Mechanical and Cytocompatibility Evaluation of UHMWPE/PCL/Bioglass® Fibrous Composite for Acetabular Labrum Implant
Abstract
:1. Introduction
2. Materials and Methods
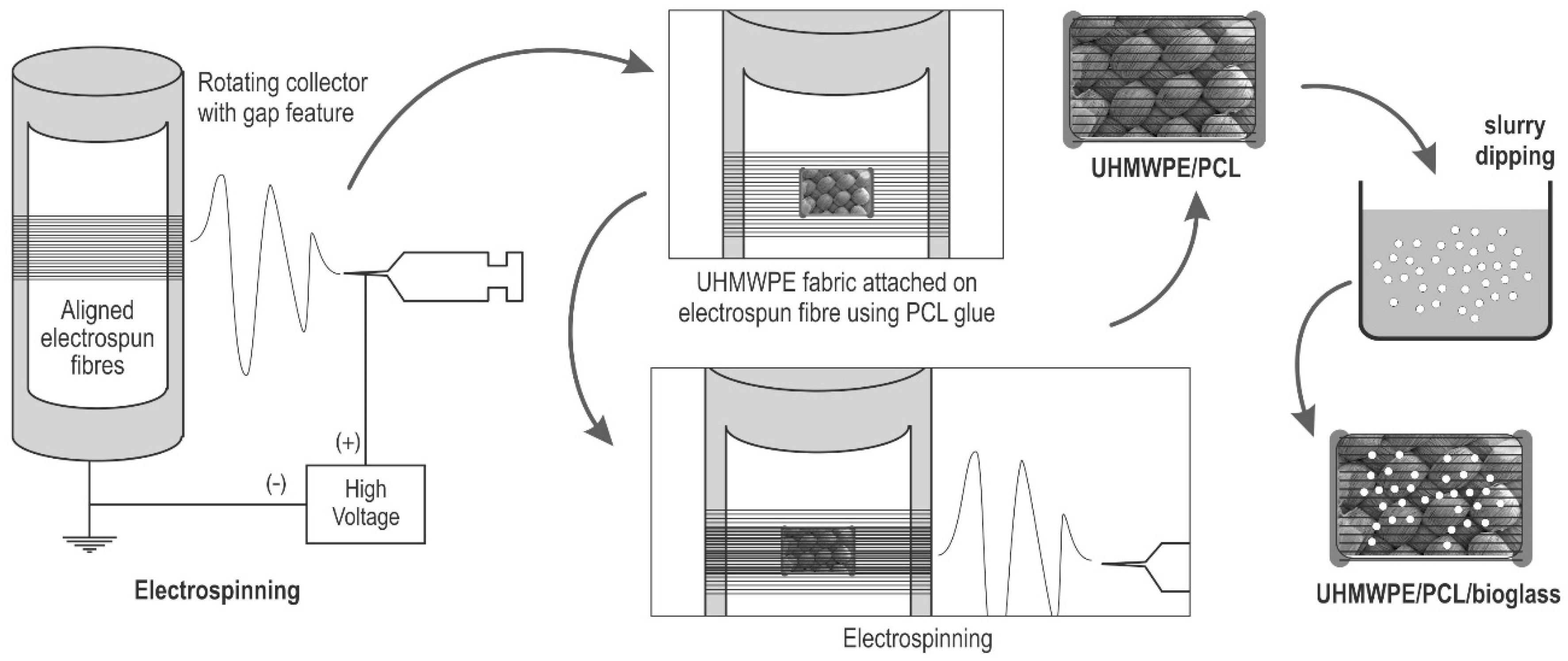
2.1. Electrospinning
2.2. Fabrication of UHMWPE/PCL
2.3. Bioglass® Coating
2.4. Cyclic Loading Test of UHMWPE Fabric
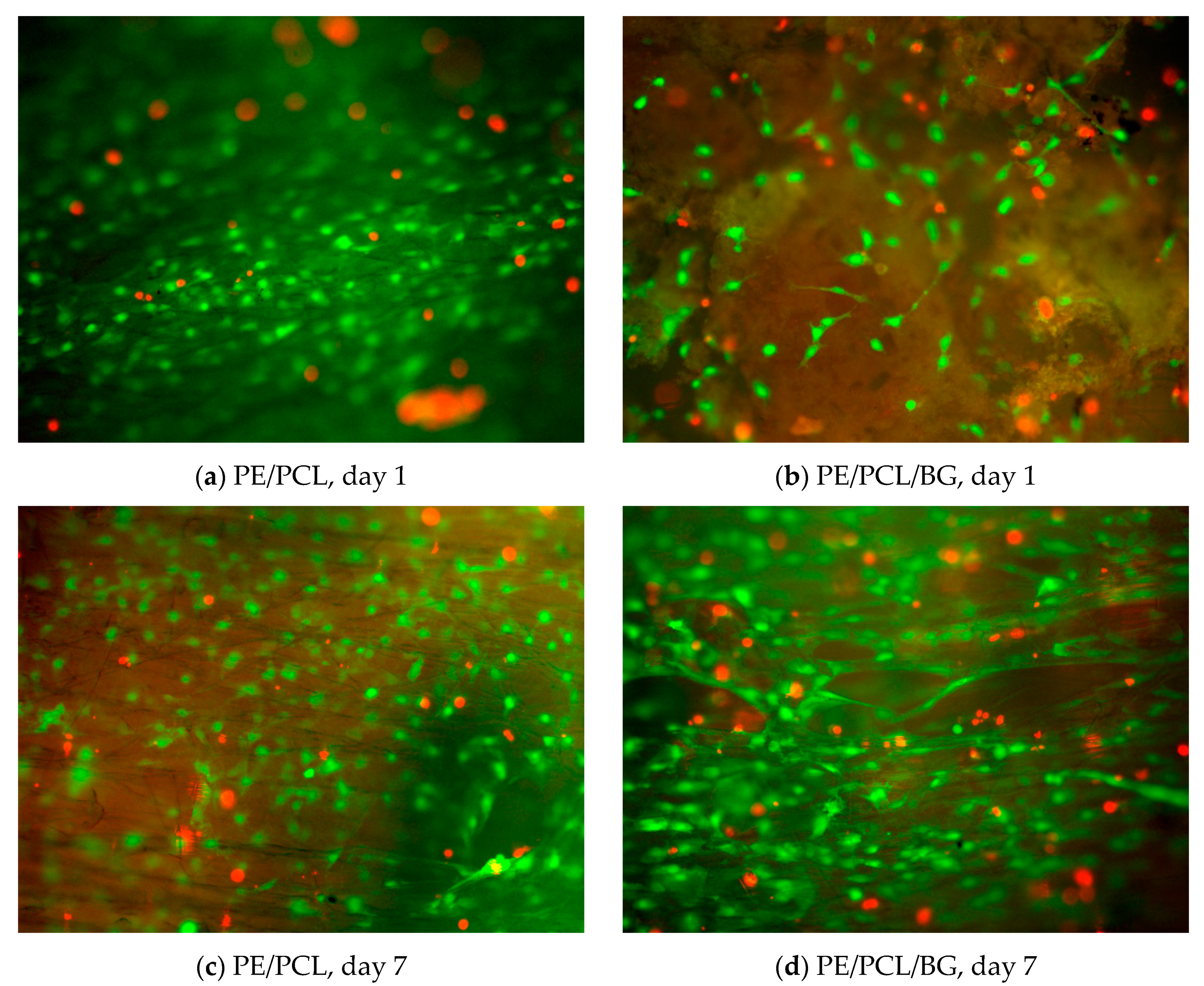
2.5. Cell Culture and Seeding
2.6. Viability Assay
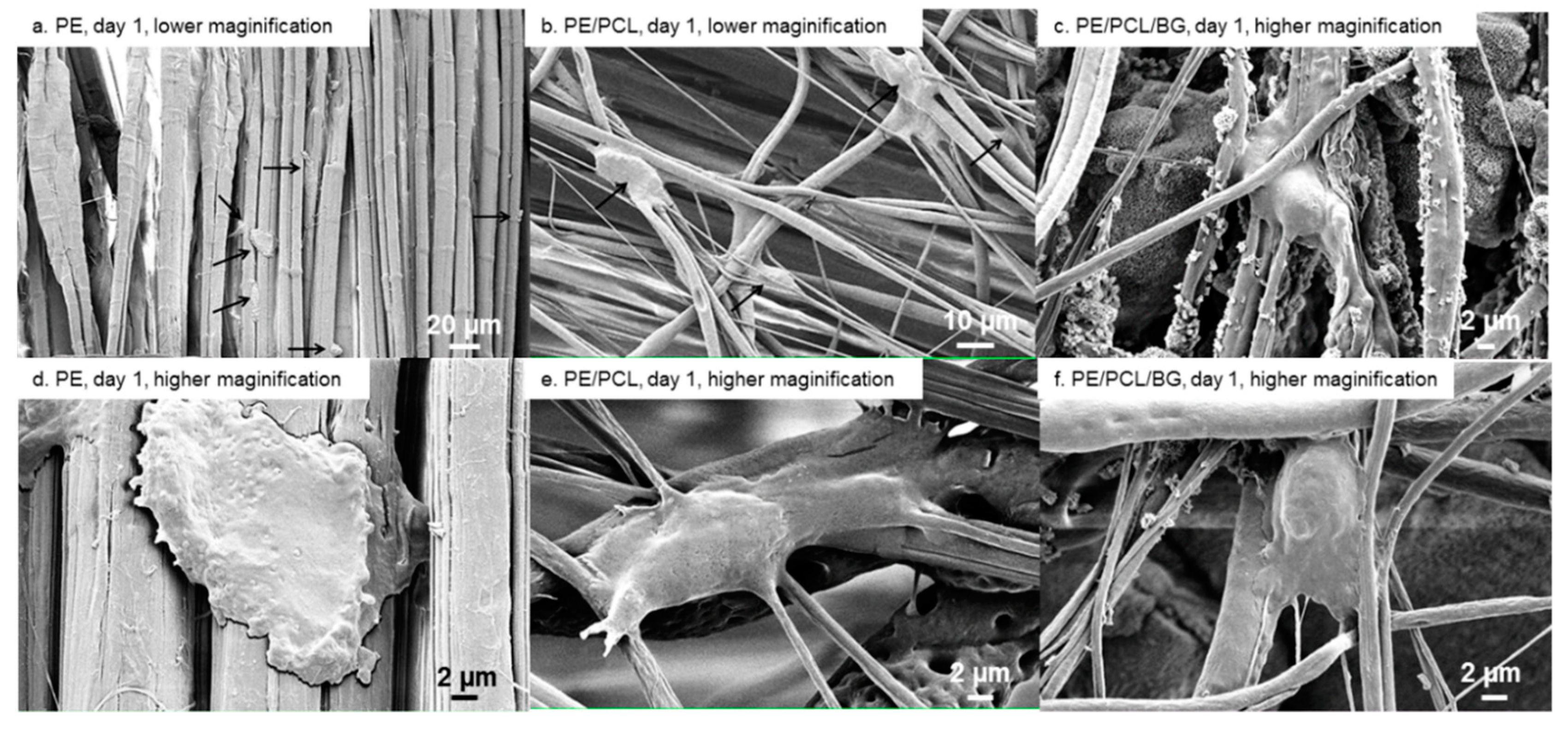
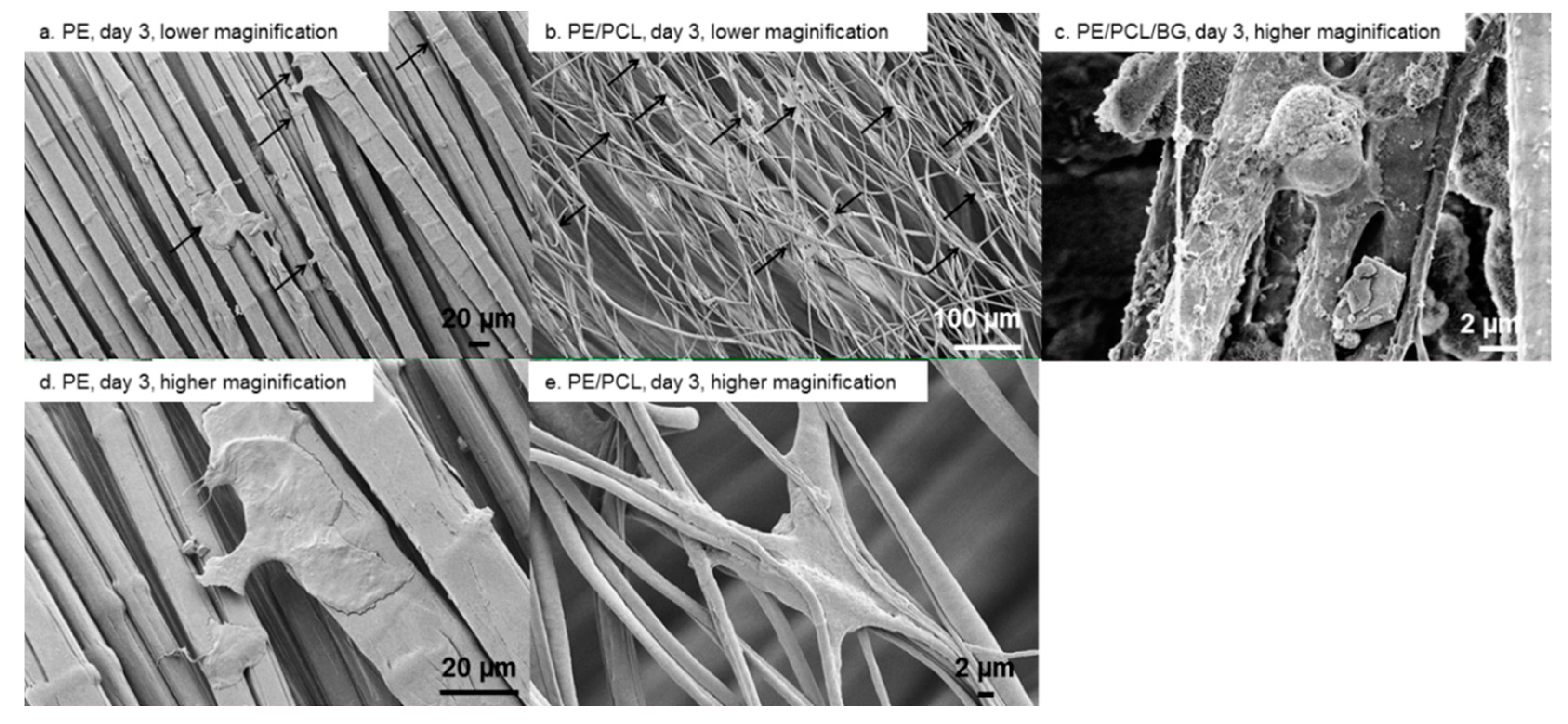
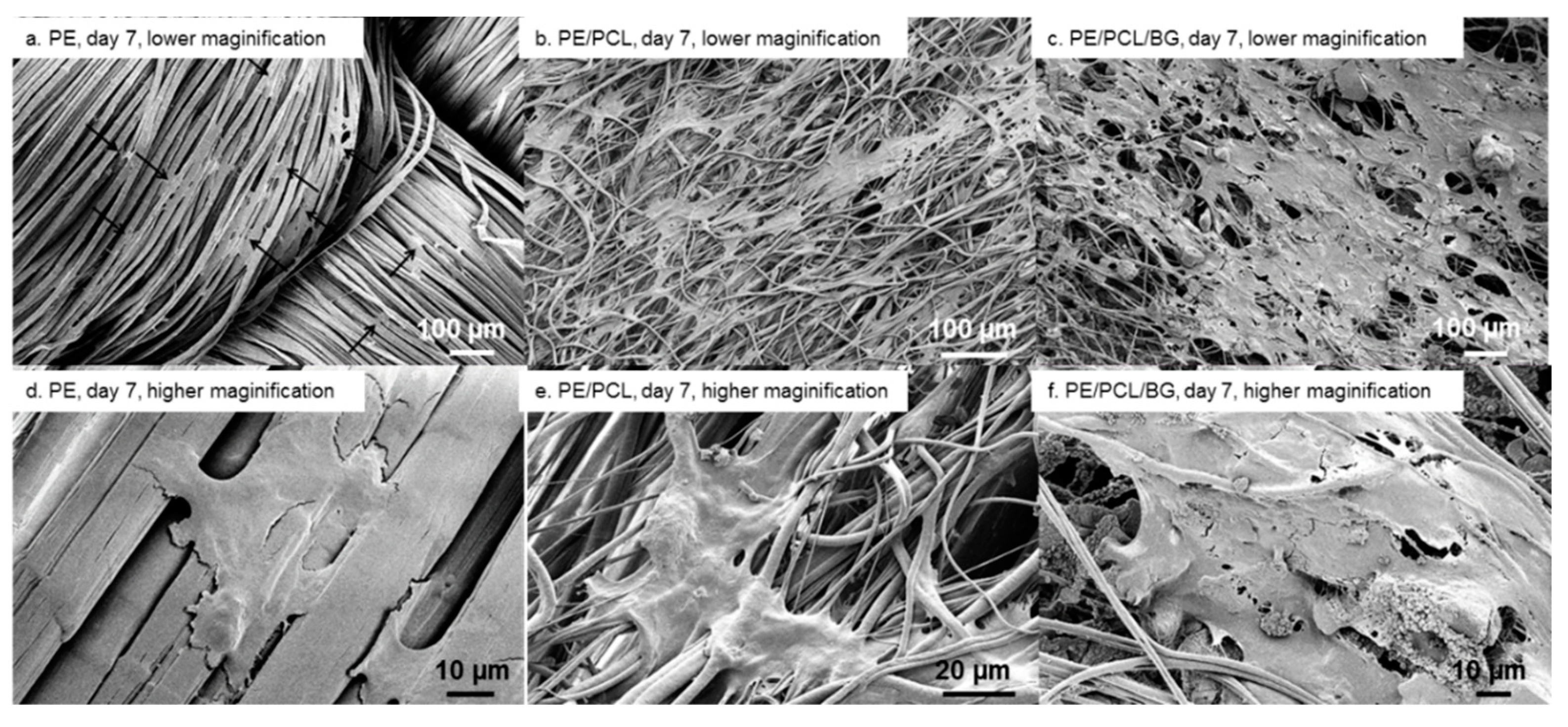
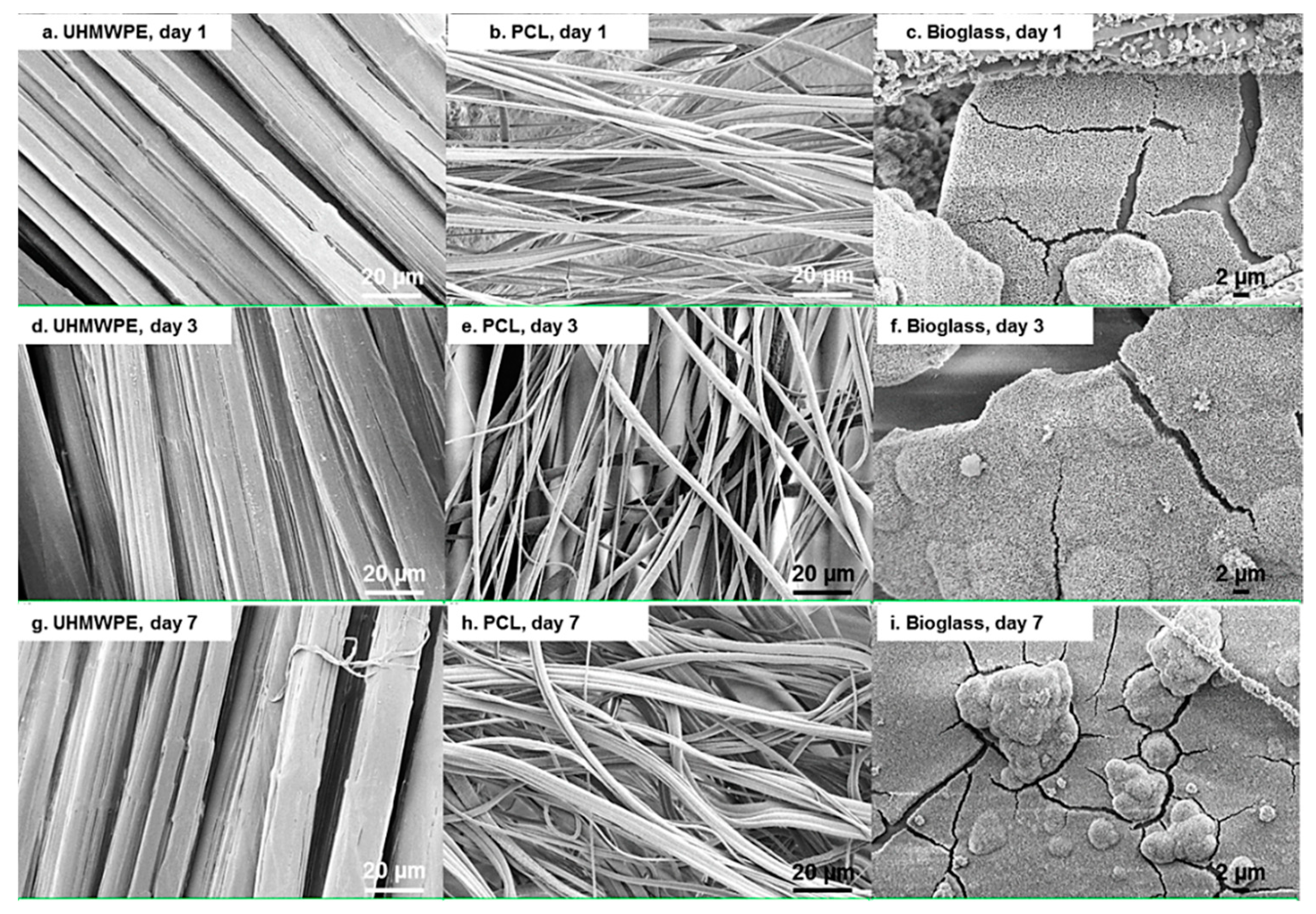
2.7. Scanning Electron Microscopy
3. Results
Cyclic Loading Test of UHMWPE Fabric
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seldes, R.M.; Tan, V.; Hunt, J.; Katz, M.; Winiarsky, R.; Robert, H.; Fitzgerald, J. Anatomy, histologic features, and vascularity of the adult acetabular labrum. Clin. Orthop. Relat. Res. 2001, 382, 232–240. [Google Scholar] [CrossRef]
- Grant, A.D.; Sala, D.A.; Davidovitch, R.I. The labrum: Structure, function, and injury with femoro-acetabular impingement. J. Child. Orthop. 2012, 6, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.J.; Bryant, J.T.; Ganz, R.; Ito, K. The acetabular labrum seal: A poroelastic finite element model. Clin. Biomech. 2000, 15, 463–468. [Google Scholar] [CrossRef]
- Ganz, R.; Parvizi, J.; Beck, M.; Leunig, M.; Notzli, H.; Siebenrock, K.A. Femoroacetabular impingement: A cause for osteoarthritis of the hip. Clin. Orthop. Relat. Res. 2003, 417, 112–120. [Google Scholar]
- McCarthy, J.C.; Noble, P.C.; Schuck, M.R.; Wright, J.; Lee, J. The otto e. Aufranc award: The role of labral lesions to development of early degenerative hip disease. Clin. Orthop. Relat. Res. 2001, 393, 25–37. [Google Scholar] [CrossRef]
- Ejnisman, L.; Philippon, M.J.; Lertwanich, P. Acetabular labral tears: Diagnosis, repair, and a method for labral reconstruction. Clin. Sports Med. 2011, 30, 317–329. [Google Scholar] [CrossRef]
- Sierra, R.; Trousdale, R. Labral reconstruction using the ligamentum teres capitis: Report of a new technique. Clin. Orthop. Relat. Res. 2009, 467, 753–759. [Google Scholar] [CrossRef]
- Matsuda, D.K. Arthroscopic labral reconstruction with gracilis autograft. Arthrosc. Tech. 2012, 1, e15–e21. [Google Scholar] [CrossRef]
- Hench, L.L. Biomaterials: A forecast for the future. Biomaterials 1998, 19, 1419–1423. [Google Scholar] [CrossRef]
- Petersen, W.; Petersen, F.; Tillmann, B. Structure and vascularization of the acetabular labrum with regard to the pathogenesis and healing of labral lesions. Arch. Orthop. Trauma Surg. 2003, 123, 283–288. [Google Scholar] [CrossRef]
- Pruitt, L.A.; Chakravartula, A.M. Mechanics of Biomaterials: Fundamantal Principles of Implant Design; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Holloway, J.L.; Lowman, A.M.; Palmese, G.R. Mechanical evaluation of poly(vinyl alcohol)-based fibrous composites as biomaterials for meniscal tissue replacement. Acta Biomaterialia 2010, 6, 4716–4724. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, K.; Kotani, Y.; Abumi, K.; Takada, T.; Shimamoto, N.; Shikinami, Y.; Kadosawa, T.; Kaneda, K. Biomechanical and morphologic evaluation of a three-dimensional fabric sheep artificial intervertebral disc in vitro and in vivoanalysis. Spine 2001, 26, 1562–1569. [Google Scholar] [CrossRef]
- Kotani, Y.; Abumi, K.; Shikinami, Y.; Takada, T.; Kadoya, K.; Shimamoto, N.; Ito, M.; Kadosawa, T.; Fujinaga, T.; Kaneda, K. Artificial intervertebral disc replacement using bioactive three-dimensional fabric: Design, development, and preliminary animal study. Spine 2002, 27, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Takahata, M.; Kotani, Y.; Abumi, K.; Shikinami, Y.; Kadosawa, T.; Kaneda, K.; Minami, A. Bone ingrowth fixation of artificial intervertebral disc consisting of bioceramic-coated three-dimensional fabric. Spine 2003, 28, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Shikinami, Y.; Kotani, Y.; Cunningham, B.W.; Abumi, K.; Kaneda, K. A biomimetic artificial disc with improved mechanical properties compared to biological intervertebral discs. Adv. Funct. Mater. 2004, 14, 1039–1046. [Google Scholar] [CrossRef]
- Shikinami, Y.; Kawabe, Y.; Yasukawa, K.; Tsuta, K.; Kotani, Y.; Abumi, K. A biomimetic artificial intervertebral disc system composed of a cubic three-dimensional fabric. Spine J. 2010, 10, 141–152. [Google Scholar] [CrossRef]
- Bach, J.S.; Detrez, F.; Cherkaoui, M.; Cantournet, S.; Ku, D.N.; Corté, L. Hydrogel fibers for ACL prosthesis: Design and mechanical evaluation of PVA and PVA/UHMWPE fiber constructs. J. Biomech. 2013, 46, 1463–1470. [Google Scholar] [CrossRef]
- Guidoin, M.-F.; Marois, Y.; Bejui, J.; Poddevin, N.; King, M.W.; Guidoin, R. Analysis of retrieved polymer fiber based replacements for the acl. Biomaterials 2000, 21, 2461–2474. [Google Scholar] [CrossRef]
- Karaman, A.I.; Kir, N.; Belli, S. Four applications of reinforced polyethylene fiber material in orthodontic practice. Am. J. Orthod. Dentofacial Orthop. 2002, 121, 650–654. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Li, W.-J.; Cooper, J.A., Jr.; Mauck, R.L.; Tuan, R.S. Fabrication and characterization of six electrospun poly(α-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomaterialia 2006, 2, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.M.; Mauck, R.L.; Shah, R.P.; Schaer, T.P.; Maher, S.A. 5.524—Biomaterials for replacement and repair of the meniscus and annulus fibrosus. In Comprehensive Biomaterials; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 317–332. [Google Scholar]
- Gantenbein-Ritter, B.; Sakai, D. 6.612—Biomaterials for intervertebral disc regeneration. In Comprehensive Biomaterials; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2011; pp. 161–169. [Google Scholar]
- Baker, B.M.; Gee, A.O.; Metter, R.B.; Nathan, A.S.; Marklein, R.A.; Burdick, J.A.; Mauck, R.L. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials 2008, 29, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Mauck, R.L. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials 2007, 28, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.B.; Henning, E.A.; Söegaard, N.; Esterhai, J.L.; Mauck, R.L. Organized nanofibrous scaffolds that mimic the macroscopic and microscopic architecture of the knee meniscus. Acta Biomaterialia 2013, 9, 4496–4504. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, N.L.; Sen, S.; Huang, A.H.; Elliott, D.M.; Mauck, R.L. Engineered disc-like angle-ply structures for intervertebral disc replacement. Spine 2010, 35, 867–873. [Google Scholar] [CrossRef]
- Li, W.-J.; Tuli, R.; Okafor, C.; Derfoul, A.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 2005, 26, 599–609. [Google Scholar] [CrossRef]
- Lebourg, M.; Sabater Serra, R.; Más Estellés, J.; Hernández Sánchez, F.; Gómez Ribelles, J.L.; Suay Antón, J. Biodegradable polycaprolactone scaffold with controlled porosity obtained by modified particle-leaching technique. J. Mater. Sci. Mater. Med. 2008, 19, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Mauck, R.L.; Cooper, J.A.; Yuan, X.; Tuan, R.S. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J. Biomech. 2007, 40, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, L.; Remund, T.; Bao, J.; Neufeld, D.; Fong, H.; Deng, Y. Tissue engineering of annulus fibrosus using electrospun fibrous scaffolds with aligned polycaprolactone fibers. J. Biomed. Mater. Res. Part A 2011, 99A, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, L.; Zhang, L.; Neufeld, D.; Fong, H.; Deng, Y. Electrospun nanofibrous polycaprolactone scaffolds for tissue engineering of annulus fibrosus. Macromol. Biosci. 2011, 11, 391–399. [Google Scholar] [CrossRef]
- Thorvaldsson, A.; Stenhamre, H.; Gatenholm, P.; Walkenström, P. Electrospinning of highly porous scaffolds for cartilage regeneration. Biomacromolecules 2008, 9, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of bioglass. J. Mater. Sci. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R. Review of bioactive glass: From hench to hybrids. Acta Biomaterialia 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive glasses: Where are we and where are we going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Pigott, G.H.; Schoen, F.J.; Hench, L.L. Toxicology and biocompatibility of bioglasses. J. Biomed. Mater. Res. 1981, 15, 805–817. [Google Scholar] [CrossRef]
- Sola, A.; Bellucci, D.; Cannillo, V. Functionally graded materials for orthopedic applications—An update on design and manufacturing. Biotechnol. Adv. 2016, 34, 504–531. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M.; Boccaccini, A.R.; Shurey, S.; Roether, J.A.; Forbes, A.; Hench, L.L.; Gabe, S.M. Assessment of polyglycolic acid mesh and bioactive glass for soft-tissue engineering scaffolds. Biomaterials 2004, 25, 5857–5866. [Google Scholar] [CrossRef] [PubMed]
- Verrier, S.; Blaker, J.J.; Maquet, V.; Hench, L.L.; Boccaccini, A.R. PDLLA/Bioglass® composites for soft-tissue and hard-tissue engineering: An in vitro cell biology assessment. Biomaterials 2004, 25, 3013–3021. [Google Scholar] [CrossRef]
- Stamboulis, A.; Hench, L.L.; Boccaccini, A.R. Mechanical properties of biodegradable polymer sutures coated with bioactive glass. J. Mater. Sci. Mater. Med. 2002, 13, 843–848. [Google Scholar] [CrossRef]
- Li, H.; Chen, S.; Wu, Y.; Jiang, J.; Ge, Y.; Gao, K.; Zhang, P.; Wu, L. Enhancement of the osseointegration of a polyethylene terephthalate artificial ligament graft in a bone tunnel using 58s bioglass. Int. Orthop. 2012, 36, 191–197. [Google Scholar] [CrossRef]
- Suominen, E.; Aho, A.J.; Vedel, E.; Kangasniemi, I.; Uusipaikka, E.; Yli-Urpo, A. Subchondral bone and cartilage repair with bioactive glasses, hydroxyapatite, and hydroxyapatite-glass composite. J. Biomed. Mater. Res. 1996, 32, 543–551. [Google Scholar] [CrossRef]
- Bal, B.S.; Rahaman, M.N.; Jayabalan, P.; Kuroki, K.; Cockrell, M.K.; Yao, J.Q.; Cook, J.L. In vivo outcomes of tissue-engineered osteochondral grafts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93B, 164–174. [Google Scholar]
- Tellisi, N.; Ashammakhi, N. Comparison of meshes, gels and ceramic for cartilage tissue engineering in vitro. Eur. J. Plast. Surg. 2012, 35, 159–170. [Google Scholar] [CrossRef]
- Wu, J.; Xue, K.; Li, H.; Sun, J.; Liu, K. Improvement of PHBV scaffolds with bioglass for cartilage tissue engineering. PLoS ONE 2013, 8, e71563. [Google Scholar] [CrossRef]
- Isa, I.L.M.; Günay, B.; Joyce, K.; Pandit, A. Tissue engineering: Biomaterials for disc repair. Curr. Mol. Biol. Rep. 2018, 4, 161–172. [Google Scholar] [CrossRef]
- Zur, G.; Linder-Ganz, E.; Elsner, J.; Shani, J.; Brenner, O.; Agar, G.; Hershman, E.; Arnoczky, S.; Guilak, F.; Shterling, A. Chondroprotective effects of a polycarbonate-urethane meniscal implant: Histopathological results in a sheep model. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 255–263. [Google Scholar] [CrossRef]
- Messner, K. Meniscal substitution with a teflon-periosteal composite graft: A rabbit experiment. Biomaterials 1994, 15, 223–230. [Google Scholar] [CrossRef]
- Messner, K. The concept of a permanent synthetic meniscus prosthesis: A critical discussion after 5 years of experimental investigation using dacron and teflon implants. Biomaterials 1994, 15, 243–250. [Google Scholar] [CrossRef]
- Anindyajati, A.; Boughton, P.; Ruys, A. Fabrication and microstructure evaluation of fibrous composite for acetabular labrum implant. Mater. Sci. Forum 2017, 900, 17–22. [Google Scholar] [CrossRef]
- Ferro, F.P.; Philippon, M.J.; Rasmussen, M.T.; Smith, S.D.; LaPrade, R.F.; Wijdicks, C.A. Tensile properties of the human acetabular labrum and hip labral reconstruction grafts. Am. J. Sports Med. 2015, 43, 1222–1227. [Google Scholar] [CrossRef]
- Hunter, A.; Archer, C.W.; Walker, P.S.; Blunn, G.W. Attachment and proliferation of osteoblasts and fibroblasts on biomaterials for orthopaedic use. Biomaterials 1995, 16, 287–295. [Google Scholar] [CrossRef]
- Silver, I.A.; Erecinska, M. Interactions of osteoblastic and other cells with bioactive glasses and silica in vitro and in vivo. Materialwissenschaft Werkstofftechnik 2003, 34, 1069–1075. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Anesi, A.; Salvatori, R.; Chiarini, L.; Cannillo, V. Bioactive glass/hydroxyapatite composites: Mechanical properties and biological evaluation. Mater. Sci. Eng. C 2015, 51, 196–205. [Google Scholar] [CrossRef]
- Carvalho, S.; Oliveira, A.; Andrade, V.; De Fatima Leite, M.; Goes, A.; Pereira, M. Comparative effect of the ionic products from bioactive glass dissolution on the behavior of cementoblasts, osteoblasts, and fibroblasts. Key Eng. Mater. 2009, 396–398, 55–59. [Google Scholar] [CrossRef]
- Detsch, R.; Stoor, P.; Grünewald, A.; Roether, J.A.; Lindfors, N.C.; Boccaccini, A.R. Increase in VEGF secretion from human fibroblast cells by bioactive glass S53P4 to stimulate angiogenesis in bone. J. Biomed. Mater. Res. Part A 2014, 102, 4055–4061. [Google Scholar] [CrossRef] [PubMed]
- Helen, W.; Gough, J.E. Cell viability, proliferation and extracellular matrix production of human annulus fibrosus cells cultured within pdlla/bioglass® composite foam scaffolds in vitro. Acta Biomaterialia 2008, 4, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-J.; Lu, P.-S.; Hsieh, C.-H.; Chen, W.-C.; Chen, J.-C. Effects of bioglass powders with and without mesoporous structures on fibroblast and osteoblast responses. Appl. Surf. Sci. 2014, 314, 967–972. [Google Scholar] [CrossRef]
- Alcaide, M.; Portolés, P.; López-Noriega, A.; Arcos, D.; Vallet-Regí, M.; Portolés, M.T. Interaction of an ordered mesoporous bioactive glass with osteoblasts, fibroblasts and lymphocytes, demonstrating its biocompatibility as a potential bone graft material. Acta Biomaterialia 2010, 6, 892–899. [Google Scholar] [CrossRef]
- Staniewicz-Brudnik, B.; Lekka, M.; Bączek, E.; Wodnicka, K.; Miller, T.; Wilk, W. Biocomposites with submicrocrystalline sintered corundum and bioglass system as substrates and their structural and physical properties. Short- and long-term cultures of the fibroblast human skin on these substrates. Optica Applicata 2012, 42, 387–397. [Google Scholar]
- Day, R.M. Bioactive glass stimulates the secretion of angiogenic growth factors and angiogenesis in vitro. Tissue Eng. 2005, 11, 768–777. [Google Scholar] [CrossRef]
- Yu, H.F.; Peng, J.L.; Xu, Y.H.; Chang, J.; Li, H.Y. Bioglass activated skin tissue engineering constructs for wound healing. ACS Appl. Mater. Interfaces 2016, 8, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Choee, J.H.; Lee, S.J.; Lee, Y.M.; Rhee, J.M.; Lee, H.B.; Khang, G. Proliferation rate of fibroblast cells on polyethylene surfaces with wettability gradient. J. Appl. Polym. Sci. 2004, 92, 599–606. [Google Scholar] [CrossRef]
- Soon Hee, K.; Hyun Jung, H.; Youn Kyung, K.; Sun Jung, Y.; Rhee, J.M.; Moon Suk, K.; Hai Bang, L.; Khang, G. Correlation of proliferation, morphology and biological responses of fibroblasts on ldpe with different surface wettability. J. Biomater. Sci. Polym. Ed. 2007, 18, 609–622. [Google Scholar]
- Novotná, Z.; Rimpelová, S.; Juřík, P.; Veselý, M.; Kolská, Z.; Hubáček, T.; Ruml, T.; Švorčík, V. The interplay of plasma treatment and gold coating and ultra-high molecular weight polyethylene: On the cytocompatibility. Mater. Sci. Eng. C 2017, 71, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Rimpelová, S.; Kasálková, N.S.; Slepička, P.; Lemerová, H.; Švorčík, V.; Ruml, T. Plasma treated polyethylene grafted with adhesive molecules for enhanced adhesion and growth of fibroblasts. Mater. Sci. Eng. C 2013, 33, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Soparkar, C.N.S.; Wong, J.F.; Patrinely, J.R.; Appling, D. Epidermal and fibroblast growth factors enhance fibrovascular integration of porous polyethylene implants. Ophthalmic Plast. Reconstr. Surg. 2000, 16, 337–340. [Google Scholar] [CrossRef]
- Anindyajati, A.; Boughton, P.; Ruys, A. The effect of rotating collector design on tensile properties and morphology of electrospun polycaprolactone fibres. MATEC Web Conf. 2015, 27, 02002. [Google Scholar] [CrossRef]
- Roether, J.A.; Boccaccini, A.R.; Hench, L.L.; Maquet, V.; Gautier, S.; Jerjme, R. Development and in vitro characterisation of novel bioresorbable and bioactive composite materials based on polylactide foams and bioglass for tissue engineering applications. Biomaterials 2002, 23, 3871–3878. [Google Scholar] [CrossRef]
- Stamboulis, A.G.; Boccaccini, A.R.; Hench, L.L. Novel biodegradable polymer/bioactive glass composites for tissue engineering applications. Adv. Eng. Mater. 2002, 4, 105–109. [Google Scholar] [CrossRef]
- Chatterjee, K.; Hung, S.; Kumar, G.; Simon, C.G. Time-dependent effects of pre-aging 3D polymer scaffolds in cell culture medium on cell proliferation. J. Funct. Biomater. 2012, 3, 372–381. [Google Scholar] [CrossRef]
- Thevenot, P.; Nair, A.; Dey, J.; Yang, J.; Tang, L. Method to analyze three-dimensional cell distribution and infiltration in degradable scaffolds. Tissue Eng. Part C Methods 2008, 14, 319–331. [Google Scholar] [CrossRef]
- Sobieraj, M.C.; Rimnac, C.M. Ultra high molecular weight polyethylene: Mechanics, morphology, and clinical behavior. J. Mech. Behav. Biomed. Mater. 2009, 2, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, G.; Goudouri, O.M.; Kontonasaki, E.; Chatzistavrou, X.; Papadopoulou, L.; Kantiranis, N.; Paraskevopoulos, K.M. Comparative bioactivity study of 45S5 and 58S bioglasses in organic and inorganic environment. Bioceram. Dev. Appl. 2011, 1, 4. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Efthymiou, A.; Salih, V.; Boccaccini, A.R. Bioglass®-derived glass–ceramic scaffolds: Study of cell proliferation and scaffold degradation in vitro. J. Biomed. Mater. Res. Part A 2008, 84A, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Rohanova, D.; Boccaccini, A.R.; Horkavcova, D.; Bozdechova, P.; Bezdicka, P.; Castoralova, M. Is non-buffered DMEM solution a suitable medium for in vitro bioactivity tests? J. Mater. Chem. B 2014, 2, 5068–5076. [Google Scholar] [CrossRef] [Green Version]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of bioactive glasses on angiogenesis: A review of in vitro and in vivo evidences. Tissue Eng. Part B Rev. 2010, 16, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Shepherd, E.F.; Jackson, W.O.; Dorey, F.J.; Schmalzried, T.P. Average patient walking activity approaches 2 million cycles per year. J. Arthroplast. 2002, 17, 693–697. [Google Scholar] [CrossRef]
- Reznickova, A.; Novotna, Z.; Kolska, Z.; Kasalkova, N.S.; Rimpelova, S.; Svorcik, V. Enhanced adherence of mouse fibroblast and vascular cells to plasma modified polyethylene. Mater. Sci. Eng. C 2015, 52, 259–266. [Google Scholar] [CrossRef]
- Matsuda, T.; Yamauchi, K.; Ito, G. The influence of bioglass on the growth of fibroblasts. J. Biomed. Mater. Res. 1987, 21, 499–507. [Google Scholar] [CrossRef]
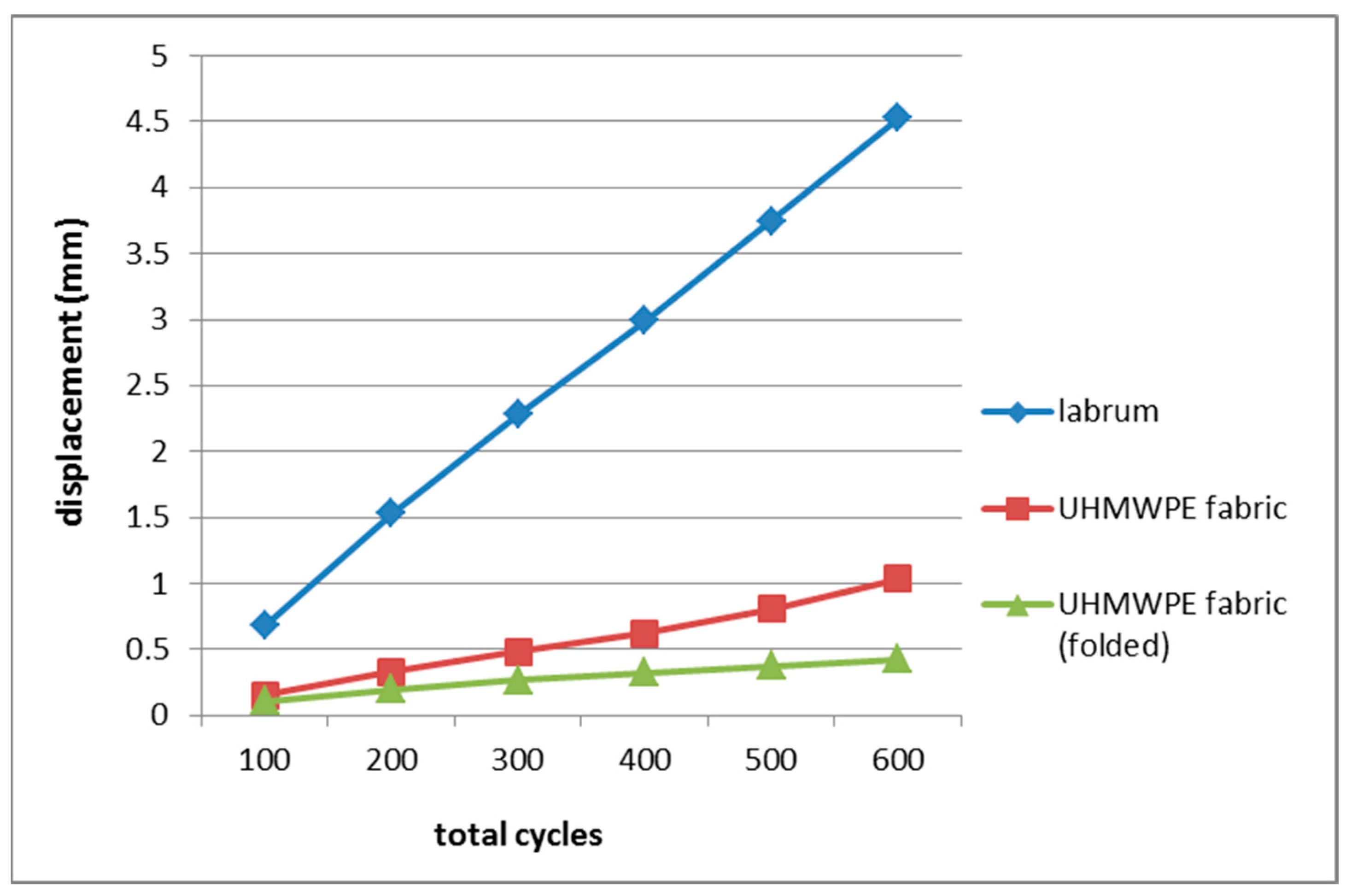
| Cycle (Load) | Mean Displacement (mm) | ||
|---|---|---|---|
| Labrum [53] | UHMWPE Fabric | Folded UHMWPE Fabric | |
| 0–100 (20–50 N) | 0.68 | 0.153 | 0.106 |
| 0–200 (20–100 N) | 1.53 | 0.331 | 0.200 |
| 0–300 (20–150 N) | 2.28 | 0.486 | 0.269 |
| 0–400 (20–200N) | 2.99 | 0.625 | 0.326 |
| 0–500 (20–250 N) | 3.75 | 0.806 | 0.378 |
| 0–600 (20–300 N) | 4.53 | 1.038 | 0.426 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anindyajati, A.; Boughton, P.; Ruys, A.J. Mechanical and Cytocompatibility Evaluation of UHMWPE/PCL/Bioglass® Fibrous Composite for Acetabular Labrum Implant. Materials 2019, 12, 916. https://doi.org/10.3390/ma12060916
Anindyajati A, Boughton P, Ruys AJ. Mechanical and Cytocompatibility Evaluation of UHMWPE/PCL/Bioglass® Fibrous Composite for Acetabular Labrum Implant. Materials. 2019; 12(6):916. https://doi.org/10.3390/ma12060916
Chicago/Turabian StyleAnindyajati, Adhi, Philip Boughton, and Andrew J. Ruys. 2019. "Mechanical and Cytocompatibility Evaluation of UHMWPE/PCL/Bioglass® Fibrous Composite for Acetabular Labrum Implant" Materials 12, no. 6: 916. https://doi.org/10.3390/ma12060916
APA StyleAnindyajati, A., Boughton, P., & Ruys, A. J. (2019). Mechanical and Cytocompatibility Evaluation of UHMWPE/PCL/Bioglass® Fibrous Composite for Acetabular Labrum Implant. Materials, 12(6), 916. https://doi.org/10.3390/ma12060916






