. Surf. Coat. Technol. 2015, 190, 15–24. [Google Scholar] [CrossRef]
- Ocylok, S.; Weisheit, A.; Kelbassa, I. Functionally graded multi-layers by laser cladding for increased wear and corrosion protection. Physics Proc. 2010, 5, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Shah, K.; Haq, I.; Khan, A.; Shah, S.; Khan, M.; Pinkerton, A. Parametric study of development of Inconel-steel functionally graded materials by laser direct metal deposition. Mater. Design 2014, 54, 531–538. [Google Scholar] [CrossRef]
- Uenishi, K.; Kobayashi, K. Formation of surface layer based on Al3Ti on aluminum by laser cladding and its compatibility with ceramics. Intermetallics 1999, 7, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Liu, W.; Kan, Y.; Zhong, M. Microstructure and wear properties of laser cladding Ti–Al–Fe–B coatings on AA2024 aluminum alloy. Mater. Design. 2006, 27, 405–410. [Google Scholar] [CrossRef]
- Guo, B.; Zhou, J.; Zhang, S.; Zhou, H.; Pu, Y.; Chen, J. Phase composition and tribological properties of Ti–Al coatings produced on pure Ti by laser cladding. Appl. Surf. Sci. 2007, 253, 9301–9310. [Google Scholar] [CrossRef]
- Alemohammad, H.; Esmaeili, S.; Toyserkani, E. Deposition of Co–Ti alloy on mild steel substrate using laser cladding. Mater. Sci. Eng. A-Struct. 2007, 456, 156–161. [Google Scholar] [CrossRef]
- Kar, A.; Mazumder, J. Extended solid solution and nonequilibrium phase diagram for Ni-Al alloy formed during laser cladding. Metall. Trans. A. 1989, 20, 363–371. [Google Scholar] [CrossRef]
- Duraiselvam, J.; Galun, R.; Wesling, V.; Mordike, B.; Reiter, R.; Oligmüller, R. Cavitation erosion resistance of AISI 420 martensitic stainless steel laser-clad with nickel aluminide intermetallic composites and matrix composites with TiC reinforcement. Surf. Coat. Technol. 2006, 201, 1289–1295. [Google Scholar] [CrossRef]
- Day, J.; Huang, X.; Yao, M. Study on Composition-Induced Microstructural Variation in the Interface Between Co-Based Hardfacing Alloys and IN738 Ni-Based Superalloy. J. Mater. Eng. Perform. 2004, 13, 158–166. [Google Scholar] [CrossRef]
- Corbin, S.; Toyserkani, E.; Khajepour, A. Cladding of an Fe-aluminide coating on mild steel using pulsed laser assisted powder deposition. Mater. Sci. Eng. A-Struct. 2003, 354, 48–57. [Google Scholar] [CrossRef]
- Choi, J.; Choudhuri, S.; Mazumder, J. Role of preheating and specific energy input on the evolution of microstructure and wear properties of laser clad Fe-Cr-C-W alloys. J. Mater. Sci. 2000, 35, 3213–3219. [Google Scholar] [CrossRef]
- Pogson, S.; Fox, P.; Oneill, W.; Sutcliffe, C. The direct metal laser remelting of copper and tool steel powders. Mater. Sci. Eng. A-Struct. 2004, 386, 453–459. [Google Scholar] [CrossRef]
- Beal, V.; Erasenthiran, P.; Hopkinson, N.; Dickens, P.; Ahrens, C. Optimisation of processing parameters in laser fused H13/Cu materials using response surface method (RSM). J. Mater. Process. Technol. 2006, 174, 145–154. [Google Scholar] [CrossRef]
- Jeng, S.L.; Chang, Y.H. Microstructure and flow behavior of Ni–Cr–Fe welds with Nb and Mo additions. Mater. Sci. Eng. A 2013, 560, 343–350. [Google Scholar] [CrossRef]
- Dinda, G.; Dasgupta, A.; Mazumder, J. Laser aided direct metal deposition of Inconel 625 superalloy: Microstructural evolution and thermal stability. Mater. Sci. Eng. A-Struct. 2009, 509, 98–104. [Google Scholar] [CrossRef]
- Verdi, D.; Garrido, M.; Múnez, C.; Poza, P. Microscale evaluation of laser cladded Inconel 625 exposed at high temperature in air. Mater. Design. 2017, 114, 326–338. [Google Scholar] [CrossRef]
- Silva, C.; Miranda, H.; Motta, M.; Farias, J.; Afonso, C.; Ramirez, A. New insight on the solidification path of an alloy 625 weld overlay. J. Mater. Res. Technol. 2013, 2, 228–237. [Google Scholar] [CrossRef] [Green Version]

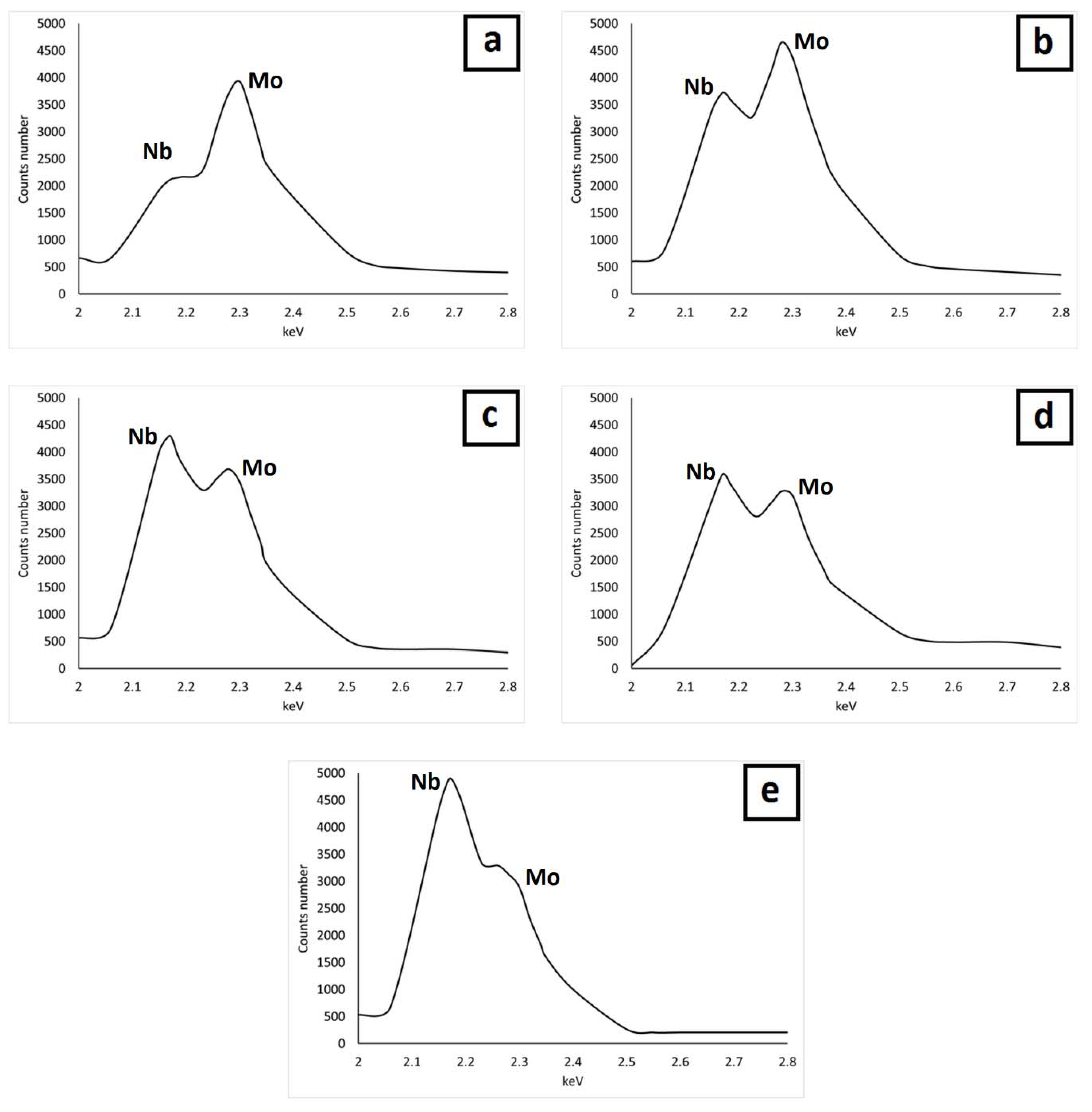

| Material | % C | % Si | % Mn | % Cr | % Mo | % Nb | % V | % Fe | % Ni |
|---|---|---|---|---|---|---|---|---|---|
| AISI H13 | 0.40 | 0.90 | 0.21 | 5.20 | 1.47 | - | 0.98 | Bal. | 0.12 |
| Inconel 625 | 0.027 | - | 0.45 | 21.3 | 2.73 | 2.6 | - | 11.00 | Bal. |
| Measurement uncertainty KI = 2 | ±0.01 | ±0.04 | ±0.01 | ±0.01 | ±0.05 | ±0.001 | ±0.004 | ±0.01 | ±0.01 |
| Process Parameters | |
|---|---|
| Power (kW) | 1.5 |
| Scanning speed (mm min−1) | 900 |
| Spot diameter (mm) | 2.7 |
| Powder feed rate (g min−1) | 20 |
| Overlap distance (mm) | 1.0 |
| Shielding gas | Ar |
| Shielding gas flow (l min−1) | 20 |
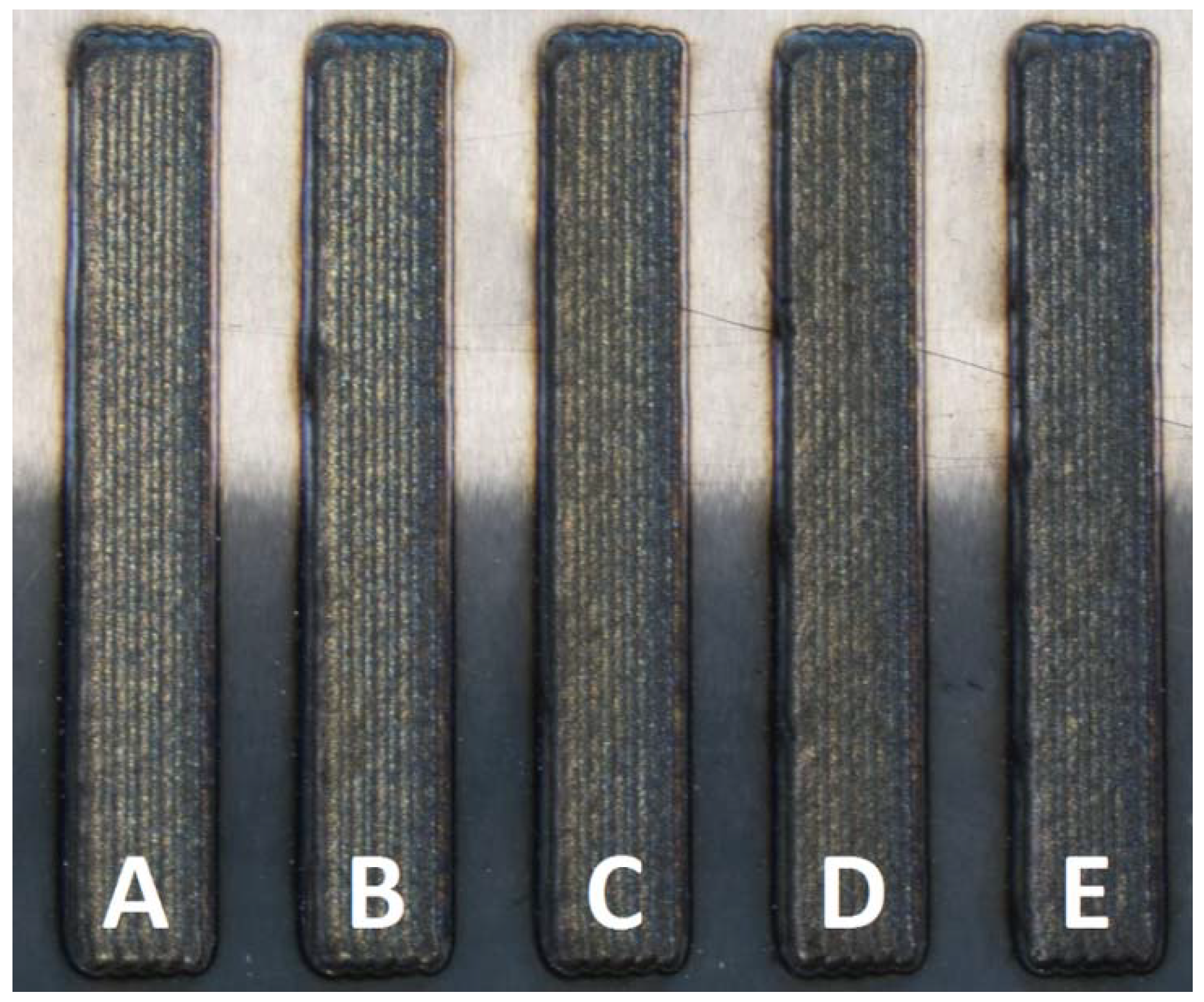
| Sample | %AISI H13 | %C | %Si | %Mn | %Cr | %Mo | %Nb | %V | %Fe | %Ni |
|---|---|---|---|---|---|---|---|---|---|---|
| A theoretical | 10 | 0.06 | 0.09 | 0.43 | 19.7 | 2.60 | 2.34 | 0.10 | 19.0 | 55.7 |
| A | 0.06 | 0.09 | 0.44 | 19.6 | 2.61 | 2.30 | 0.09 | 18.8 | 56.0 | |
| B theoretical | 20 | 0.10 | 0.18 | 0.40 | 18.1 | 2.48 | 2.08 | 0.20 | 26.9 | 49.54 |
| B | 0.09 | 0.20 | 0.39 | 18.2 | 2.52 | 2.09 | 0.18 | 26.7 | 49.6 | |
| B theoretical | 30 | 0.14 | 0.27 | 0.38 | 16.5 | 2.35 | 1.82 | 0.29 | 34.9 | 43.3 |
| C | 0.13 | 0.26 | 0.39 | 16.5 | 2.40 | 1.78 | 0.27 | 34.8 | 43.5 | |
| C theoretical | 40 | 0.18 | 0.36 | 0.35 | 14.9 | 2.23 | 1.56 | 0.39 | 42.9 | 37.2 |
| D | 0.17 | 0.35 | 0.35 | 14.6 | 2.25 | 1.53 | 0.36 | 42.6 | 37.8 | |
| D theoretical | 50 | 0.21 | 0.45 | 0.33 | 13.3 | 2.10 | 1.30 | 0.49 | 50.9 | 31.0 |
| E | 0.20 | 0.44 | 0.34 | 13.3 | 2.15 | 1.33 | 0.47 | 50.6 | 31.2 | |
| Measurement uncertainty KI = 2 | ±0.01 | ±0.04 | ±0.01 | ±0.01 | ±0.05 | ±0.001 | ±0.004 | ±0.01 | ±0.01 | |
| Sample | Hardness HV 0.3 | ||
|---|---|---|---|
| Clad Zone | HAZ | Base Metal | |
| A (10% H13) | 290 ± 5.5 | 535 ± 5.5 | 549 ± 5.5 |
| B (20% H13) | 272 ± 5.5 | 505 ± 5.5 | 542 ± 5.5 |
| C (30% H13) | 245 ± 5.5 | 514 ± 5.5 | 555 ± 5.5 |
| D (40% H13) | 241 ± 5.5 | 529 ± 5.5 | 547 ± 5.5 |
| E (50% H13) | 230 ± 7.2 | 527 ± 5.5 | 554 ± 5.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muro, M.; Leunda, J.; Artola, G.; Soriano, C. Microstructural Tuning of a Laser-Cladding Layer by Means of a Mix of Commercial Inconel 625 and AISI H13 Powders. Materials 2019, 12, 544. https://doi.org/10.3390/ma12030544
Muro M, Leunda J, Artola G, Soriano C. Microstructural Tuning of a Laser-Cladding Layer by Means of a Mix of Commercial Inconel 625 and AISI H13 Powders. Materials. 2019; 12(3):544. https://doi.org/10.3390/ma12030544
Chicago/Turabian StyleMuro, Maider, Josu Leunda, Garikoitz Artola, and Carlos Soriano. 2019. "Microstructural Tuning of a Laser-Cladding Layer by Means of a Mix of Commercial Inconel 625 and AISI H13 Powders" Materials 12, no. 3: 544. https://doi.org/10.3390/ma12030544





