Noble Metal Composite Porous Silk Fibroin Aerogel Fibers
Abstract
:1. Introduction
2. Materials and Methods
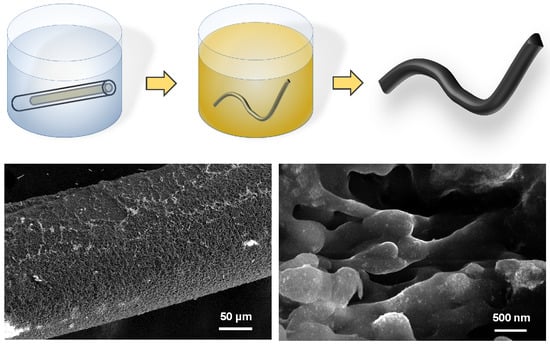
2.1. Silk Fibroin Fiber Aerogel Synthesis
2.2. Scanning Electron Microscopy (SEM)
2.3. X-ray Diffractometry (XRD)
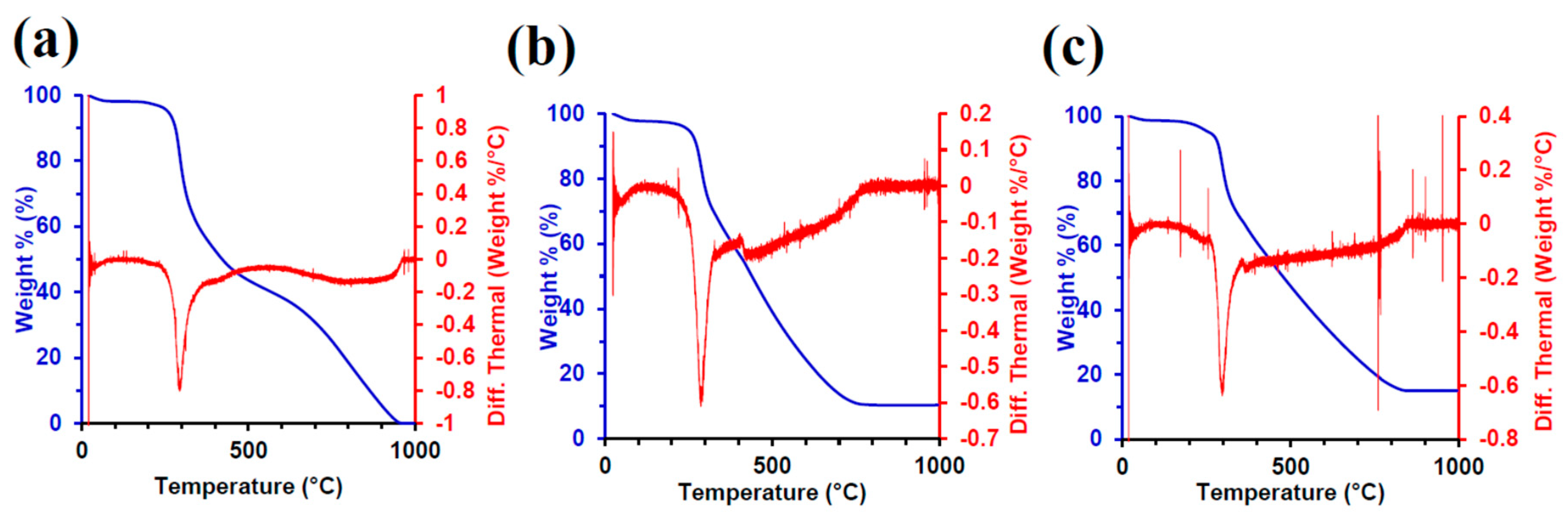
2.4. Thermal Gravimetric Analysis (TGA)
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
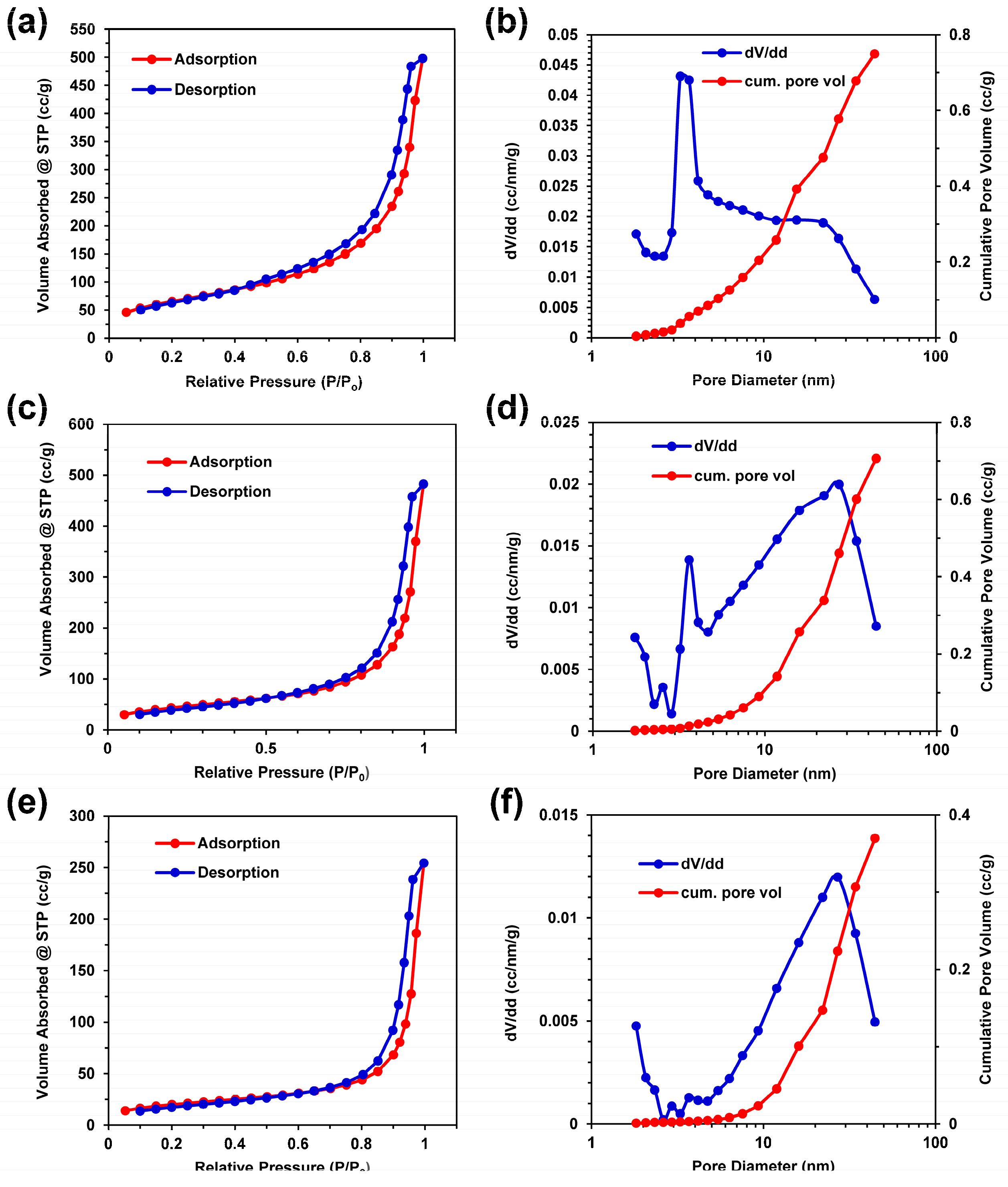
2.6. Porosity and Surface Area Analysis
3. Results and Discussion
3.1. Silk Fibroin Aerogel Synthesis
3.2. Aerogel Morphology and Noble Metal Nanoparticles
3.3. XRD Characterization
3.4. FTIR Characterization
3.5. TGA Characterization
3.6. Porosity and Surface Area Characterization
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, J.; Saltzman, W.M. Biomaterials with Hierarchically Defined Micro- and Nanoscale Structure. Biomaterials 2004, 25, 3593–3601. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.H. Preparation and Characterization of Regenerated Bombyx Mori Silk Fibroin Fiber with High Strength. eXPRESS Polym. Lett. 2008, 2, 885–889. [Google Scholar] [CrossRef]
- Burpo, F.J.; Mitropoulos, A.N.; Nagelli, E.A.; Rye, M.Y.; Palmer, J.L. Gelatin Biotemplated Platinum Aerogels. MRS Adv. 2018, 3, 2875–2880. [Google Scholar] [CrossRef]
- Burpo, F.J.; Mitropoulos, A.N.; Nagelli, E.A.; Palmer, J.L.; Morris, L.A.; Ryu, M.Y.; Kenneth Wickiser, J. Cellulose Nanofiber Biotemplated Palladium Composite Aerogels. Molecules 2018, 23, 1405. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-Based Biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Lawrence, B.D.; Cronin-Golomb, M.; Georgakoudi, I.; Kaplan, D.L.; Omenetto, F.G. Bioactive Silk Protein Biomaterial Systems for Optical Devices. Biomacromolecules 2008, 9, 1214–1220. [Google Scholar] [CrossRef]
- Lu, Q.; Hu, X.; Wang, X.; Kluge, J.A.; Lu, S.; Cebe, P.; Kaplan, D.L. Water-Insoluble Silk Films with Silk I Structure. Acta Biomater. 2010, 6, 1380–1387. [Google Scholar] [CrossRef]
- Perry, H.; Gopinath, A.; Kaplan, D.L.; Dal Negro, L.; Omenetto, F.G. Nano- and Micropatterning of Optically Transparent, Mechanically Robust, Biocompatible Silk Fibroin Films. Adv. Mater. 2008, 20, 3070–3072. [Google Scholar] [CrossRef]
- Jin, H.J.; Park, J.; Karageorgiou, V.; Kim, U.J.; Valluzzi, R.; Cebe, P.; Kaplan, D.L. Water-Stable Silk Films with Reduced β-Sheet Content. Adv. Funct. Mater. 2005, 15, 1241–1247. [Google Scholar] [CrossRef]
- Wang, X.; Yucel, T.; Lu, Q.; Hu, X.; Kaplan, D.L. Silk Nanospheres and Microspheres from Silk/pva Blend Films for Drug Delivery. Biomaterials 2010, 31, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.-J.; Park, J.; Li, C.; Jin, H.-J.; Valluzzi, R.; Kaplan, D.L. Structure and Properties of Silk Hydrogels. Biomacromolecules 2004, 5, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Floren, M.L.; Spilimbergo, S.; Motta, A.; Migliaresi, C. Carbon Dioxide Induced Silk Protein Gelation for Biomedical Applications. Biomacromolecules 2012, 13, 2060–2072. [Google Scholar] [CrossRef] [PubMed]
- Mallepally, R.R.; Marin, M.A.; McHugh, M.A. CO2-Assisted Synthesis of Silk Fibroin Hydrogels and Aerogels. Acta Biomater. 2014, 10, 4419–4424. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.A.; Mallepally, R.R.; McHugh, M.A. Silk Fibroin Aerogels for Drug Delivery Applications. J. Supercrit. Fluids 2014, 91, 84–89. [Google Scholar] [CrossRef]
- Tseng, P.; Napier, B.; Zhao, S.; Mitropoulos, A.N.; Applegate, M.B.; Marelli, B.; Kaplan, D.L.; Omenetto, F.G. Directed Assembly of Bio-Inspired Hierarchical Materials with Controlled Nanofibrillar Architectures. Nat. Nanotechnol. 2017, 12, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yao, J.; Masuda, H.; Kishore, R.; Asakura, T. Structural Characterization and Artificial Fiber Formation of Bombyx Mori Silk Fibroin in Hexafluoro-Iso-Propanol Solvent System. Biopolymers 2003, 69, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shao, Z.; Knight, D.P.; Vollrath, F. Conformation Transition Kinetics of Bombyx Mori Silk Protein. Proteins 2007, 68, 223–231. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Hirota, N.; Mizuno, K.; Goto, Y. Cooperative Alpha-Helix Formation of Beta-Lactoglobulin and Melittin Induced by Hexafluoroisopropanol. Protein Sci. 1997, 6, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Roccatano, D.; Fioroni, M.; Zacharias, M.; Colombo, G. Effect of Hexafluoroisopropanol Alcohol on the Structure of Melittin: A Molecular Dynamics Simulation Study. Protein Sci. 2005, 14, 2582–2589. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Grinberg, A.; Seok Gil, E.; Panilaitis, B.; Kaplan, D.L. High-Strength Silk Protein Scaffolds for Bone Repair. Proc. Natl. Acad. Sci. USA 2012, 109, 7699–7704. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Qin, Z.; Li, C.; Huang, W.; Kaplan, D.L.; Buehler, M.J. Polymorphic Regenerated Silk Fibers Assembled through Bioinspired Spinning. Nat. Commun. 2017, 8, 1387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ahluwalia, I.P.; Literman, R.; Kaplan, D.L.; Yelick, P.C. Human Dental Pulp Progenitor Cell Behavior on Aqueous and Hexafluoroisopropanol Based Silk Scaffolds. J. Biomed. Mater. Res. Part A 2011, 97, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Kluge, J.A.; Rockwood, D.N.; Rajkhowa, R.; Wang, L.; Wang, X.; Kaplan, D.L. Mechanical Improvements to Reinforced Porous Silk Scaffolds. J. Biomed. Mater. Res. Part A 2011, 99, 16–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone, G.S.; Leisk, G.G.; Lo, T.J.; Moreau, J.E.; Haas, D.S.; Papenburg, B.J.; Golden, E.B.; Partlow, B.P.; Fox, S.E.; Ibrahim, A.M.S.; et al. The Use of Silk-Based Devices for Fracture Fixation. Nat. Commun. 2014, 5, 3385. [Google Scholar] [CrossRef] [PubMed]
- Tsotsas, E.; Mujumdar, A.S. (Eds.) Modern Drying Technology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Williams, J.R.; Clifford, A.A.; Al-Saidi, S.H.R. Supercritical Fluids and Their Applications in Biotechnology and Related Areas. Mol. Biotechnol. 2002, 22, 263–286. [Google Scholar] [CrossRef]
- Brunner, G. Applications of Supercritical Fluids. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials Fabrication from Bombyx Mori Silk Fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Burpo, F.J.; Nagelli, E.A.; Morris, L.A.; McClure, J.P.; Ryu, M.Y.; Palmer, J.L. Direct Solution-Based Reduction Synthesis of Au, Pd, and Pt Aerogels. J. Mater. Res. 2017, 32, 4153–4165. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Jin, H.-J.; Kaplan, D.L. Mechanism of Silk Processing in Insects and Spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, L.; Mourdikoudis, S.; Pastoriza-Santos, I.; Perez-Juste, J. Nanocrystal Engineering of Noble Metals and Metal Chalcogenides: Controlling the Morphology, Composition and Crystallinity. CrystEngComm 2015, 17, 2727–2762. [Google Scholar] [CrossRef]
- Jewell, L.L.; Davis, B.H. Review of Absorption and Adsorption in the Hydrogen-Palladium System. Appl. Catal. A Gen. 2006, 310, 1–15. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.L.; Dai, F.Y.; Zhang, H.H.; Ni, B.; Zhou, W.; Yang, X.; Wu, Y.Z. Preparation and Characterization of Silk Fibroin as a Biomaterial with Potential for Drug Delivery. J. Transl. Med. 2012, 10, 117. [Google Scholar] [CrossRef]
- Lu, S.; Li, J.; Zhang, S.; Yin, Z.; Xing, T.; Kaplan, D.L. The Influence of the Hydrophilic-Lipophilic Environment on the Structure of Silk Fibroin Protein. J. Mater. Chem. B 2015, 3, 2599–2606. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhang, Y.Q. Effect of Regeneration of Liquid Silk Fibroin on Its Structure and Characterization. Soft Matter 2013, 9, 138–145. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Dynamic Protein-Water Relationships during Beta-Sheet Formation. Macromolecules 2008, 41, 3939–3948. [Google Scholar] [CrossRef]
- Lamoolphak, W.; De-Eknamkul, W.; Shotipruk, A. Hydrothermal Production and Characterization of Protein and Amino Acids from Silk Waste. Bioresour. Technol. 2008, 99, 7678–7685. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.Y.; Chun, B.S. Behavior of Hydrothermal Decomposition of Silk Fibroin to Amino Acids in near-Critical Water. Korean J. Chem. Eng. 2004, 21, 654–659. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitropoulos, A.N.; Burpo, F.J.; Nguyen, C.K.; Nagelli, E.A.; Ryu, M.Y.; Wang, J.; Sims, R.K.; Woronowicz, K.; Wickiser, J.K. Noble Metal Composite Porous Silk Fibroin Aerogel Fibers. Materials 2019, 12, 894. https://doi.org/10.3390/ma12060894
Mitropoulos AN, Burpo FJ, Nguyen CK, Nagelli EA, Ryu MY, Wang J, Sims RK, Woronowicz K, Wickiser JK. Noble Metal Composite Porous Silk Fibroin Aerogel Fibers. Materials. 2019; 12(6):894. https://doi.org/10.3390/ma12060894
Chicago/Turabian StyleMitropoulos, Alexander N., F. John Burpo, Chi K. Nguyen, Enoch A. Nagelli, Madeline Y. Ryu, Jenny Wang, R. Kenneth Sims, Kamil Woronowicz, and J. Kenneth Wickiser. 2019. "Noble Metal Composite Porous Silk Fibroin Aerogel Fibers" Materials 12, no. 6: 894. https://doi.org/10.3390/ma12060894