Corrosion Initiation and Propagation on Carburized Martensitic Stainless Steel Surfaces Studied via Advanced Scanning Probe Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electron Microscopy
2.3. Scanning Probe Microscopy
2.3.1. Ex situ Scanning Probe Microscopy (SPM)
2.3.2. In Situ SPM
2.3.3. Image Processing
2.4. Electrochemical Corrosion Testing
3. Results
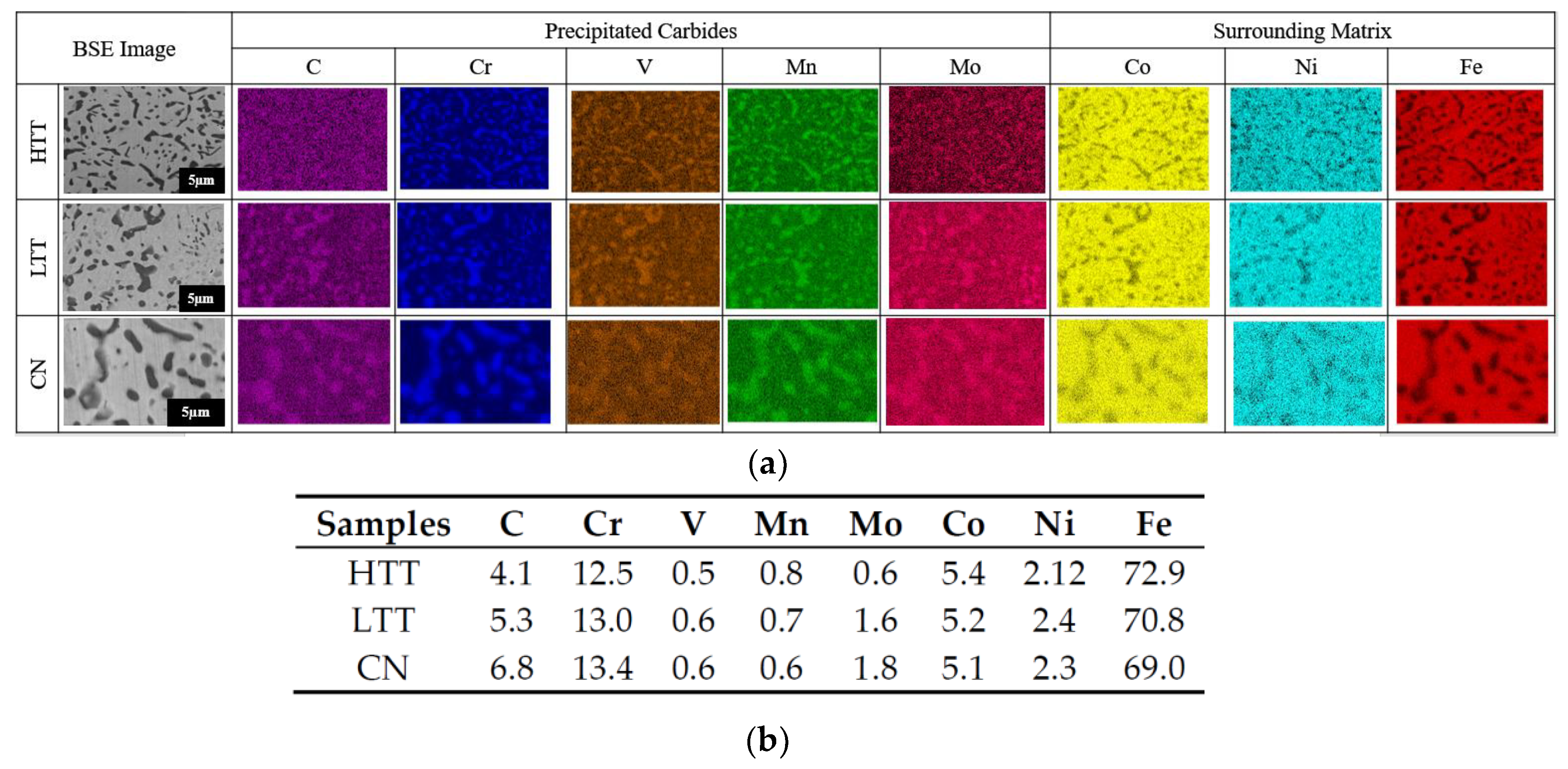
3.1. Surface Composition
3.2. Scanning Probe Microscopy
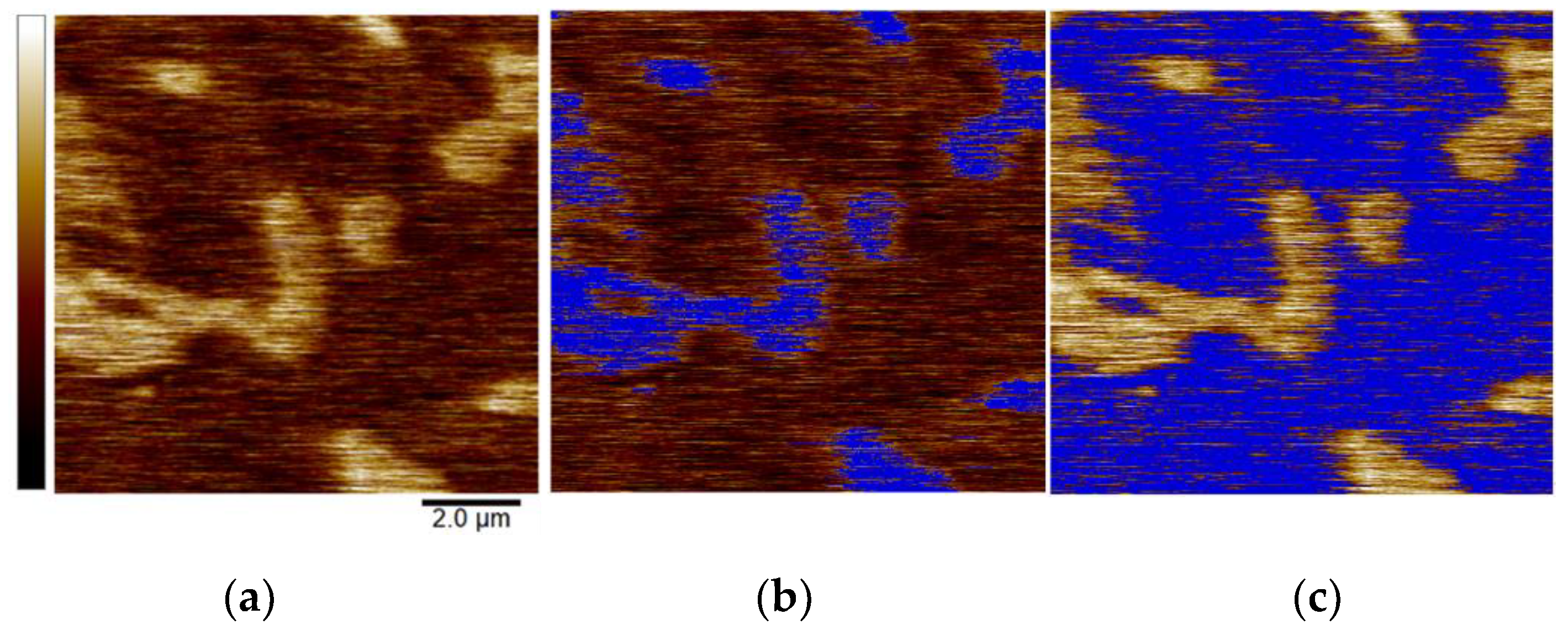
3.2.1. Magnetic Force Microscopy (MFM)
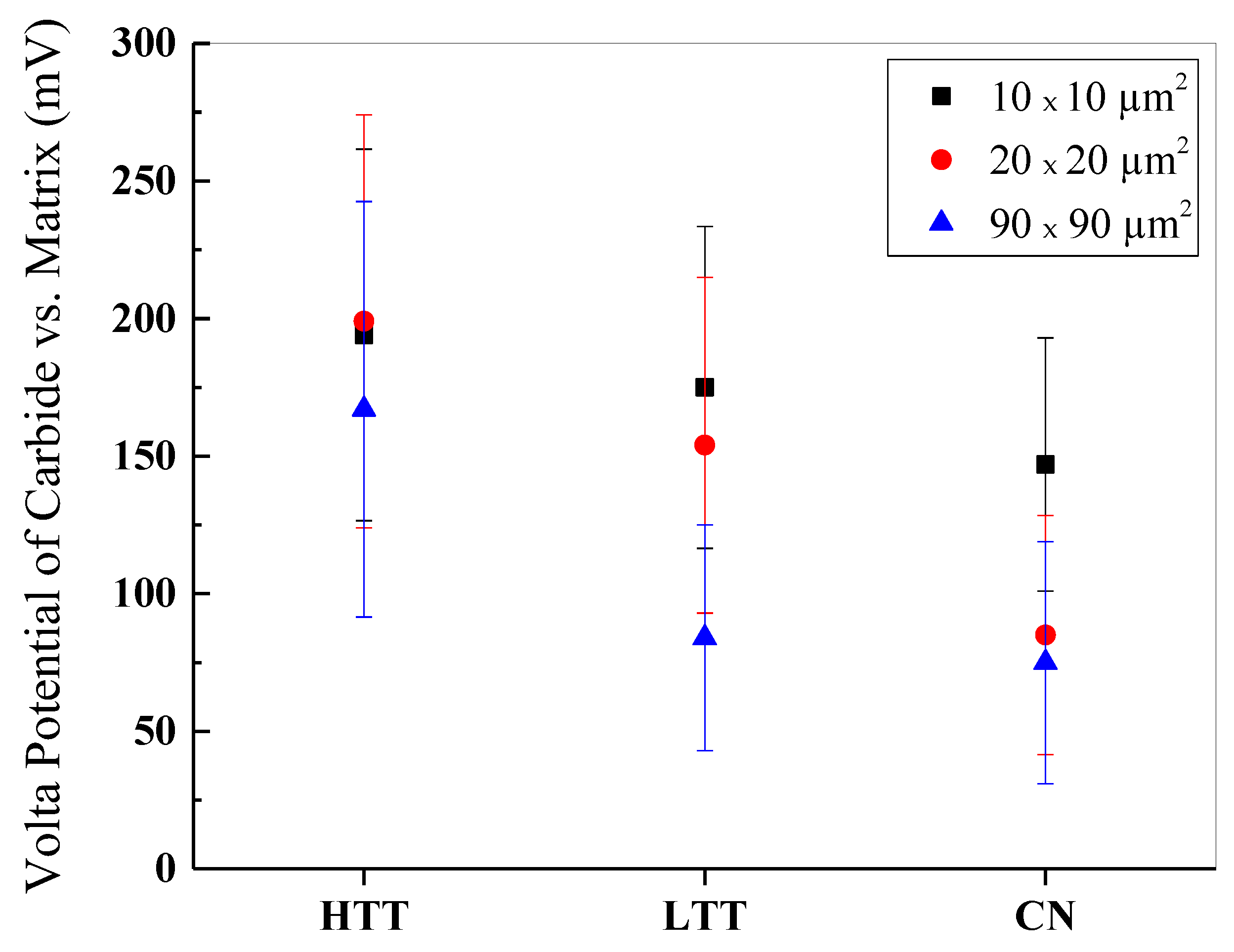
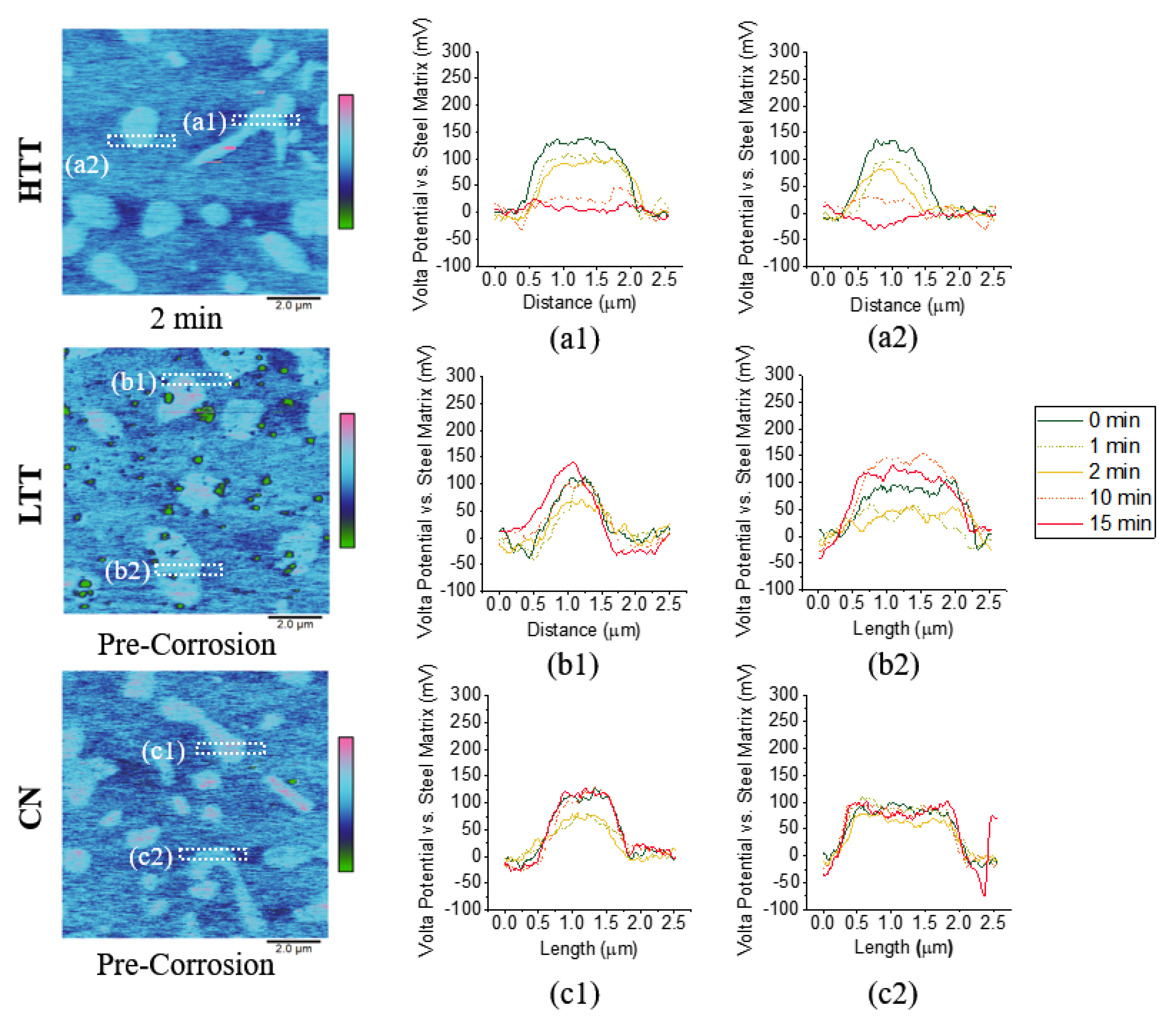
3.2.2. Inert Environment Scanning Kelvin Probe Microscopy (SKPFM)
3.2.3. Intermittent SKPFM
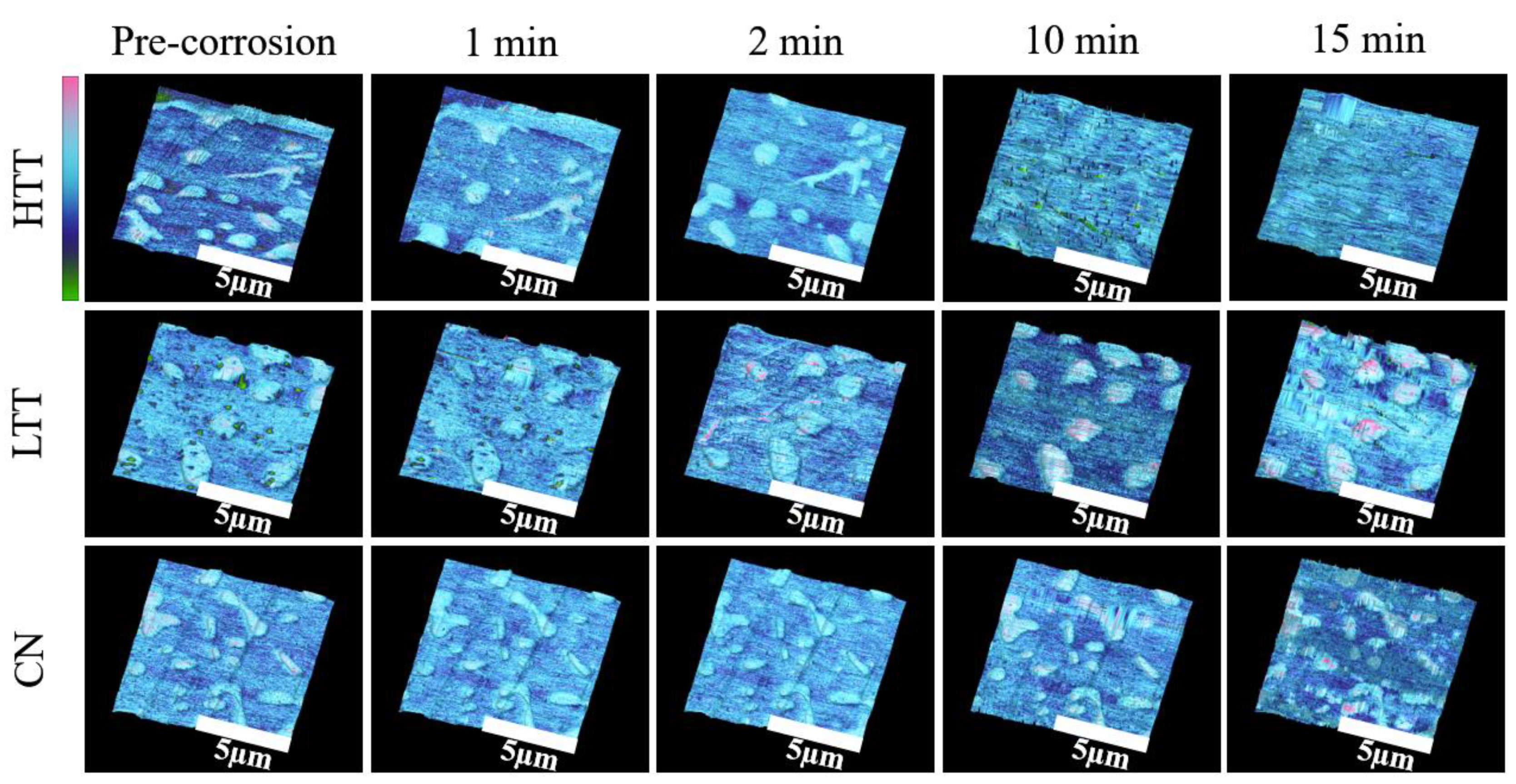
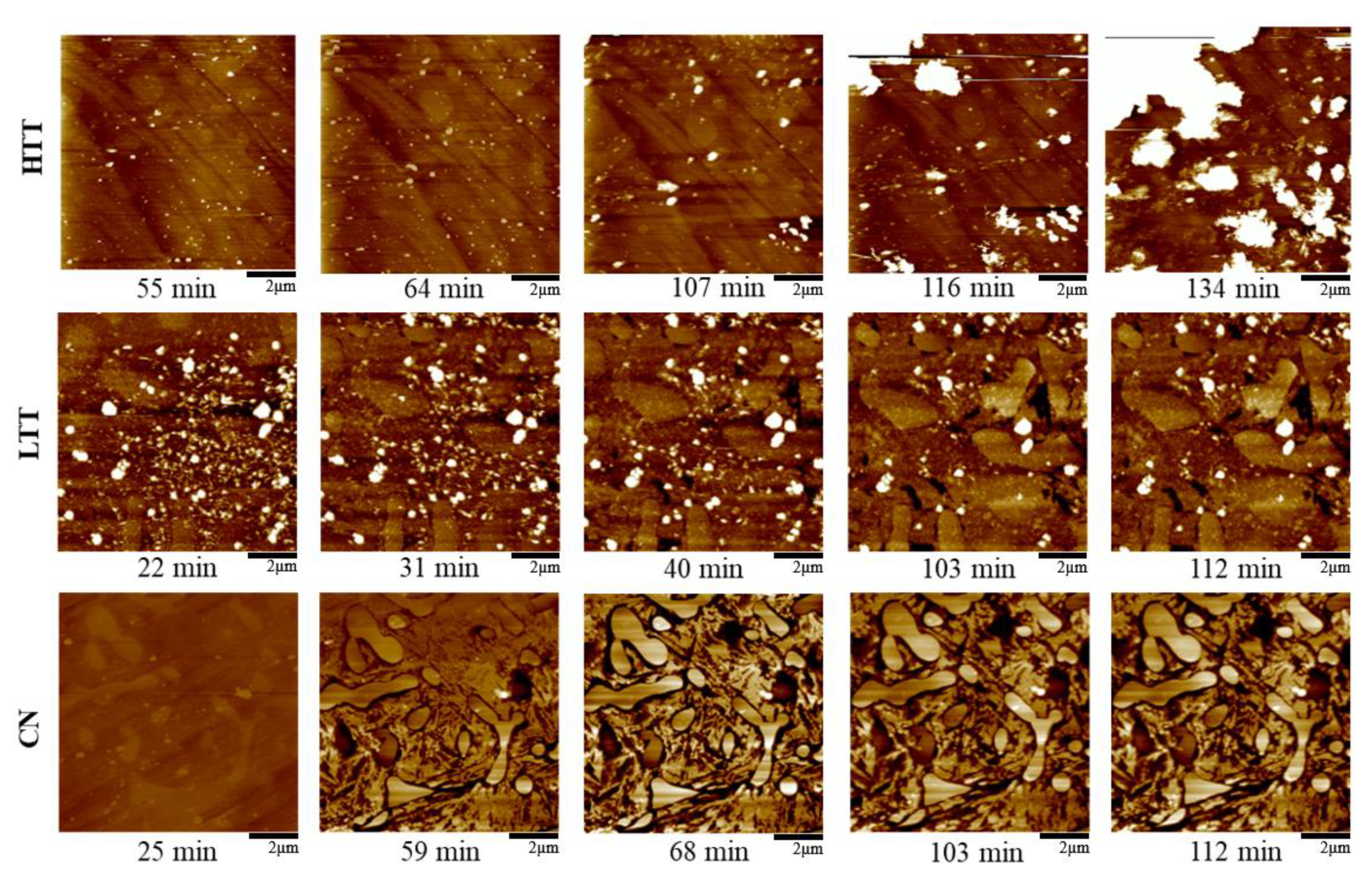
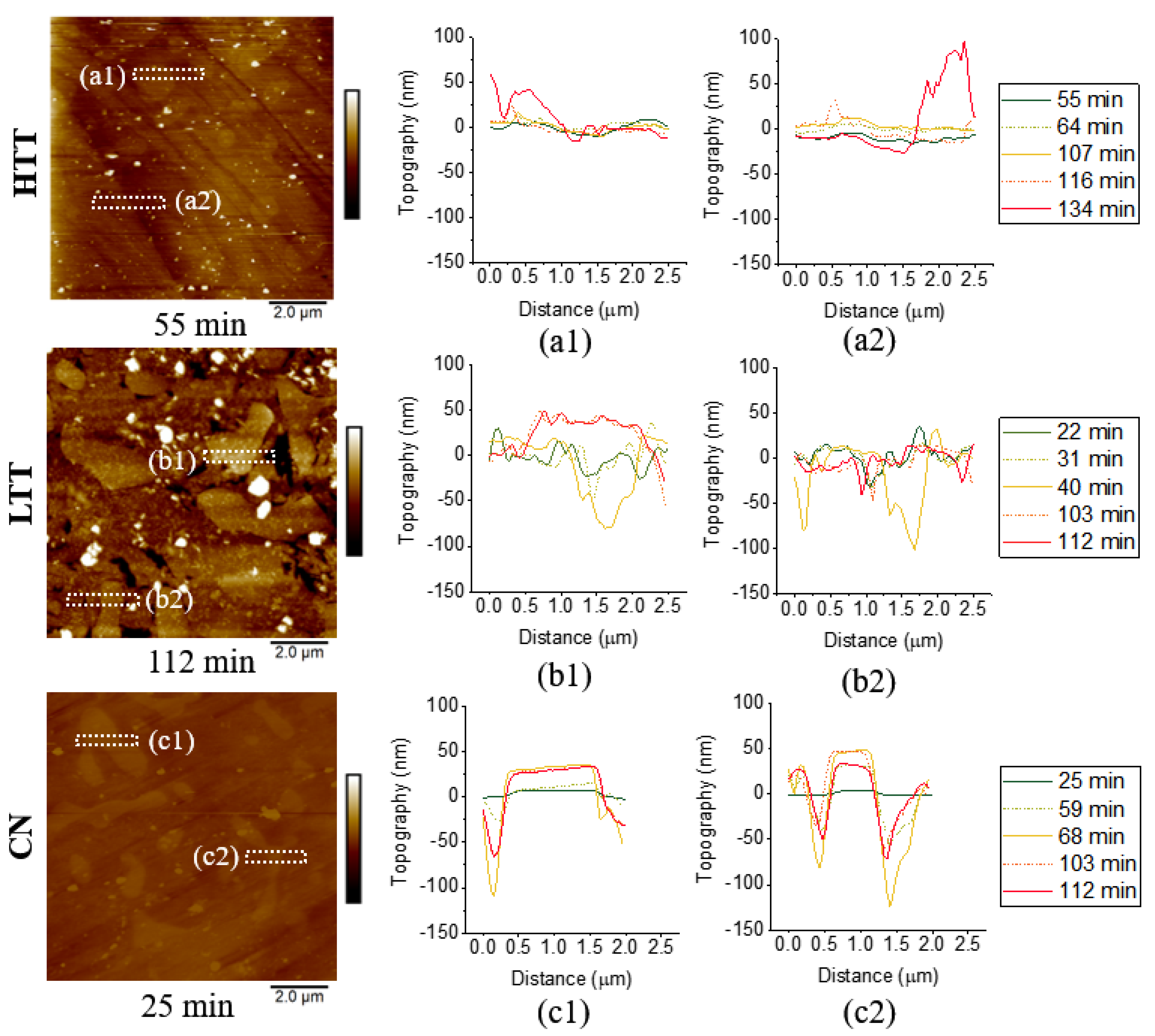
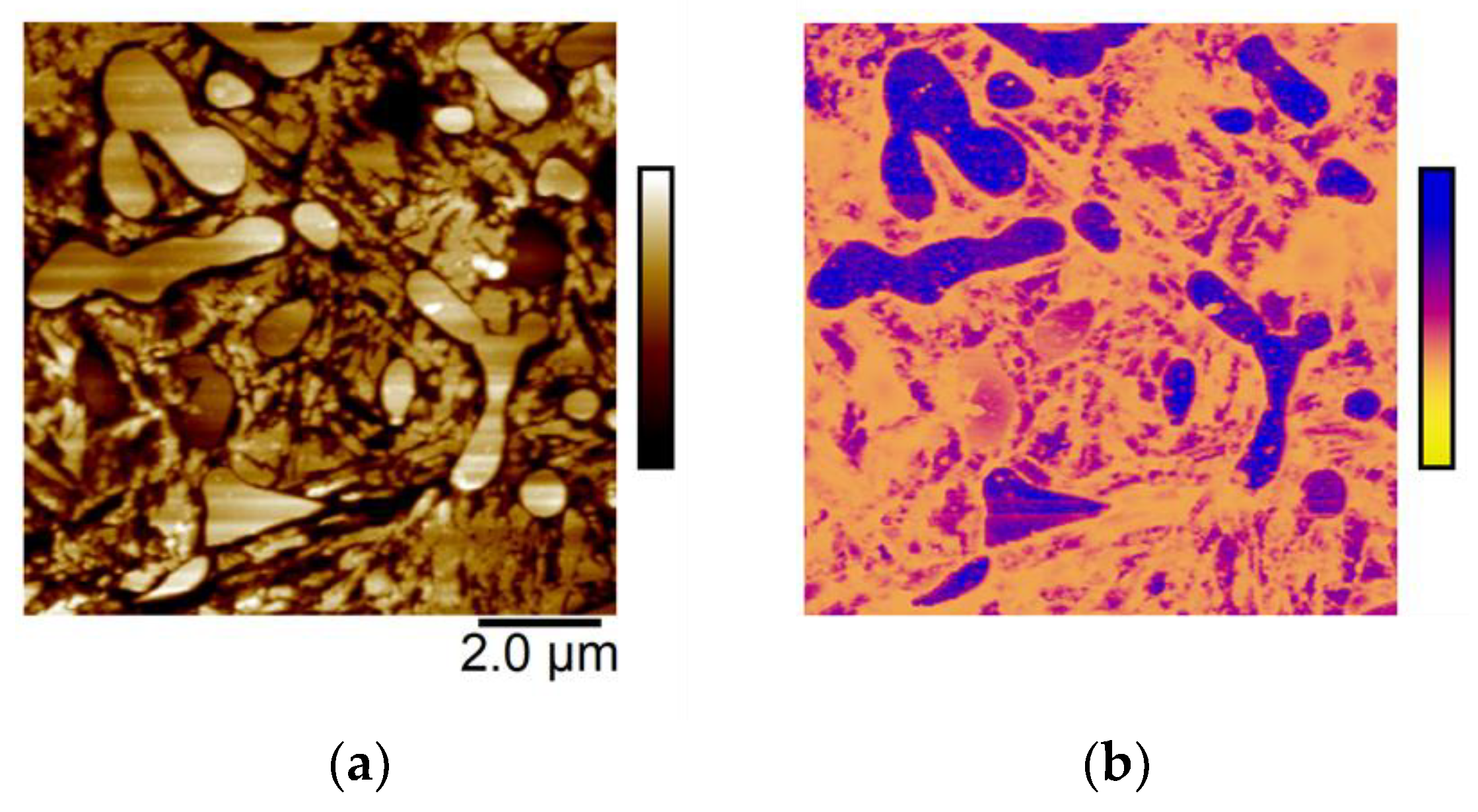
3.2.4. In Situ Atomic Force Microscopy (AFM)
3.3. Electrochemical Corrosion Testing
4. Discussion
4.1. Nanoscale Origins of Corrosion Initiation
4.2. Corrosion Propagation
4.3. SPM Characterization and Implications on Wear
5. Conclusions
- MFM imaging distinguished local differences in magnetic properties where precipitated carbides exhibited a larger magnetic moment than the matrix, likely due to the presence of chromium relative to the chromium-depleted matrix.
- SKPFM VPD measurements in an inert environment showed HTT as the thermodynamically most favorable to experience microgalvanic corrosion between the chromium-rich precipitated carbides and the surrounding martensitic matrix, with a measured carbide-matrix VPD of 200 mV, while LTT (150 mV) and CN (90 mV) were less.
- Intermittent SKPFM showed the HTT sample behaved differently during corrosion than the LTT and CN samples; by the end of the testing period, there was minimal VPD between the HTT carbides and the surrounding matrix, whereas the carbides present in the LTT and CN samples retained their relative nobility throughout testing.
- Corrosion propagation was also monitored in real time via in situ AFM and revealed that HTT underwent the most rapid spread of corrosion attack across the sample, while LTT and CN were less affected and showed much more localized, intergranular attack and adjacent to carbides.
- Bulk electrochemical testing results agreed with in situ AFM results, with LTT and CN showing distinct passive regions as compared to HTT, confirming the nanoscale differences in corrosion behavior observed between the steel heat treatments investigated.
Author Contributions
Funding
Conflicts of Interest
References
- Bhadeshia, H.K.D.H. Steels for bearings. Prog. Mater. Sci. 2012, 57, 268–435. [Google Scholar] [CrossRef]
- Davies, D.P. Gear materials in helicopter transmissions. Metals Mater. 1986, 2, 342–348. [Google Scholar]
- Zaretsky, E.V. Rolling bearing steels—A technical and historical perspective. Mater. Sci. Technol. 2012, 28, 58–69. [Google Scholar] [CrossRef]
- Zaretsky, E.V. Bearing and gear steels for aerospace applications. In NASA Technical Memorandum 102529; 1990. Available online: https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19900011075.pdf (accessed on 16 March 2019).
- Hurley, M.F.; Marx, B.M.; Allahar, K.N.; Smith, C.P.; Chin, H.A.; Ogden, W.P.; Butt, D.P. Corrosion Assessment and Characterization of Aerospace-Bearing Steels in Seawater and Ester-Based Lubricants. Corrosion 2012, 68, 645–661. [Google Scholar] [CrossRef]
- Popgoshev, D.; Valori, R. Rolling Contact Fatigue Evaluation of Advanced Bearing Steels STP 771; ASTM International: West Conshohocken, PA, USA, 1982. [Google Scholar]
- Kvryan, A.; Faulkner, E.; Lysne, D.; Carter, N.; Acharya, S.; Rafla, V.; Trivedi, H.K.; Hurley, M.F. Electrochemical Corrosion Test Methods for Rapid Assessment of Aerospace Bearing Steel Performance. In Bearing Steel Technologies:11th Volume, Progress in Steel Technologies and Bearing Steeel Quality Assurance, ASTM STP1600; ASTM International: West Conshohocken, PA, USA, 2017; pp. 466–486. [Google Scholar]
- Zaretsky, E.V.; Branzai, E.V. Rolling Bearing Service Life Based on Probable Cause for Removal—A Tutorial. Tribol. Trans. 2017, 60, 300–312. [Google Scholar] [CrossRef]
- Trivedi, H.; Otto, F.; McCoy, B. Low Temperature Plasma Nitriding of Pyrowear 675. In Bearing steel Technologies: 10th Volume, Advances in Steel Technologies for Rolling Bearings, STP 1580; ASTM International: West Conshocken, PA, USA, 2015; Volume 10, pp. 444–464. [Google Scholar]
- Trivedi, H.; Monahan, R. Low Temperature Plasma Nitriding of Pyrowear 675. In Bearing steel Technologies: 10th Volume, Advances in Steel Technologies for Rolling Bearings, STP 1580; ASTM International: West Conshocken, PA, USA, 2015; Volume 10, pp. 444–464. [Google Scholar]
- Dalmau, A.; Richard, C.; Igual–Muñoz, A. Degradation mechanisms in martensitic stainless steels: Wear, corrosion and tribocorrosion appraisal. Tribol. Int. 2018, 121, 167–179. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.; Liu, C.; Subramanian, S. Microstructure and properties of 13Cr5Ni1Mo0. 025Nb0. 09V0. 06N super martensitic stainless steel. Mater. Sci. Eng. A 2012, 539, 271–279. [Google Scholar] [CrossRef]
- Thibault, D.; Bocher, P.; Thomas, M. Residual stress and microstructure in welds of 13% Cr–4% Ni martensitic stainless steel. J. Mater. Process. Technol. 2009, 209, 2195–2202. [Google Scholar] [CrossRef]
- Li, C.; Bell, T. Corrosion properties of plasma nitrided AISI 410 martensitic stainless steel in 3.5% NaCl and 1% HCl aqueous solutions. Corros. Sci. 2006, 48, 2036–2049. [Google Scholar] [CrossRef]
- Smallman, R.E.; Ngan, A.H.W. Physical Metallurgy and Advanced Materials, 7th ed.; Elsevier Ltd.: Burlington, MA, USA, 2007. [Google Scholar]
- Klueh, R.; Nelson, A. Ferritic/martensitic steels for next-generation reactors. J. Nuclear Mater. 2007, 371, 37–52. [Google Scholar] [CrossRef]
- Zinkle, S.J. Advanced materials for fusion technology. Fusion Eng. Des. 2005, 74, 31–40. [Google Scholar] [CrossRef]
- Puli, R.; Ram, G.J. Microstructures and properties of friction surfaced coatings in AISI 440C martensitic stainless steel. Surf. Coat. Technol. 2012, 207, 310–318. [Google Scholar] [CrossRef]
- Anantha, K.H.; Örnek, C.; Ejnermark, S.; Medvedeva, A.; Sjöström, J.; Pan, J. Correlative Microstructure Analysis and In Situ Corrosion Study of AISI 420 Martensitic Stainless Steel for Plastic Molding Applications. J. Electrochem. Soc. 2017, 164, C85–C93. [Google Scholar] [CrossRef]
- Klecka, M.A. Microstructure-Property Relationships and Constitutive Response of Plastically Graded Case Hardened Steels; University of Florida: Gainesville, FL, USA, 2011. [Google Scholar]
- Schneider, J.M.; Chatterjee, M. Introduction to Surface Hardening of Steels; ASM International: West Conshohocken, PA, USA, 2013; Volume 4A, pp. 389–398. [Google Scholar]
- Godec, M.; Batic, B.S.; Mandrino, D.; Nagode, A.; Leskovsek, V.; Skapin, S.D.; Jenko, M. Characterization of the carbides and the martensite phase in powder-metallurgy high-speed steel. Mater. Charact. 2010, 61, 452–458. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An Introduction; Wiley: New York, NY, USA, 2007; Volume 7. [Google Scholar]
- Johnson, M.; Laritz, J.; Rhoads, M. Thin Dense Chrome Bearing Insertion Program: Pyrowear 675 and Cronidur Wear Testing; GE Advanced Engineering Technologies Dept: Cincinnati, OH, USA, 1998. [Google Scholar]
- Wert, D.E. Development of a carburizing stainless steel alloy. Adv. Mater. Process. 1994, 145, 89–91. [Google Scholar]
- Trivedi, H.K.; Wedeven, V.; Black, W. Effect of Silicon Nitride Ball on Adhesive Wear of Martensitic Stainless Steel Pyrowear 675 and AISI M-50 Races with Type II Ester Oil. Tribol. Trans. 2016, 59, 363–374. [Google Scholar] [CrossRef]
- Hetzner, D.W.; Van Geertruyden, W. Crystallography and metallography of carbides in high alloy steels. Mater. Charact. 2008, 59, 825–841. [Google Scholar] [CrossRef]
- Goldschmidt, H. The structure of carbides in alloy steels. Part 2-Carbide formation in high-speed steels. J. Iron Steel Inst. 1952, 170, 189. [Google Scholar]
- Trivedi, H.; Rosado, L.; Gerardi, D.; Givan, G.; McCoy, B. Fatigue Life Performance of Hybrid Angular Contact Pyrowear 675 Bearings. In Bearing Steel Technologies: 11th Volume, Progress in Steel Technologies and Bearing Steel Quality Assurance, ASTM STP 1600; ASTM International: West Conshohocken, PA, USA, 2017; pp. 275–295. [Google Scholar]
- Kirsch, M.; Trivedi, H. Microstructural Changes in Aerospace Bearing Steels under Accelerated Rolling Contact Fatigue Life Testing. In Bearing Steel Technologies: 11th Volume, Progress in Steel Technologies and Bearing Steel Quality Assurance, ASTM STP 1600; ASTM International: West Conshohocken, PA, USA, 2017; pp. 92–107. [Google Scholar]
- Reynaud-Laporte, I.; Vayer, M.; Kauffmann, J.-P.; Erre, R. An electrochemical-AFM study of the initiation of the pitting corrosion of a martensitic stainless steel. Microsc. Microanal. Microstr. 1997, 8, 175–185. [Google Scholar] [CrossRef]
- Leblanc, P.P.; Frankel, G. Investigation of filiform corrosion of epoxy-coated 1045 carbon steel by scanning Kelvin probe force microscopy. J. Electrochem. Soc. 2004, 151, B105–B113. [Google Scholar] [CrossRef]
- Schmutz, P.; Frankel, G.S. Characterization of AA2024-T3 by scanning Kelvin probe force microscopy. J. Electrochem. Soc. 1998, 145, 2285–2295. [Google Scholar] [CrossRef]
- Campestrini, P.; van Westing, E.P.M.; van Rooijen, H.W.; de Wit, J.H.W. Relation between microstructural aspects of AA2024 and its corrosion behaviour investigated using AFM scanning potential technique. Corros. Sci. 2000, 42, 1853–1861. [Google Scholar] [CrossRef]
- de Wit, J.H.W. Local potential measurements with the SKPFM on aluminium alloys. Electrochim. Acta 2004, 49, 2841–2850. [Google Scholar] [CrossRef]
- Larignon, C.; Alexis, J.; Andrieu, E.; Lacroix, L.; Odemer, G.; Blanc, C. Combined Kelvin probe force microscopy and secondary ion mass spectrometry for hydrogen detection in corroded 2024 aluminium alloy. Electrochim. Acta 2013, 110, 484–490. [Google Scholar] [CrossRef] [Green Version]
- Hurley, M.F.; Efaw, C.M.; Davis, P.H.; Croteau, J.R.; Graugnard, E.; Birbilis, N. Volta Potentials Measured by Scanning Kelvin Probe Force Microscopy as Relevant to Corrosion of Magnesium Alloys. Corrosion 2015, 71, 160–170. [Google Scholar] [CrossRef]
- Kvryan, A.; Livingston, K.; Efaw, C.M.; Knori, K.; Jaques, B.J.; Davis, P.H.; Butt, D.P.; Hurley, M.F. Microgalvanic Corrosion Behavior of Cu-Ag Active Braze Alloys Investigated with SKPFM. Metals 2016, 6, 91. [Google Scholar] [CrossRef]
- Guillaumin, V.; Schmutz, P.; Frankel, G.S. Characterization of Corrosion Interfaces by the Scanning Kelvin Probe Force Microscopy Technique. Electrochem. Soc. 2001, 148, 163–173. [Google Scholar] [CrossRef]
- Andreatta, F.; Terryn, H.; De Wit, J. Corrosion behaviour of different tempers of AA7075 aluminium alloy. Electrochim. Acta 2004, 49, 2851–2862. [Google Scholar] [CrossRef]
- Örnek, C.; Leygraf, C.; Pan, J. Passive film characterisation of duplex stainless steel using scanning Kelvin prove force microscopy in combination with electrochemical measurements. NPJ Mater. Degrad. 2019, 3, 8. [Google Scholar] [CrossRef]
- Anantha, K.H.; Örnek, C.; Ejnermark, S.; Medvedeva, A.; Sjöström, J.; Pan, J. In Situ AFM Study of Localized Corrosion Processes of Tempered AISI 420 Martensitic Stainless Steel: Effect of Secondary Hardening. J. Electrochem. Soc. 2017, 164, C810–C818. [Google Scholar] [CrossRef]
- Ramírez-Salgado, J.; Domínguez-Aguilar, M.; Castro-Domínguez, B.; Hernández-Hernández, P.; Newman, R. Detection of secondary phases in duplex stainless steel by magnetic force microscopy and scanning Kelvin probe force microscopy. Mater. Charact. 2013, 86, 250–262. [Google Scholar] [CrossRef]
- Sathirachinda, N.; Pettersson, R.; Pan, J.S. Depletion effects at phase boundaries in 2205 duplex stainless steel characterized with SKPFM and TEM/EDS. Corros. Sci. 2009, 51, 1850–1860. [Google Scholar] [CrossRef]
- Femenia, M.; Canalias, C.; Pan, J.; Leygraf, C. Scanning Kelvin probe force microscopy and magnetic force microscopy for characterization of duplex stainless steels. J. Electrochem. Soc. 2003, 150, B274–B281. [Google Scholar] [CrossRef]
- Birbilis, N.; Meyer, K.; Muddle, B.; Lynch, S. In situ measurement of corrosion on the nanoscale. Corros. Sci. 2009, 51, 1569–1572. [Google Scholar] [CrossRef]
- Davoodi, A.; Pan, J.; Leygraf, C.; Norgren, S. In situ investigation of localized corrosion of aluminum alloys in chloride solution using integrated EC-AFM/SECM techniques. Electrochem. Solid-State Lett. 2005, 8, B21–B24. [Google Scholar] [CrossRef]
- Guo, L.; Li, M.; Shi, X.L.; Yan, Y.; Li, X.; Qiao, L. Effect of annealing temperature on the corrosion behavior of duplex stainless steel studied by in situ techniques. Corros. Sci. 2011, 53, 3733–3741. [Google Scholar] [CrossRef]
- Park, J.; Kalnaus, S.; Han, S.; Lee, Y.K.; Less, G.B.; Dudney, N.J.; Daniel, C.; Sastry, A.M. In situ atomic force microscopy studies on lithium (de) intercalation-induced morphology changes in LixCoO2 micro-machined thin film electrodes. J. Power Sources 2013, 222, 417–425. [Google Scholar] [CrossRef]
- Leblanc, P.; Frankel, G.S. A study of corrosion and pitting initiation of AA2024-T3 using atomic force microscopy. J. Electrochem. Soc. 2002, 149, B239–B247. [Google Scholar] [CrossRef]
- Schmutz, P.; Frankel, G.S. Corrosion study of AA2024-T3 by scanning Kelvin probe force microscopy and in situ atomic force microscopy scratching. J. Electrochem. Soc. 1998, 145, 2295–2306. [Google Scholar] [CrossRef]
- Efaw, C.M.; da Silva, T.; Davis, P.H.; Li, L.; Graugnard, E.; Hurley, M.F. Toward Improving Ambient Volta Potential Measurements with SKPFM for Corrosion Studies. J. Electrochem. Soc. 2019, 166, C3018–C3027. [Google Scholar] [CrossRef]
- Klecka, M.A.; Subhash, G.; Arakere, N.K. Microstructure-Property Relationships in M50-NiL and P675 Case-Hardened Bearing Steels. Tribol. Trans. 2013, 56, 1046–1059. [Google Scholar] [CrossRef]
- Lind, M.A. The Infrared Reflectivity of Chromium and Chromium-Aluminum Alloys; Iowa State University: Ames, IA, USA, 1972. [Google Scholar]
- Fawcett, E.; Alberts, H.; Galkin, V.Y.; Noakes, D.; Yakhmi, J. Spin-density-wave antiferromagnetism in chromium alloys. Rev. Modern Phys. 1994, 66, 25. [Google Scholar] [CrossRef]
- Iga, A.; Tawara, Y. Magnetic properties of molybdenum-and wolfram-Modified Mn3B4. J. Phys. Soc. Jpn. 1968, 24, 28–35. [Google Scholar] [CrossRef]
- Mouritz, A.P. Introduction to Aerospace Materials; Woodhead Publishing: Philadelphia, PA, USA, 2012. [Google Scholar]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control; Elsevier: Burlington, MA, USA, 2006. [Google Scholar]
- Lauter, V.; Lauter, H.; Glavic, A.; Toperverg, B. Reference Module in Materials Science and Materials Engineering; Elsevier: Burlington, MA, USA, 2016. [Google Scholar]
- Forman, C.M.; Verchot, E.A. Practical Galvanic Series; RS-TR-67-11; Report No. RS-TR-67-11; Army Missile Command Redstone Arsenal AL Systems Research Directorate: Redstone Arsenal, AL, USA, 1967. [Google Scholar]
- Sathirachinda, N.; Gubner, R.; Pan, J.; Kivisäkk, U. Characterization of phases in duplex stainless steel by magnetic force microscopy/scanning Kelvin probe force microscopy. Electrochem. Solid-State Lett. 2008, 11, C41–C45. [Google Scholar] [CrossRef]
- Mészáros, I.; Szabo, P. Complex magnetic and microstructural investigation of duplex stainless steel. Non-destruct. Test. Eval. Int. 2005, 38, 517–521. [Google Scholar] [CrossRef]
- Tavares, S.; Da Silva, M.; Neto, J. Magnetic property changes during embrittlement of a duplex stainless steel. J. Alloys Compd. 2000, 313, 168–173. [Google Scholar] [CrossRef]
- Tavares, S.; Pedrosa, P.; Teodosio, J.; Da Silva, M.; Neto, J.; Pairis, S. Magnetic properties of the UNS S39205 duplex stainless steel. J. Alloys Compd. 2003, 351, 283–288. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Muller, V.M.; Toporov, Y.P. Effect of contact deformations on the adhesion of particles. J. Colloid Interface Sci. 1975, 53, 314–326. [Google Scholar] [CrossRef]




| Steel | C | Mn | Cr | Mo | Si | Ni | S | V | Co |
|---|---|---|---|---|---|---|---|---|---|
| P675 (AMS 5930B) | 0.07 | 0.75 | 13 | 2 | 0.4 | 2.5 | 0.010 | 0.6 | 6.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kvryan, A.; Efaw, C.M.; Higginbotham, K.A.; Maryon, O.O.; Davis, P.H.; Graugnard, E.; Trivedi, H.K.; Hurley, M.F. Corrosion Initiation and Propagation on Carburized Martensitic Stainless Steel Surfaces Studied via Advanced Scanning Probe Microscopy. Materials 2019, 12, 940. https://doi.org/10.3390/ma12060940
Kvryan A, Efaw CM, Higginbotham KA, Maryon OO, Davis PH, Graugnard E, Trivedi HK, Hurley MF. Corrosion Initiation and Propagation on Carburized Martensitic Stainless Steel Surfaces Studied via Advanced Scanning Probe Microscopy. Materials. 2019; 12(6):940. https://doi.org/10.3390/ma12060940
Chicago/Turabian StyleKvryan, Armen, Corey M. Efaw, Kari A. Higginbotham, Olivia O. Maryon, Paul H. Davis, Elton Graugnard, Hitesh K. Trivedi, and Michael F. Hurley. 2019. "Corrosion Initiation and Propagation on Carburized Martensitic Stainless Steel Surfaces Studied via Advanced Scanning Probe Microscopy" Materials 12, no. 6: 940. https://doi.org/10.3390/ma12060940






