Copper/Epoxy Joints in Printed Circuit Boards: Manufacturing and Interfacial Failure Mechanisms
Abstract
:1. Introduction
2. Manufacturing of Copper/Epoxy Joints
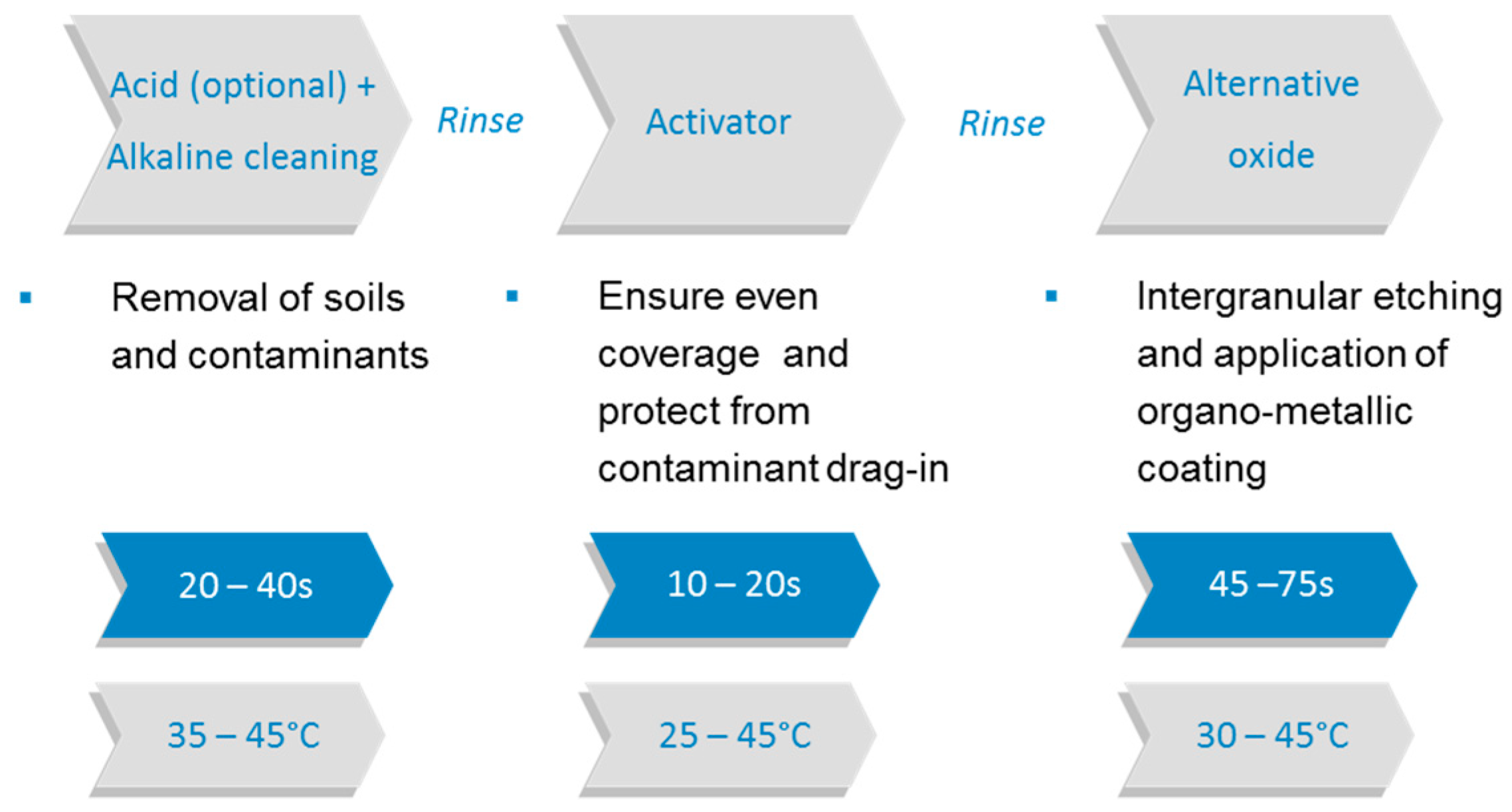
2.1. Etching Technologies
2.1.1. Black Oxide
- oxidizing agent (e.g., sodium chlorite, potassium persulfate),
- sodium hydroxide,
- electrolyte (e.g., trisodium phosphate or sodium sulfite).
2.1.2. Oxide Replacement Technologies
2.2. Chemical Bonding Technologies
2.2.1. Non-Etching Adhesion Promoters
- Sn2+ source (e.g., SnCl2, SnSO4),
- Complexing agent (thiourea),
- Acid (e.g., H2SO4, HCl, methanesulphonate, buffer solution),
- Temperature (30–60 °C),
- Optional: additives (e.g., reduction agent, bath stabilizers).
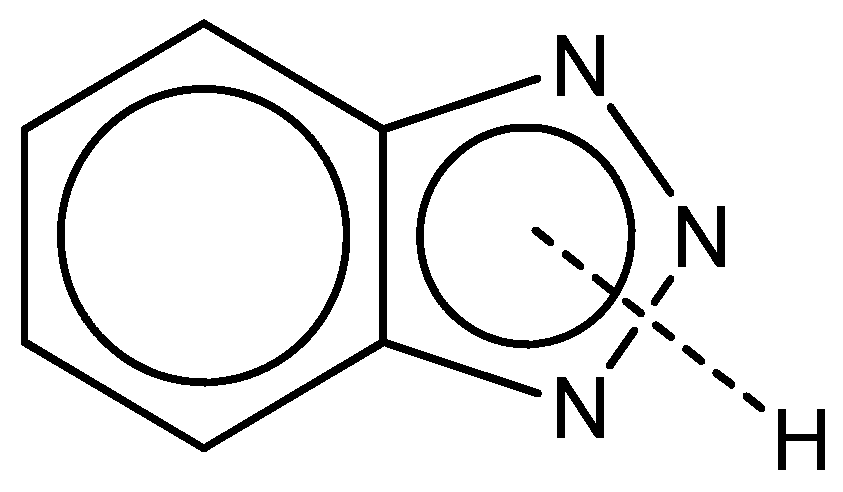
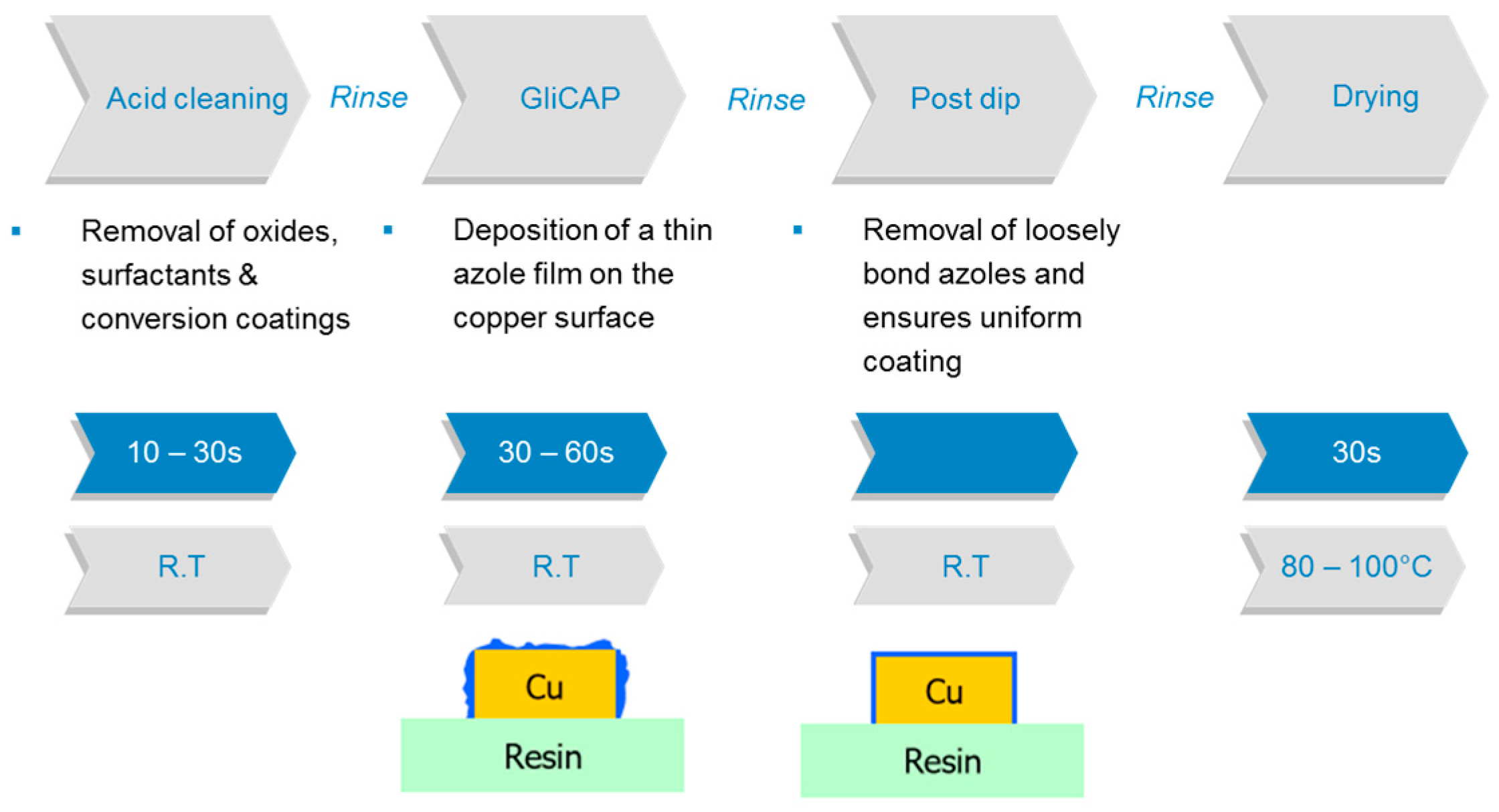
2.2.2. Azole Derivatives
3. Investigations on Interfacial Adhesion Failure
3.1. Oxidative Degradation of Polymers
3.2. Weak Boundary Layer
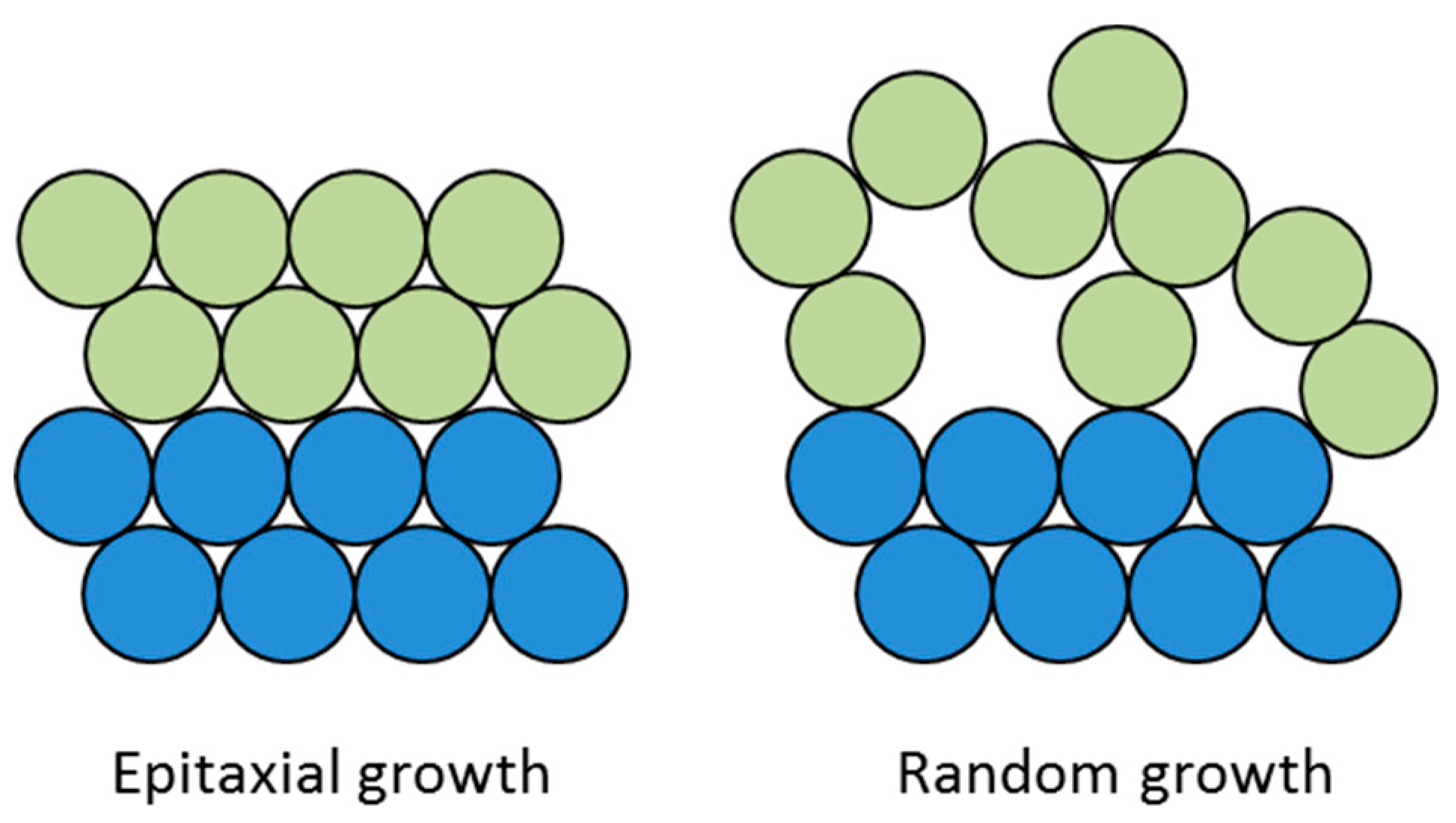
3.3. Mismatches at the Cu/Cu2O/CuO Interfaces
- temperature,
- environmental conditions,
- heating duration,
- surface impurities or contaminants and the surface finish of the metal.
3.4. Surface Tension, Wettability and Moisture Uptake
3.5. Differences in Coefficient of Thermal Expansion
3.6. Microvoid Formation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPC. World PCB Production Report for the Year 2016. Available online: www.ipc.org (accessed on 24 January 2019).
- Garimella, S.V.; Fleischer, A.S.; Murthy, J.Y.; Keshavarzi, A.; Prasher, R.; Patel, C.; Bhavnani, S.H.; Venkatasubramanian, R.; Mahajan, R.; Joshi, Y.; et al. Thermal challenges in next-generation electronic systems. IEEE Trans. Compon. Packag. Technol. 2008, 31, 801–815. [Google Scholar] [CrossRef]
- Uehara, K.; Sakurai, M. Bonding strength of adhesives and surface roughness of joined parts. J. Mater. Process. Technol. 2002, 127, 178–181. [Google Scholar] [CrossRef]
- Sekercioglu, T.; Rende, H.; Gulsoz, A.; Meran, C. The effect of surface roughness on strength of adhesively bonded cylindrical components. J. Mater. Process. Technol. 2003, 142, 82–86. [Google Scholar] [CrossRef]
- Shahid, M.; Hashim, S.A. Effect of surface roughness on the strength of cleavage joints. Int. J. Adhes. Adhes. 2002, 22, 235–244. [Google Scholar] [CrossRef]
- Tews, D.; Hülsmann, T.; Atotech Deutschland GmBH, Berlin, Germany. Discussion about Bondfilm. Personal Communication, 2015. [Google Scholar]
- Kinloch, A.J. The science of adhesion—Part 1 surface and interfacial aspects. J. Mater. Sci. 1980, 15, 2141–2166. [Google Scholar] [CrossRef]
- Massey, R.; Zee, A. Use of non-etching adhesion promoters in advanced PCB applications. In Proceedings of the 3rd Electronics System Integration Technology Conference ESTC, Berlin, Germany, 13–16 September 2010. [Google Scholar]
- Lebbai, M.; Kim, J.-K.; Szeto, W.K. Surface characteristics and adhesion performance of black oxide coated copper substrates with epoxy resins. J. Adhes. Sci. Technol. 2003, 17, 1543–1560. [Google Scholar] [CrossRef]
- Evans, J.R.G.; Packham, D.E. Adhesion of polyethylene to metals: The role of surface topography. J. Adhes. 1979, 10, 177–191. [Google Scholar] [CrossRef]
- Evans, J.R.G.; Packham, D.E. Adhesion of polyethylene to copper: Reactions between copper oxides and the polymer. J. Adhes. 1978, 9, 267–277. [Google Scholar] [CrossRef]
- Yun, H.K.; Cho, K.; An, J.H.; Park, C.E. Adhesion improvement of copper/epoxy joints. J. Mater. Sci. 1992, 27, 5811–5817. [Google Scholar] [CrossRef]
- Love, B.J.; Packman, P.F. Effects of surface modifications on the peel strength of copper-based polymer-metal interfaces with characteristic morphologies. J. Adhesi. 1993, 40, 139–150. [Google Scholar] [CrossRef]
- Lee, H.; Yu, J. Effects of oxidation treatments on the fracture toughness of lead frame/epoxy interfaces. Mater. Sci. Eng. A 2000, 277, 154–160. [Google Scholar] [CrossRef]
- Hine, P.J.; Muddarris, S.E.; Packham, D.E. Adhesion of microfibrous surfaces on steel and copper to epoxy resins. J. Adhes. Sci. Technol. 1987, 1, 69–78. [Google Scholar] [CrossRef]
- Webster, H.F.; Wightman, J.P.; Johnson, T.W. Analysis of the molecular structure at the PPS/copper interphase and its role in adhesion. J. Ahes. 1995, 53, 229–244. [Google Scholar] [CrossRef]
- Brooks, P.; Fuerhaupter, H.; Hechler, J.; Day, B.; Yang, H. High Performance Multilayer Bonding Systems. Available online: https://www.researchgate.net/publication/237329847 (accessed on 24 July 2017).
- Lee, H.; Yu, J. Measurements of the adhesion strength Cu/epoxy interfaces. Mater. Res. Soc. Symp. Proc. 2000, 586, 213–218. [Google Scholar] [CrossRef]
- Wetherhold, R.C.; Harry, Z. A rapid chemical method for improving peel strength at Cu-epoxy interfaces. Theor. Appl. Fract. Mech. 2010, 53, 42–46. [Google Scholar] [CrossRef]
- Hong, S.; Wang, T.; Hong, C. Investigation of primers reducing the pink-ring formation in multilayer printed circuit boards. Macromol. Chem. Phys. 1995, 231, 91–108. [Google Scholar]
- Xue, G.; Wu, P.; Dai, Q.; Cheng, R. The coupling mechanism of benzotriazole pretreated copper metal and epoxy-resin. Angew. Chem. Int. Ed. 1991, 188, 51–61. [Google Scholar]
- Ding, J.; Chen, C.; Xue, G. The dynamic mechanical analysis of epoxy copper-powder composites using azole compounds as coupling agents. J. Appl. Polym. Sci. 1991, 42, 1459–1464. [Google Scholar] [CrossRef]
- Tromans, D.; Sun, R. Anodic polarization behavior of copper in aqueous chloride/benzotriazole solutions. J. Electrochem. Soc. 1991, 138, 3235–3244. [Google Scholar] [CrossRef]
- Dugdale, I.; Cotton, J.B. An electrochemical investigation on the prevention of staining of copper by benzotriazole. Corros. Sci. 1963, 3, 69–74. [Google Scholar] [CrossRef]
- Polewska, W.; Vogt, M.R.; Magnussen, O.M.; Behm, R.J. In situ STM study of Cu(111) surface structure and corrosion in pure and benzotriazole-containing sulfuric acid solution. J. Phys. Chem. B 1999, 103, 10440–10451. [Google Scholar] [CrossRef]
- Polo, J.L.; Pinilla, P.; Cano, E.; Bastidas, J.M. Triphenylmethane compounds as copper corrosion inhibitors in hydrochloric acid solution. Corrosion 2003, 59, 414–423. [Google Scholar] [CrossRef]
- Allam, N.K.; Hegazy, H.S.; Ashour, E.A. Adsorption–Desorption kinetics of benzotriazole on cathodically polarized copper. J. Electrochem. Soc. 2010, 157, C174–C177. [Google Scholar] [CrossRef]
- Ashour, E.A.; Hegazy, H.S.; Ateya, B.G. Effect of blends of benzotriazole and thiourea on the anodic dissolution of copper. Adsorpt. Sci. Technol. 2002, 20, 485–494. [Google Scholar] [CrossRef]
- Allam, N.K.; Nazeer, A.A.; Ashour, E.A. A review of the effects of benzotriazole on the corrosion of copper and copper alloys in clean and polluted environments. J. Appl. Electrochem. 2009, 39, 961–969. [Google Scholar] [CrossRef]
- Antonijevic, M.M.; Petrovic, M.B. Copper corrosion inhibitors. A review. Int. J. Electrochem. Sci. 2008, 3, 1–28. [Google Scholar]
- Modestov, A.D.; Zhou, G.D.; Wu, Y.P.; Notoya, T.; Schweinsberg, D.P. A study of the electrochemical formation of Cu(I)-BTA films on copper electrodes and the mechanism of copper corrosion inhibition in aqueous chloride/benzotriazole solutions. Corros. Sci. 1994, 36, 1931–1946. [Google Scholar] [CrossRef]
- Youda, R.; Nishihara, H.; Aramaki, K. SERS and impedance study of the equilibrium between complex formation and adsorption of benzotriazole and 4-hydroxybenzotriazole on a copper electrode in sulphate solutions. Electrochim. Acta 1990, 35, 1011–1017. [Google Scholar] [CrossRef]
- Roberts, R. X-ray photoelectron spectroscopic characterization of copper oxide surfaces treated with benzotriazole. J. Electron Spectrosc. Relat. Phen. 1974, 4, 273–291. [Google Scholar] [CrossRef]
- Tews, D.; Hülsmann, T.; Atotech Deutschland GmBH, Berlin, Germany. Introduction into surface treatment technologies. Trainee Program. Personal Communication, 2013. [Google Scholar]
- Bastidas, J.M.; Pinilla, P.; Cano, E.; Polo, J.L.; Miguel, S. Copper corrosion inhibition by triphenylmethane derivatives in sulphuric acid media. Corros. Sci. 2003, 45, 427–449. [Google Scholar] [CrossRef] [Green Version]
- Bartley, J.; Huynh, N.; Bottle, S.E.; Flitt, H.; Notoya, T.; Schweinsberg, D.P. Computer simulation of the corrosion inhibition of copper in acidic solution by alkyl esters of 5-carboxybenzotriazole. Corros. Sci. 2003, 45, 81–96. [Google Scholar] [CrossRef]
- Frignani, A.; Tommesani, L.; Brunoro, G.; Monticelli, C.; Fogagnolo, M. Influence of the alkyl chain on the protective effects of 1,2,3-benzotriazole towards copper corrosion.: Part I: Inhibition of the anodic and cathodic reactions. Corros. Sci. 1999, 41, 1205–1215. [Google Scholar] [CrossRef]
- Bolger, J.C.; Molvar, H.E. The chemical composition of metal and oxide surfaces and how these interact with polymeric materials. Soc. Plast. Eng. Tech. Pap. 1972, 18, 408. [Google Scholar]
- Park, J.M.; Bell, J.P. Epoxy adhesion to copper. In Adhesion Aspects of Polymeric Coatings; Mittal, K.L., Ed.; Springer: Boston, MA, USA, 1983; pp. 205–224. [Google Scholar]
- Villamil, R.F.V.; Cordeiro, G.G.O.; Matos, J.; D’Elia, E.; Agostinho, S.M.L. Effect of sodium dodecylsulfate and benzotriazole on the interfacial behavior of Cu/Cu(II), H2SO4. Mater. Chem. Phys. 2003, 78, 448–452. [Google Scholar] [CrossRef]
- Zee, A.; Massey, R. Advantage of non-etching adhesion promoter on high frequency signal loss. In Proceedings of the Microsystems Packaging Assembly and Circuits Technology Conference (IMPACT), Taipei, Taiwan, 20–22 October 2010. [Google Scholar]
- Brooks, D. Skin Effect. Printed Circuit Design and Manufacturing. Available online: http://www.ultracad.com (accessed on 8 April 2018).
- Quality, P.; The, F.; Age, D. Electrical Losses due to Skin Effect and Proximity Effect. Available online: ep2000.com/uploads/EP_WhitePaper_SkinEffect.pdf (accessed on 6 June 2017).
- Lee, L.-H. Fundamentals of Adhesion; Springer: Boston, MA, USA, 1991. [Google Scholar]
- Molenaar, A.; de Bakker, J.W.G. Autocatalytic deposition of tin. J. Electrochem. Soc. 1989, 136, 378–382. [Google Scholar] [CrossRef]
- Huttunen-Saarivirta, E.; Tiainen, T. Autocatalytic tin plating in the fabrication of tin-coated copper tube. J. Mater. Process. Technol. 2005, 170, 211–219. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Wang, W.; Naotoshi, M.; Chen, Z. Sustained immersion tin deposition on copper from choline chloride based aqueous solution without reducing agent. J. Electrochem. Soc. 2013, 160, D295–D299. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Shi, T. Investigation of deposition mechanism and characteristics of electroless Sn plating. Adv. Mater. Res. 2010, 139–141, 410–413. [Google Scholar] [CrossRef]
- Zhao, J.; Li, N.; Cui, G.; Zhao, J. Study on immersion tin process by electrochemical methods and molecular orbital theory. J. Electrochem. Soc. 2006, 153, C848–C853. [Google Scholar] [CrossRef]
- Huttunen-Saarivirta, E. Observations on the uniformity of immersion tin coatings on copper. Surf. Coat. Technol. 2002, 160, 288–294. [Google Scholar] [CrossRef]
- Lee, B.-Z.; Lee, D.N. Spontaneous growth mechanism of tin whiskers. Acta Mater. 1998, 46, 3701–3714. [Google Scholar] [CrossRef]
- Schetty, R. Minimization of tin whisker formation for lead-free electronics finishing. Circuit World 2001, 27, 17–20. [Google Scholar] [CrossRef]
- Shozo, M.; Takayuki, M.; Naoto, O.; Miya, T.; Masato, K.; Noriaki, Y. Azole Silane Compound, Surface Treatment Solution, Surface Treatment Method, and Use Thereof. WO Patent 2015002158 (A1), 8 January 2015. [Google Scholar]
- Deflorian, F.; Rossi, S.; Fedrizzi, L.; Fedel, M. Integrated electrochemical approach for the investigation of silane pre-treatments for painting copper. Prog. Org. Coat. 2008, 63, 338–344. [Google Scholar] [CrossRef]
- Brooks, P.; Kumashiro, S.; Terauchi, K. Novel approach for a non-etching adhesion promoter for the next generation of IC substrates. In Proceedings of the Microsystems, Packaging, Assembly and Circuits Technology, Taipei, Taiwan, 1–3 October 2007. [Google Scholar]
- Nothdurft, P.; Feldbacher, S.; Jakopic, G.; Mühlbacher, I.; Pötz, S.; Kern, W. Surface characterization of copper substrates modified with carboxyl terminated phosphonic acids. Int. J. Adhes. Adhes. 2018, 84, 143–152. [Google Scholar] [CrossRef]
- Cabrita, J.F.; Viana, A.S.; Abrantes, L.M. Copper protection by phosphonic acid self-assembled monolayers. Corros. Prot. Mater. 2010, 29, 114–119. [Google Scholar]
- Kane, J.G.; Laibinis, P.E. Corrosion inhibition using self-assembled monolayers of alkanethiols on copper. In Organic Coatings for Corrosion Control; American Chemical Society: Washington, DC, USA, 1998; pp. 33–409. [Google Scholar]
- Caipa Campos, M.A.; Trilling, A.K.; Yang, M.; Giesbers, M.; Beekwilder, J.; Paulusse, J.M.J.; Zuilhof, H. Self-assembled functional organic monolayers on oxide-free copper. Langmuir 2011, 27, 8126–8133. [Google Scholar] [CrossRef] [PubMed]
- Antonijević, M.M.; Milić, S.M.; Petrović, M.B. Films formed on copper surface in chloride media in the presence of azoles. Corros. Sci. 2009, 51, 1228–1237. [Google Scholar] [CrossRef]
- Lalitha, A.; Ramesh, S.; Rajeswari, S. Surface protection of copper in acid medium by azoles and surfactants. Electrochim. Acta 2005, 51, 47–55. [Google Scholar] [CrossRef]
- Kokalj, A.; Kovačević, N.; Peljhan, S.; Finšgar, M.; Lesar, A.; Milošev, I. Triazole, benzotriazole, and naphthotriazole as copper corrosion inhibitors: I. Molecular electronic and adsorption properties. ChemPhysChem 2011, 12, 3547–3555. [Google Scholar] [CrossRef]
- Bai, S.Q.; Young, D.J.; Hor, T.S.A. Nitrogen-rich azoles as ligand spacers in coordination polymers. Chem. Asian J. 2011, 6, 292–304. [Google Scholar] [CrossRef]
- Chem, S. Chemical Adhesion Process GliCAP GC-703. Available online: https://www.shikoku.co.jp (accessed on 4 June 2018).
- Sharpe, L.H. The interphase in adhesion. J. Adhes. 1972, 4, 51–64. [Google Scholar] [CrossRef]
- Chan, M.G.; Allara, D.L. Infrared reflection studies of metal-polymer interfaces. Polym. Eng. Sci. 1974, 14, 12–15. [Google Scholar] [CrossRef]
- Kelley, K.; Ishino, Y.; Ishida, H. Fourier transform IR reflection techniques for characterization of polyimide films on copper substrates. Thin Solid Films 1987, 154, 271–279. [Google Scholar] [CrossRef]
- Inagaki, N.; Tasaka, S.; Ohmori, H.; Mibu, S. Improvement in the adhesion between copper metal and polyimide substrate by plasma polymer deposition of cyano compounds onto polyimide. J. Adhes. Sci. Technol. 1996, 10, 243–256. [Google Scholar] [CrossRef]
- Burrell, M.C.; Codella, P.J.; Fontana, J.A.; Chera, J.J. Interfacial reactions at copper surfaces coated with polymer films. J. Vac. Sci. Technol. 1989, 7, 1778–1783. [Google Scholar] [CrossRef]
- McElhaney, R.D.; Castner, D.G.; Ratner, B.D. Characterization of the poly(ether ether ketone)—Copper interface. Met. Poly. Am. Chem. Soc. 1990, 440, 370–378. [Google Scholar]
- Allara, D.L.; White, C.W.; Meek, R.L.; Briggs, T.H. Mechanism of oxidation at a copper–polyethylene interface. II. Penetration of copper ions in the polyethylene matrix. J. Polym. Sci. Polym. Chem. Ed. 1976, 14, 93–104. [Google Scholar] [CrossRef]
- Sánchez, G.; Brito, Z.; Mujica, V.; Perdomo, G. The thermal behaviour of cured epoxy-resins. The influence of metallic fillers. Polym. Degrad. Stab. 1993, 40, 109–114. [Google Scholar] [CrossRef]
- Allara, D.L.; Roberts, R.P. Catalysis-inhibition effects of oxidized copper surfaces in the autoxidation of hexadecane. J. Catal. 1976, 45, 54–67. [Google Scholar] [CrossRef]
- Yoshida, S.; Ishida, H. A FT-IR reflection-absorption spectroscopic study of an epoxy coating on imidazole-treated copper. J. Adhes. 1984, 16, 217–232. [Google Scholar] [CrossRef]
- Patrick, R.L. Interface conversion for polymer coatings. Polym. Lett. 1970, 8, 309–311. [Google Scholar] [CrossRef]
- Racich, J.L.; Koutsky, J.A. Boundary layers in thermosets. In Chemistry and Properties of Crosslinked Polymers; Labana, S.S., Ed.; Elsevier: Amsterdam, The Netherlands, 1977; pp. 303–323. [Google Scholar]
- Chung, J.; Munz, M.; Sturm, H. Amine-cured epoxy surface morphology and interphase with copper: An approach employing electron beam lithography and scanning force microscopy. J. Adhes. Sci. Technol. 2005, 19, 1263–1276. [Google Scholar] [CrossRef]
- Van Ooij, W.J. Metal-polymer interfaces. In Industrial Adhesion Problems; Brewis, D.M., Briggs, D., Eds.; Wiley: New York, NY, USA, 1985; p. 90. [Google Scholar]
- Brockmann, W. A rapid durability test method for adhesives. In Durability of Structural Adhesives; Kinloch, A.J., Ed.; Springer: London, UK, 1983. [Google Scholar]
- Miller, C.W.; Laberge, P.C. Surface characterization of the copper–epoxy adhesion interface from production printed circuit boards. J. Vac. Sci. Technol. A Vac. Surf. Film. 1989, 7, 1818–1822. [Google Scholar] [CrossRef]
- Boerio, F.J.; Hong, P.P. Non-destructive characterization of epoxy-dicyandiamide interphases using surface-enhanced Raman scattering. Mater. Sci. Eng. A 1990, 126, 245–252. [Google Scholar] [CrossRef]
- Hong, S.; Wang, T. Effect of copper oxides on the thermal oxidative degradation of the epoxy resin. J. Appl. Polym. Sci. 1994, 52, 1339–1351. [Google Scholar] [CrossRef]
- Hong, S.; Wang, T. The effect of copper oxides on the curing of brominated epoxy resins. Thermochim. Acta 1994, 237, 305–316. [Google Scholar] [CrossRef]
- Bikerman, J.J. Causes of poor adhesion: weak boundary layers. Ind. Eng. Chem. 1967, 59, 40–44. [Google Scholar] [CrossRef]
- Lee, S.-K.; Hsu, H.-C.; Tuan, W.-H. Oxidation behavior of copper at a temperature below 300 °C and the methodology for passivation. Mater. Res. 2016, 19, 51–56. [Google Scholar] [CrossRef]
- Cathcart, J.V. High-temperature oxidation of metals. Science 1967, 157, 415. [Google Scholar] [CrossRef]
- Cho, S.J.; Paik, K.W.; Kim, Y.G. The effect of the oxidation of Cu-base leadframe on the interface adhesion between Cu metal and epoxy molding compound. IEEE Trans. Compon. Packag. Manuf. Technol. Part B 1997, 20, 167–175. [Google Scholar]
- Kim, S. The role of plastic package adhesion in performance. IEEE Trans. Compon. Hybrids Manuf. Technol. 1991, 14, 809–817. [Google Scholar] [CrossRef]
- Yoshioka, O.; Okabe, N.; Nagayama, S.; Yamagishi, R. Improvement of moisture resistance in plastic encapsulants MOS-IC by surface finishing copper leadframe. In Proceedings of the 39th Electronic Components Conference, Houston, TX, USA, 22–24 May 1989. [Google Scholar]
- Ohsuga, H.; Suzuki, H.; Aihara, T.; Hamano, T. Development of molding compounds suited for copper leadframes. In Proceedings of the 44th Electronic Components and Technology Conference, Washington, DC, USA, 1–4 May 1994. [Google Scholar]
- Cho, K.; Cho, E.C. Effect of the microstructure of copper oxide on the adhesion behavior of epoxy/copper leadframe joints. J. Adhes. Sci. Technol. 2000, 14, 1333–1353. [Google Scholar] [CrossRef]
- Lawless, K.R.; Gwathmey, A.T. The structure of oxide films on different faces of a single crystal of copper. Acta Metall. 1956, 4, 153–163. [Google Scholar] [CrossRef]
- Lebbai, M.; Lam, O.Y.M.; Kim, J.-K.K. Effects of moisture and temperature ageing on reliability of interfacial adhesion with black copper oxide surface. In Proceedings of the 2nd International IEEE Conference on Polymers and Adhesives in Microelectronics and Photonics. POLYTRONIC, Zalaegerszeg, Hungary, 23–26 June 2002. [Google Scholar]
- Kim, J.K.; Woo, R.S.C.; Hung, P.Y.P.; Lebbai, M. Adhesion performance of black oxide coated copper substrates: Effects of moisture sensitivity test. Surf. Coat. Technol. 2006, 201, 320–328. [Google Scholar] [CrossRef]
- Dai, Q.; Lu, Y. Adhesion promotion for epoxy resin/copper and polyimide/copper joints by a two-component coupling system of poly (4-vinylimidazole) and cystamine. Angewandte Makromolekulare Chemie 1995, 227, 121–129. [Google Scholar] [CrossRef]
- Weide-Zaage, K.; Horaud, W.; Frémont, H. Moisture diffusion in printed circuit boards: Measurements and finite-element-simulations. Microelectron. Reliab. 2005, 45, 1662–1667. [Google Scholar] [CrossRef]
- Kitano, M.; Nishimura, A.; Kawai, S.; Nishi, K. Analysis of package cracking during reflow soldering process. In Proceedings of the 26th Annual Proceedings Reliability Physics Symposium, Monterey, CA, USA, 12–14 April 1988. [Google Scholar]
- Cai, X.; Huang, W.; Xu, B.; Kaltenpoth, G.; Cheng, Z. A study of moisture diffusion in plastic packaging. J. Electron. Mater. 2002, 31, 449–455. [Google Scholar] [CrossRef]
- Lebbai, M.; Szeto, W.K.; Kim, J.-K. Optimization of black oxide coating thickness as adhesion promoter for copper substrate. In Proceedings of the International Symposium on Electronic Materials and Packaging, Hong Kong, China, 30 November–2 December 2000; pp. 206–213. [Google Scholar]
- Kinloch, A.J. Adhesion and Adhesives: Science and Technology; Springer: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Adamson, M.J. Thermal expansion and swelling of cured epoxy resin used in graphite/epoxy composite materials. J. Mater. Sci. 1980, 15, 1736–1745. [Google Scholar] [CrossRef]
- Soles, C.L.; Yee, A.F. A discussion of the molecular mechanisms of moisture transport in epoxy resins. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 792–802. [Google Scholar] [CrossRef]
- Tencer, M. Moisture ingress into nonhermetic enclosures and packages. A quasi-steady state model for diffusion and attenuation of ambient humidity variations. In Proceedings of the 44th Electronic Components and Technology Conference, Washington, DC, USA, 1–4 May 1994. [Google Scholar]
- Dudek, R. The ELFNET Book on Failure Mechanisms, Testing Methods, and Quality Issues of Lead-Free Solder Interconnects; Grossmann, G., Zardini, C., Eds.; Springer: London, UK, 2011; pp. 297–303. [Google Scholar]
- Gledhill, R.A.; Kinloch, A.J. Environmental failure of structural adhesive joints. J. Adhes. 1974, 6, 315–330. [Google Scholar] [CrossRef]
- Rogers, C.E. Permeation of gases and vapours in polymers. In Polymer Permeability; Comyn, J., Ed.; Springer: Amsterdam, The Netherlands, 1985; pp. 11–73. [Google Scholar]
- Shirangi, M.H.; Michel, B. Mechanism of moisture diffusion, hygroscopic swelling, and adhesion degradation in epoxy molding compounds. In Moisture Sensitivity of Plastic Packages of IC Devices; Springer: New York, NY, USA, 2010; pp. 29–69. [Google Scholar]
- Davis, G.D.; Krebs, L.A.; Drzal, L.T.; Rich, M.J.; Askeland, P. Electrochemical sensors for nondestructive evaluation of adhesive bonds. J. Adhes. 2000, 72, 335–358. [Google Scholar] [CrossRef]
- Fan, H.; Chung, P.W.K.; Yuen, M.M.F.; Chan, P.C.H. An energy-based failure criterion for delamination initiation in electronic packaging. J. Adhes. Sci. Technol. 2005, 19, 1375–1386. [Google Scholar] [CrossRef] [Green Version]
- Lupinski, J.H.; Moore, R.S. Polymeric Materials for Electronics Packaging and Interconnection; American Chemical Society: Washington, DC, USA, 1989. [Google Scholar]
- Krivec, T.; AT&S AG, Hinterberg, Austria. Discussion about main interfacial failure mechanism. Personal Communication, 2016. [Google Scholar]
- Chong, C.T.; Leslie, A.; Beng, L.T.; Lee, C. Investigation on the effect of copper leadframe oxidation on package delamination. In Proceedings of the 1995 IEEE 45th Electronic Components & Technology Conference, Las Vegas, NV, USA, 21–24 May 1995; pp. 463–469. [Google Scholar]
- Cho, K.; Cho, E.C.; Park, C.E. Effect of the physical and mechanical properties of epoxy resins on the adhesion behavior of epoxy/copper leadframe joints. J. Adhes. Sci. Technol. 2001, 15, 439–456. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nothdurft, P.; Riess, G.; Kern, W. Copper/Epoxy Joints in Printed Circuit Boards: Manufacturing and Interfacial Failure Mechanisms. Materials 2019, 12, 550. https://doi.org/10.3390/ma12030550
Nothdurft P, Riess G, Kern W. Copper/Epoxy Joints in Printed Circuit Boards: Manufacturing and Interfacial Failure Mechanisms. Materials. 2019; 12(3):550. https://doi.org/10.3390/ma12030550
Chicago/Turabian StyleNothdurft, Philipp, Gisbert Riess, and Wolfgang Kern. 2019. "Copper/Epoxy Joints in Printed Circuit Boards: Manufacturing and Interfacial Failure Mechanisms" Materials 12, no. 3: 550. https://doi.org/10.3390/ma12030550





