Integrated Anode Electrode Composited Cu–Sn Alloy and Separator for Microscale Lithium Ion Batteries
Abstract
:1. Introduction
2. Experiment
2.1. Preparation of the Integrated Electrode
2.2. Characterization
2.3. Electrochemical Measurements
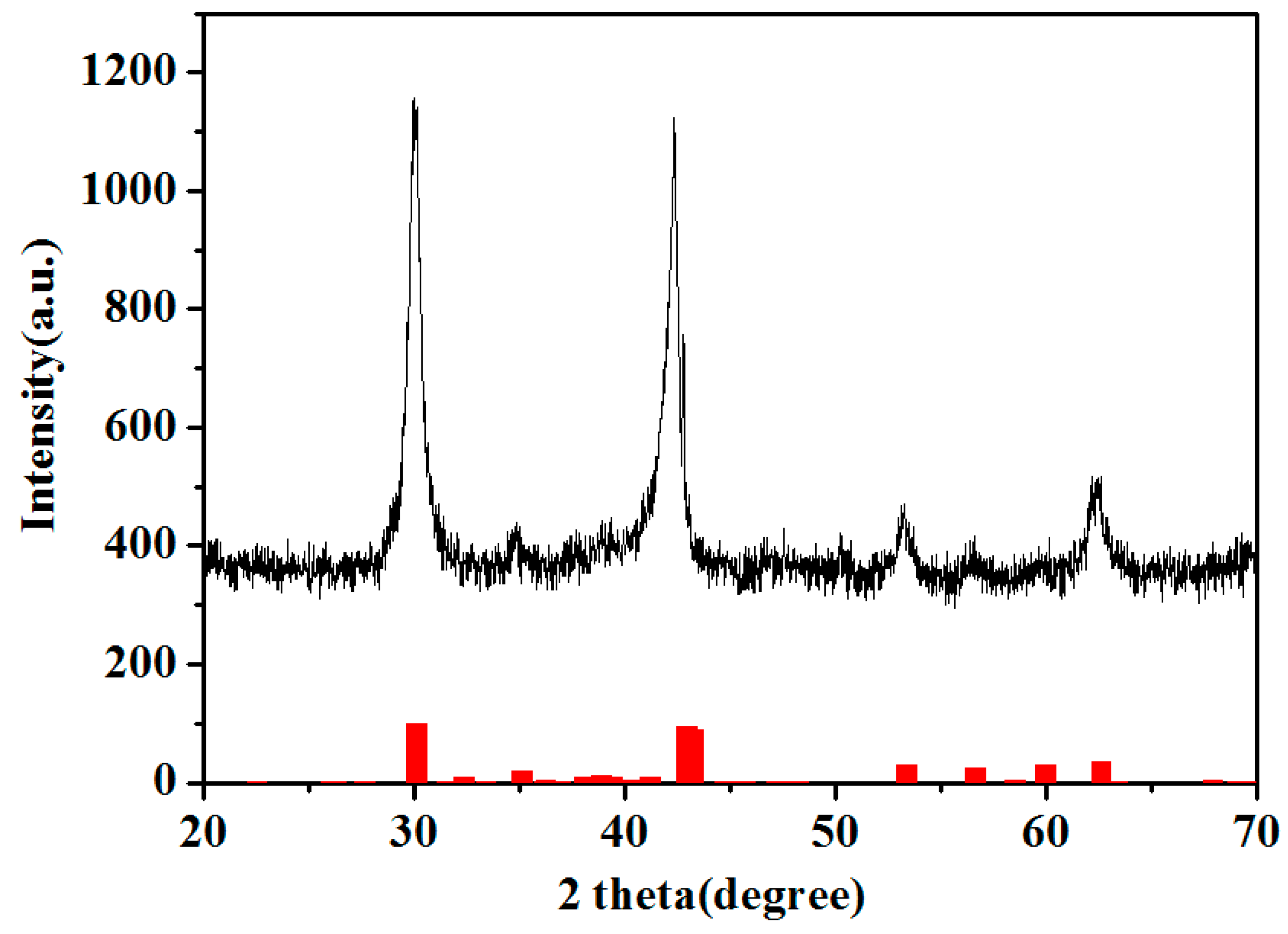
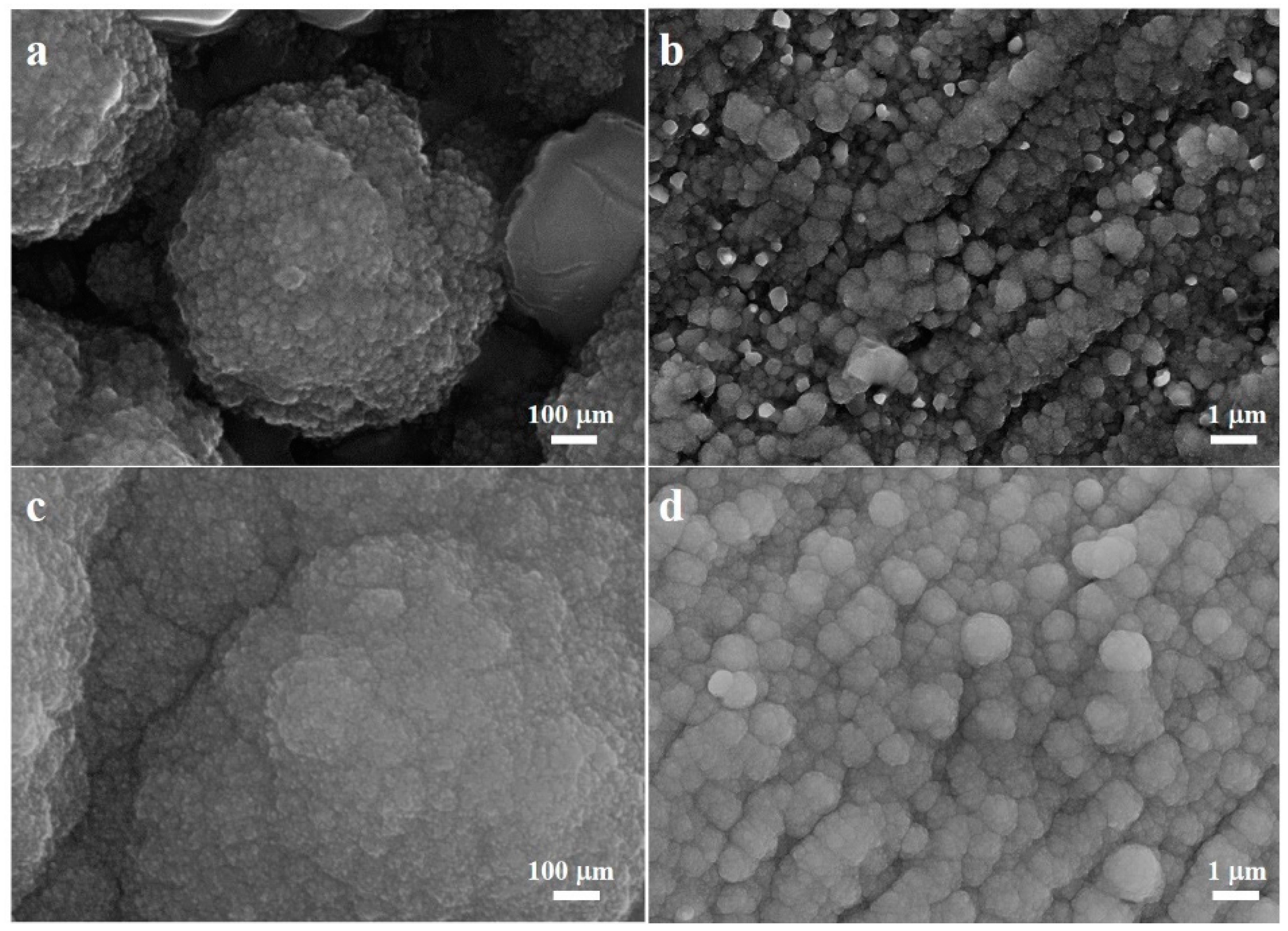
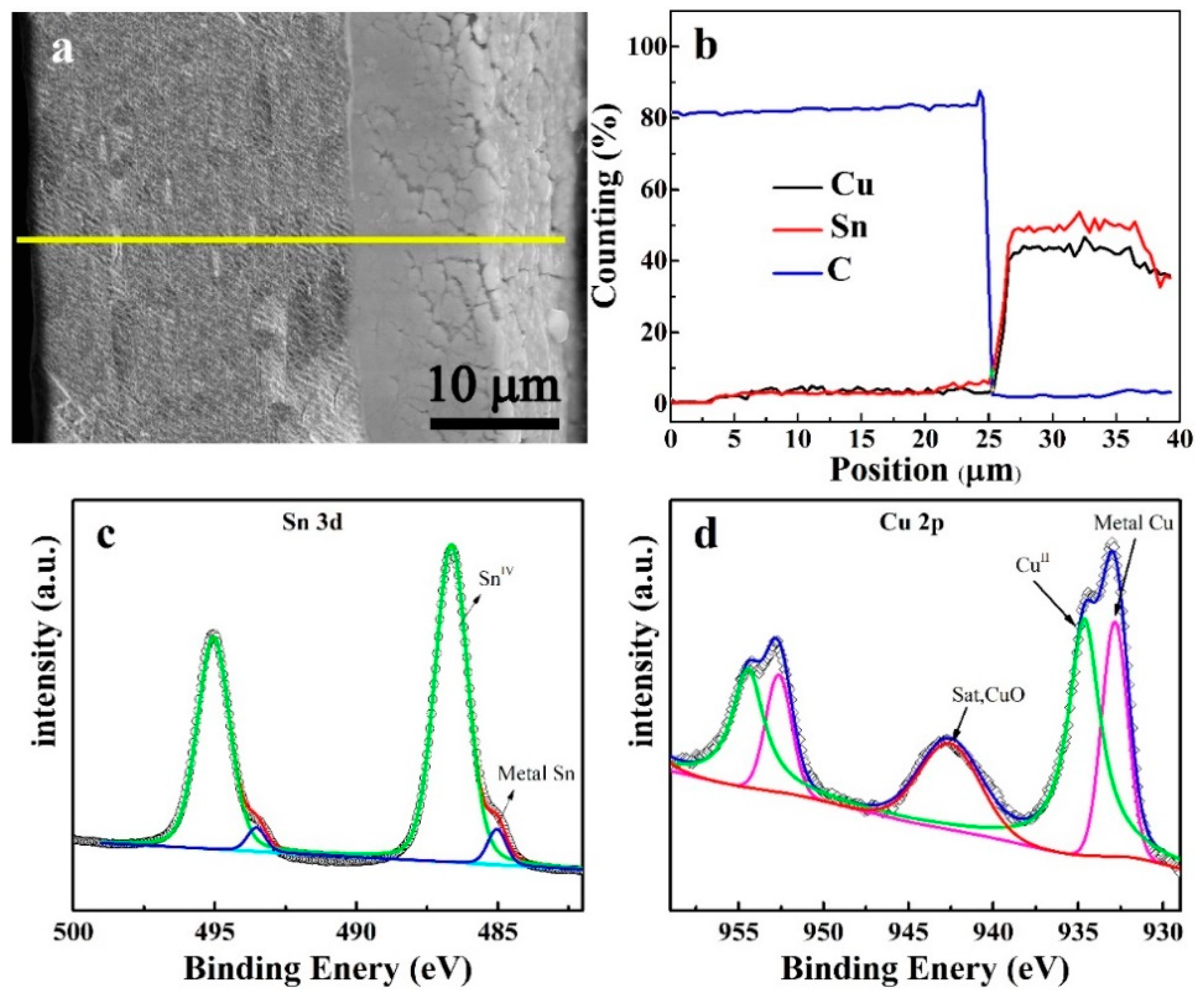
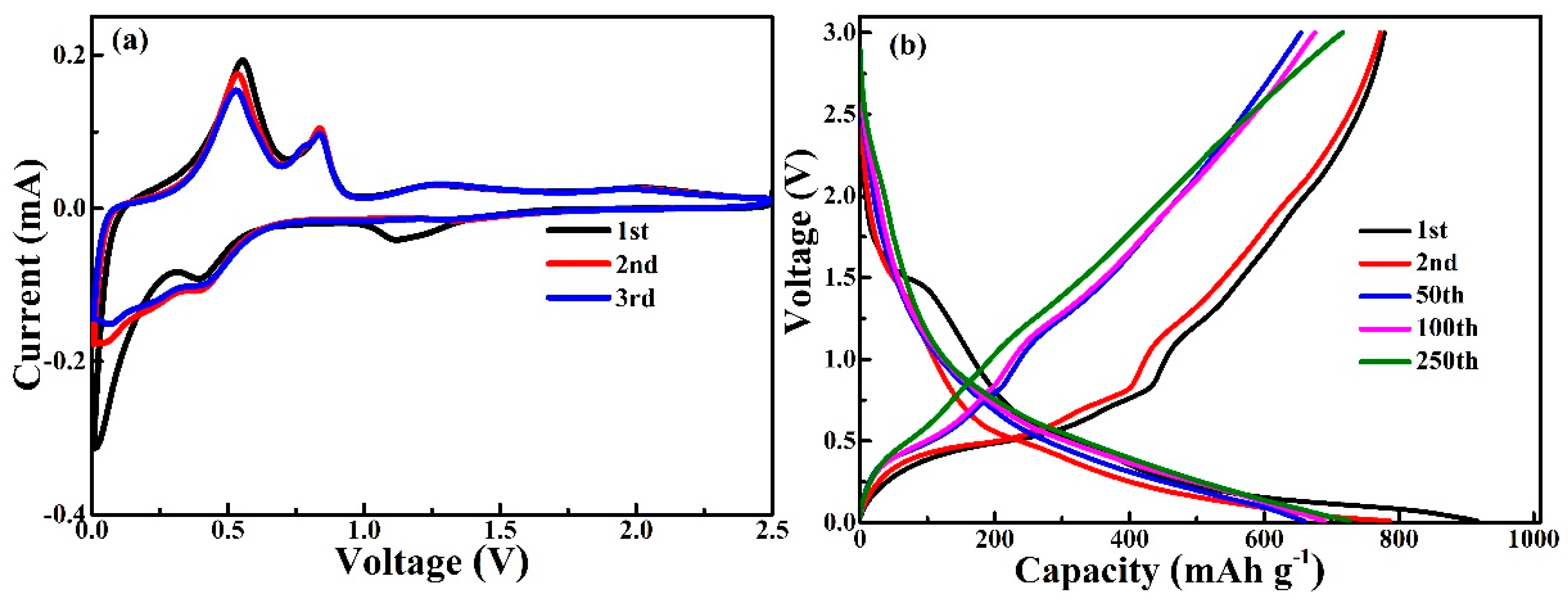
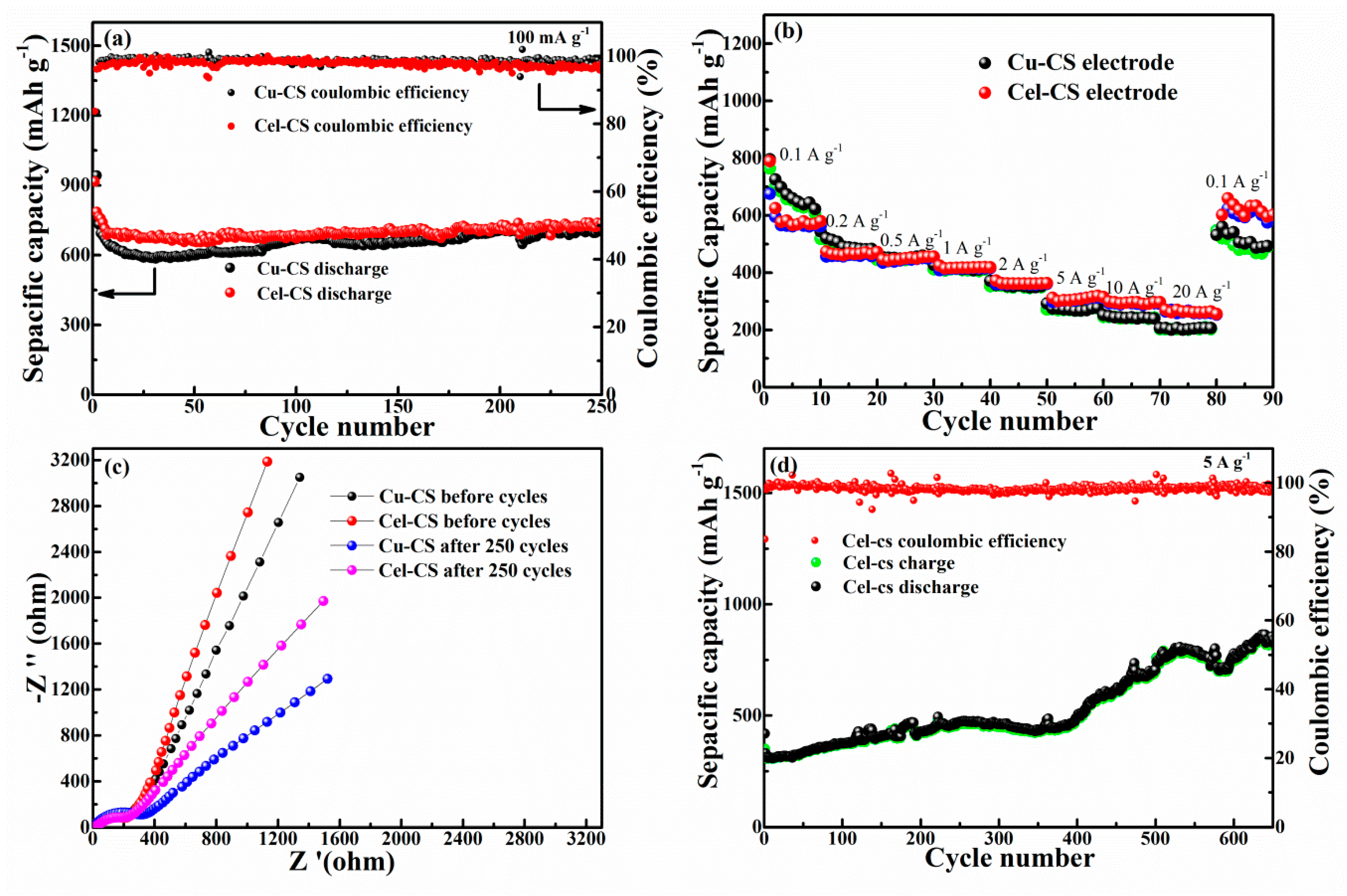
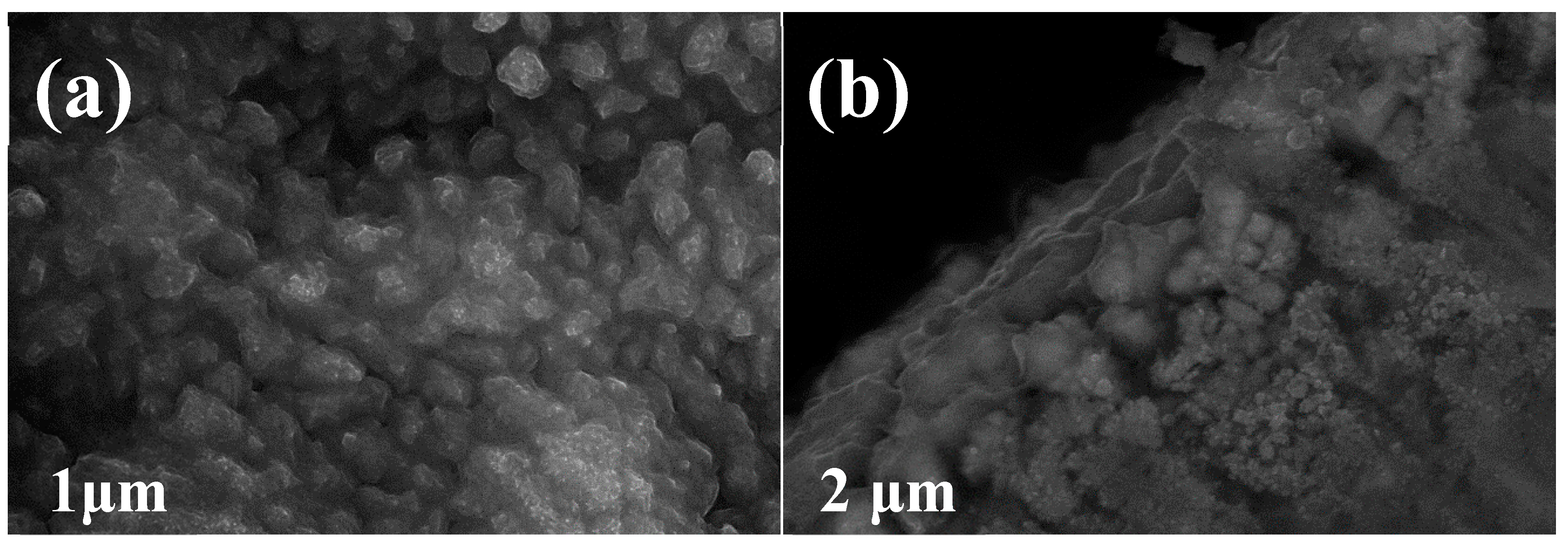
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.X.; Liu, B.; Li, Q.Y.; Cartmell, S.; Ferrara, S.; Deng, Z.D.; Xiao, J. Lithium and lithium ion batteries for applications in microelectronic devices: A review. J. Power Sources 2015, 286, 330–345. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.D.; Zhang, F.; Gan, X.F.; Huang, Q.A.; Yang, J.Z.; Lai, P.T.; Tang, W.M. Electrochemical characteristics of amorphous silicon carbide film as a lithium-ion battery anode. RSC Adv. 2018, 8, 5189–5196. [Google Scholar] [CrossRef] [Green Version]
- Cook-Chennault, K.A.; Thambi, N.; Sastry, A.M. Powering MEMS portable devices-a review of non-regenerative and regenerative power supply systems with special emphasis on piezoelectric energy harvesting systems. Smart Mater. Struct. 2008, 17, 043001. [Google Scholar] [CrossRef]
- Chen, H.; Cartmell, S.; Wang, Q.; Lozano, T.; Deng, Z.D.; Li, H.; Chen, X.; Yuan, Y.; Gross, M.E.; Carlson, T.J.; et al. Micro-battery development for juvenile salmon acoustic telemetry system applications. Sci. Rep. 2014, 4, 3790. [Google Scholar] [CrossRef] [PubMed]
- Panasonic Commercializes the Industry’s Smallest*1 Pin Shaped Lithium Ion Battery. Available online: https://news.panasonic.com/global/press/data/2014/10/en141003-2/en141003-2.html (accessed on 10 February 2019).
- Tang, C.; Zhang, Q.; Zhao, M.Q.; Tian, G.L.; Wei, F. Resilient aligned carbon nanotube/graphene sandwiches for robust mechanical energy storage. Nano Energy 2014, 7, 161–169. [Google Scholar] [CrossRef]
- Lin, J.; Wu, Y.L.; Bi, R.K.; Guo, H. All-solid-state microscale lithium-ion battery fabricated by a simple process with graphene as anode. Sensor Actuators A Phys. 2017, 253, 218–222. [Google Scholar] [CrossRef]
- Cirigliano, N.; Sun, G.; Membreno, D.; Malati, P.; Kim, C.J.; Dunn, B. 3D Architectured Anodes for Lithium-Ion Microbatteries with Large Areal Capacity. Energy Technol. 2014, 2, 362–369. [Google Scholar] [CrossRef]
- West, W.C.; Whitacre, J.F.; White, V.; Ratnakumar, B.V. Fabrication and testing of all solid-state microscale lithium batteries for microspacecraft applications. J. Micromech. Microeng. 2002, 12, 58–62. [Google Scholar] [CrossRef]
- Zhang, W.-J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 13–24. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Proietti Zaccaria, R.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Li, W.-C.; Zhu, J.-N.; Zhang, W.-P.; Lu, A.-H. Designed synthesis of nitrogen-rich carbon wrapped Sn nanoparticles hybrid anode via in-situ growth of crystalline ZIF-8 on a binary metal oxide. Nano Energy 2015, 19, 486–494. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, L.; Yu, X.-Y.; Lou, X.W.D. Encapsulating Sn Nanoparticles in Amorphous Carbon Nanotubes for Enhanced Lithium Storage Properties. Adv. Energy Mater. 2016, 6, 1601177. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Z.J.; Li, X.; Xie, W.; Wang, Q.; Liu, Q.; Fu, Y.; He, D. Group IVA Element (Si, Ge, Sn)-Based Alloying/Dealloying Anodes as Negative Electrodes for Full-Cell Lithium-Ion Batteries. Small 2017, 13, 1702000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Sun, X.; Yu, Y. Si-, Ge-, Sn-Based Anode Materials for Lithium-Ion Batteries: From Structure Design to Electrochemical Performance. Small Methods 2017, 1, 1600037. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.; Zhang, M.; Li, C.; Chen, C.; Liang, C.; Shi, Z.; Zhang, D.; Chen, G.; Chen, X.B.; Feng, S. Mercaptopropionic Acid-Capped Wurtzite Cu9Sn2Se9 Nanocrystals as High-Performance Anode Materials for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 1810–1818. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Chen, X.; Niu, J. Sn Wears Super Skin: A New Design for Long Cycling Batteries. Nano Lett. 2018, 18, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Xu, J.; Yuan, B.; Hu, R.; Yang, L.; Gao, Y.; Zhu, M. 3D Hierarchical Porous Cu-Based Composite Current Collector with Enhanced Ligaments for Notably Improved Cycle Stability of Sn Anode in Li-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 22050–22058. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Zhang, H.; Nie, Y.; Nie, K.; Lv, X.; Sun, N.; Xie, X.; Ma, Y.; Sun, X. Sn nanoparticles@nitrogen-doped carbon nanofiber composites as high-performance anodes for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 6277–6283. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, L.; Liu, D.; Hu, J.; Han, L.; Wang, Z.; Pan, F. A Conductive Binder for High-Performance Sn Electrodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Wang, S.; Fang, B.; Feng, Y.; Zhang, S. Three-dimensional nanoporous Cu6Sn5/Cu composite from dealloying as anode for lithium ion batteries. Microporous Mesoporous Mater. 2018, 261, 237–243. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, A.; Zhang, X.; Yin, H.; Wang, S.; Tang, Y.; Zhou, Y.; Wu, P. Pyrolysis of cyano-bridged hetero-metallic aerogels: A general route to immobilize Sn-M (M = Fe, Ni) alloys within a carbon matrix for stable and fast lithium storage. Nanoscale 2018, 10, 4962–4968. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, L.; Wu, H.B.; Zhang, G.; Lou, X.W. Controlled synthesis of hierarchical CoxMn3−xO4 array micro-/nanostructures with tunable morphology and composition as integrated electrodes for lithium-ion batteries. Energy Environ. Sci. 2013, 6, 2664–2671. [Google Scholar] [CrossRef]
- Lin, J.; Guo, J.; Liu, C.; Guo, H. Ultrahigh-Performance Cu2ZnSnS4 Thin Film and Its Application in Microscale Thin-Film Lithium-Ion Battery: Comparison with SnO2. ACS Appl. Mater. Interfaces 2016, 8, 34372–34378. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Jiang, K.; Yang, S. Electro-deposition preparation of self-standing Cu-Sn alloy anode electrode for lithium ion battery. J. Alloys Compd. 2019, 775, 818–825. [Google Scholar] [CrossRef]
- Fransson, L.; Nordström, E.; Edström, K.; Häggström, L.; Vaughey, J.T.; Thackeray, M.M. Structural Transformations in Lithiated η’-Cu6Sn5 Electrodes Probed by In Situ Mössbauer Spectroscopy and X-Ray Diffraction. J. Electrochem. Soc. 2002, 149, A736. [Google Scholar] [CrossRef]
- Naille, S.; Dedryvère, R.; Martinez, H.; Leroy, S.; Lippens, P.E.; Jumas, J.C.; Gonbeau, D. XPS study of electrode/electrolyte interfaces of η-Cu6Sn5 electrodes in Li-ion batteries. J. Power Sources 2007, 174, 1086–1090. [Google Scholar] [CrossRef]
- Fan, X.Y.; Ke, F.S.; Wei, G.Z.; Huang, L.; Sun, S.G. Microspherical Cu6Sn5 Alloy Anode for Lithium-Ion Battery. Electrochem. Solid-State Lett. 2008, 11, A195–A197. [Google Scholar] [CrossRef]
- Hu, R.; Waller, G.H.; Wang, Y.; Chen, Y.; Yang, C.; Zhou, W.; Zhu, M.; Liu, M. Cu6Sn5@SnO2–C nanocomposite with stable core/shell structure as a high reversible anode for Li-ion batteries. Nano Energy 2015, 18, 232–244. [Google Scholar] [CrossRef]
- Sharma, S.; Fransson, L.; Sjöstedt, E.; Nordström, L.; Johansson, B.; Edström, K. A Theoretical and Experimental Study of the Lithiation of η[sup’]-Cu[sub 6]Sn[sub 5] in a Lithium-Ion Battery. J. Electrochem. Soc. 2003, 150, A330. [Google Scholar] [CrossRef]
- Wang, P.; Hu, J.; Cao, G.; Zhang, S.; Zhang, P.; Liang, C.; Wang, Z.; Shao, G. Suppression on allotropic transformation of Sn planar anode with enhanced electrochemical performance. Appl. Surf. Sci. 2018, 435, 1150–1158. [Google Scholar] [CrossRef]
- Wang, Z.; Shan, Z.; Tian, J.; Huang, W.; Luo, D.; Zhu, X.; Meng, S. Immersion-plated Cu6Sn5/Sn composite film anode for lithium ion battery. J Mater. Sci. 2017, 52, 6020–6033. [Google Scholar] [CrossRef]
- Su, L.; Fu, J.; Zhang, P.; Wang, L.; Wang, Y.; Ren, M. Uniform core–shell Cu6Sn5@C nanospheres with controllable synthesis and excellent lithium storage performances. RSC Adv. 2017, 7, 28399–28406. [Google Scholar] [CrossRef]
- Ji, L.W.; Lin, Z.; Alcoutlabi, M.; Zhang, X.W. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; Flores, D.; Campos, H.; Villarreal, J.; Alcoutlabi, M. A comparative study on the performance of binary SnO2/NiO/C and Sn/C composite nanofibers as alternative anode materials for lithium ion batteries. Electrochim. Acta 2017, 224, 608–621. [Google Scholar] [CrossRef]
- Zuniga, L.; Agubra, V.; Flores, D.; Campos, H.; Villareal, J.; Alcoutlabi, M. Multichannel hollow structure for improved electrochemical performance of TiO2/Carbon composite nanofibers as anodes for lithium ion batteries. J. Alloys Compd. 2016, 686, 733–743. [Google Scholar] [CrossRef]
- Sun, X.; Shao, C.; Zhang, F.; Li, Y.; Wu, Q.H.; Yang, Y. SiC Nanofibers as Long-Life Lithium-Ion Battery Anode Materials. Front Chem. 2018, 6, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dou, P.; Wang, W.; Zheng, J.; Xu, X. Sn-Cu nanotubes enveloped in three-dimensional interconnected polyaniline hydrogel framework as binder-free anode for lithium-ion battery. Appl. Surf. Sci. 2017, 423, 245–254. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Samuelis, D.; Maier, J. Sustained Lithium-Storage Performance of Hierarchical, Nanoporous Anatase TiO2 at High Rates: Emphasis on Interfacial Storage Phenomena. Adv. Funct. Mater. 2011, 21, 3464–3472. [Google Scholar] [CrossRef]
- Lian, Q.; Zhou, G.; Zeng, X.; Wu, C.; Wei, Y.; Cui, C.; Wei, W.; Chen, L.; Li, C. Carbon Coated SnS/SnO2 Heterostructures Wrapping on CNFs as an Improved-Performance Anode for Li-Ion Batteries: Lithiation-Induced Structural Optimization upon Cycling. ACS Appl. Mater. Interfaces 2016, 8, 30256–30263. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Xia, F.; Huang, Y. Reconstruction of Conformal Nanoscale MnO on Graphene as a High-Capacity and Long-Life Anode Material for Lithium Ion Batteries. Adv. Funct. Mater. 2013, 23, 2436–2444. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Jiang, K.; Yang, S. Integrated Anode Electrode Composited Cu–Sn Alloy and Separator for Microscale Lithium Ion Batteries. Materials 2019, 12, 603. https://doi.org/10.3390/ma12040603
Liu Y, Jiang K, Yang S. Integrated Anode Electrode Composited Cu–Sn Alloy and Separator for Microscale Lithium Ion Batteries. Materials. 2019; 12(4):603. https://doi.org/10.3390/ma12040603
Chicago/Turabian StyleLiu, Yuxia, Kai Jiang, and Shuting Yang. 2019. "Integrated Anode Electrode Composited Cu–Sn Alloy and Separator for Microscale Lithium Ion Batteries" Materials 12, no. 4: 603. https://doi.org/10.3390/ma12040603




