Influence of PMMA on All-Inorganic Halide Perovskite CsPbBr3 Quantum Dots Combined with Polymer Matrix
Abstract
:1. Introduction
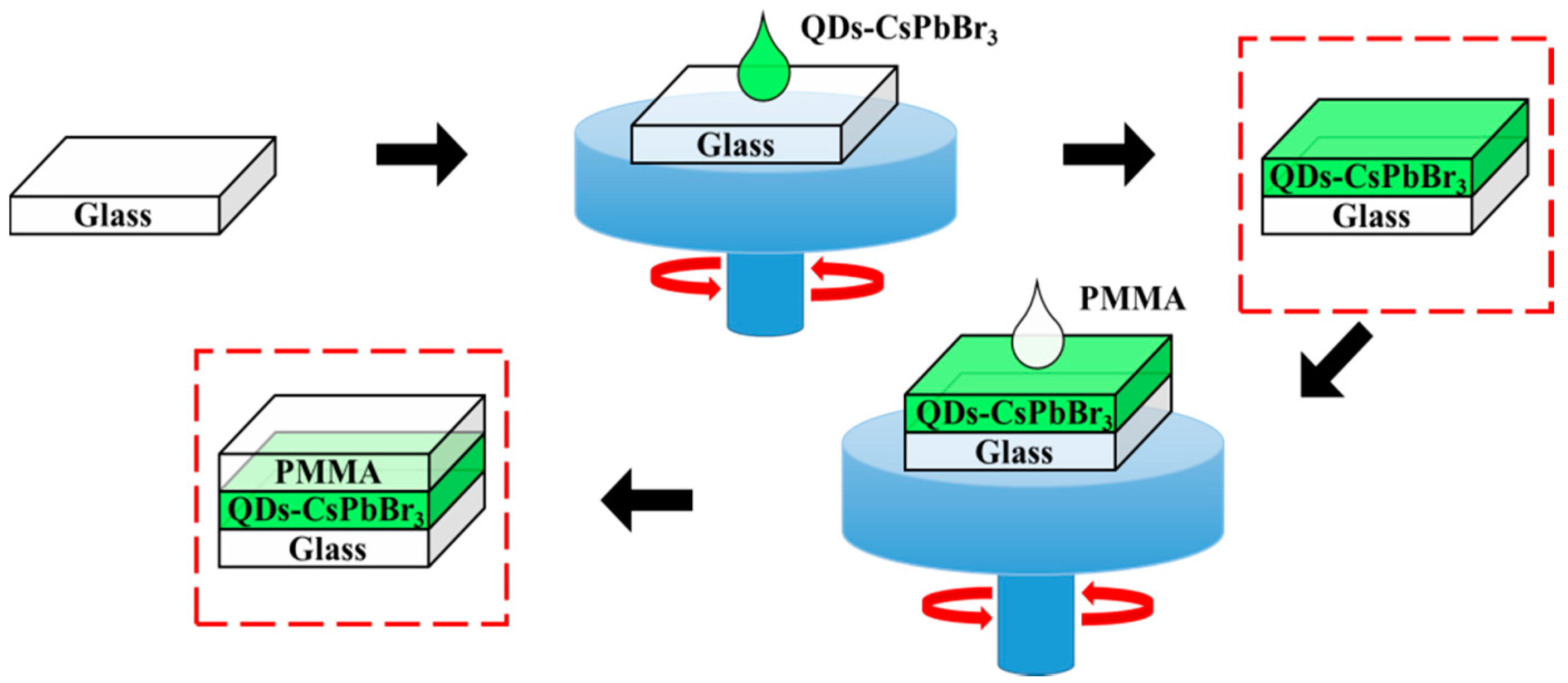
2. Materials and Methods
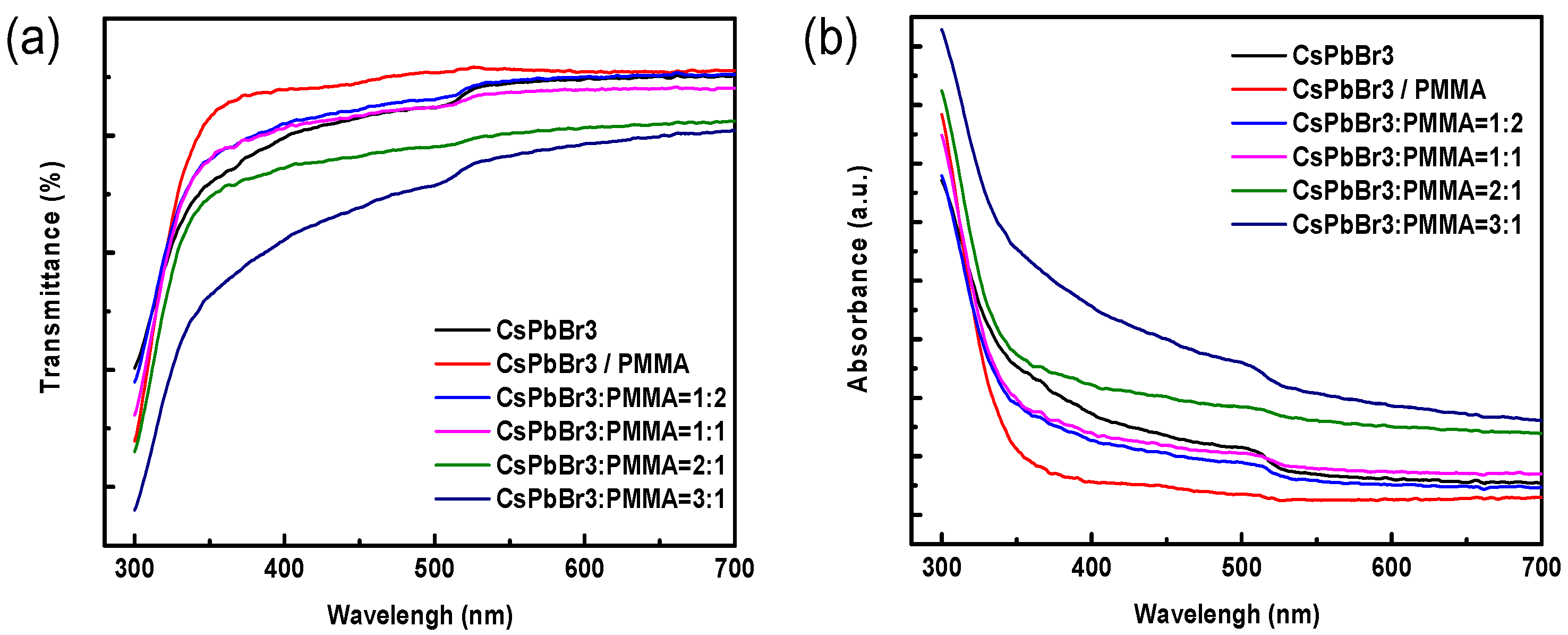
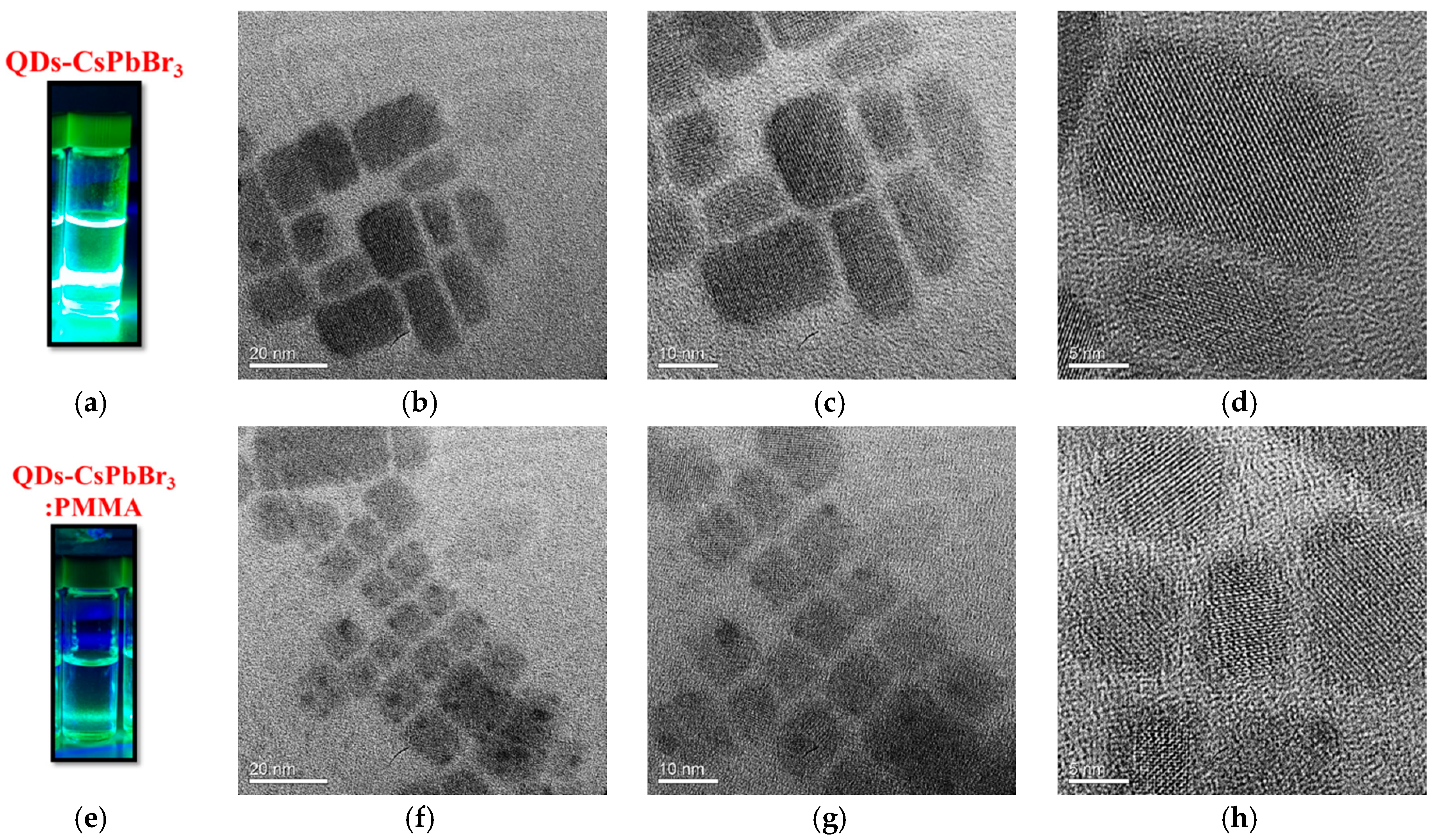
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shang, Y.; Ning, Z. Colloidal quantum-dots surface and device structure engineering for high-performance light-emitting diodes. Natl. Sci. Rev. 2017, 4, 170–183. [Google Scholar] [CrossRef]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium lead trihalide: a broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical management for colorful, efficient, and stable inorganic-organic hybrid nanostructured solar cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef]
- Lim, J.; Bae, W.K.; Kwak, J.; Lee, S.; Lee, C.; Char, K. Perspective on synthesis, device structures, and printing processes for quantum dot displays. Opt. Mater. Express 2012, 2, 594–628. [Google Scholar] [CrossRef]
- Shirasaki, Y.; Supran, G.J.; Bawendi, M.G.; Bulovic, V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photonics 2013, 7, 13–23. [Google Scholar] [CrossRef]
- Schmidt, L.C.; Pertegás, A.; González-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Espallargas, G.M.; Bolink, H.J.; Galian, R.E.; Pérez-Prieto, J. Nontemplate synthesis of CH3NH3PbBr3 perovskite nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Li, X.; Cao, F.; Yu, D.; Chen, J.; Sun, Z.; Shen, Y.; Zhu, Y.; Wang, L.; Wei, Y.; Wu, Y.; et al. All inorganic halide perovskites nanosystem: synthesis, structural features, optical properties and optoelectronic applications. Small 2017, 13, 1603996. [Google Scholar] [CrossRef]
- Butkus, J.; Vashishtha, P.; Chen, K.; Gallaher, J.K.; Prasad, S.K.K.; Metin, D.Z.; Laufersky, G.; Gaston, N.; Halpert, J.E.; Hodgkiss, J.M. The Evolution of Quantum Confinement in CsPbBr3 Perovskite Nanocrystals. Chem. Mater. 2017, 29, 3644–3652. [Google Scholar] [CrossRef]
- Aharon, S.; Etgar, L. Two dimensional organometal halide perovskite nanorods with tunable optical properties. Nano Lett. 2016, 16, 3230–3235. [Google Scholar] [CrossRef]
- Chen, L.C.; Tseng, Z.L.; Chen, S.Y.; Yang, S. An ultrasonic synthesis method for high-luminance perovskite quantum dots. Ceram. Int. 2017, 43, 16032–16035. [Google Scholar] [CrossRef]
- Chen, L.C.; Tien, C.H.; Tseng, Z.L.; Ruan, J.H. Enhance efficiency of MAPbI3 perovskite solar cells with FAPbX3 perovskite quantum dots. Nanomaterials 2019, 9, 121. [Google Scholar] [CrossRef]
- Lan, X.; Voznyy, O.; García de Arquer, F.P.; Liu, M.; Xu, J.; Proppe, A.H.; Walters, G.; Fan, F.; Tan, H.; Liu, M.; et al. 10.6% certified colloidal quantum dot solar cells via solvent polarity-engineered halide passivation. Nano Lett. 2016, 16, 4630–4634. [Google Scholar] [CrossRef]
- Kim, Y.H.; Wolf, C.; Kim, Y.T.; Cho, H.; Kwon, W.; Do, S.; Sadhanala, A.; Park, C.G.; Rhee, S.W.; Im, S.H.; et al. Highly efficient light-emitting diodes of colloidal metal-halide perovskite nanocrystals beyond quantum size. ACS Nano 2017, 11, 6586–6593. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Yuan, Z.; Tian, Y.; Wang, X.; Wang, J.C.; Xin, Y.; Hanson, K.; Ma, B.; Gao, H. Bright light-emitting diodes based on organometal halide perovskite nanoplatelets. Adv. Mater. 2016, 28, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Yang, Y.M.; You, J.; Hong, Z.; Chang, W.H.; Li, G.; Yang, Y. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Zhao, Z.X.; Li, H.Y.; Yuan, J.; Wang, Z.L.; Cao, G.Z.; Zhu, G. High-performance organolead halide perovskite-based self-powered triboelectric photodetector. ACS Nano 2015, 9, 11310–11316. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhao, X.; Xiao, L.; Zeng, H.; Sun, H. Nonlinear absorption and low-threshold multiphoton pumped stimulated emission from all-inorganic perovskite nanocrystals. Nano Lett. 2016, 16, 448–453. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, H.; Stoumpos, C.C.; Ding, Q.; Wang, J.; Kanatzidis, M.G.; Zhu, X.; Jin, S. Broad wavelength tunable robust lasing from single-crystal nanowires of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). ACS Nano 2016, 10, 7963–7972. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H. Quantum Dots: A Quantum Jump for Molecular Imaging? J. Nucl. Med. 2007, 48, 1408–1410. [Google Scholar] [CrossRef] [Green Version]
- Bentolila, L.A.; Ebenstein, Y.; Weiss, S. Quantum Dots for In Vivo Small-Animal Imaging. J. Nucl. Med. 2009, 50, 493–496. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.W.; Wu, D.Y.; Chan, Y.C.; Lin, C.C.; Chung, P.H.; Hsiao, M.; Liu, R.S. Evaluations of the chemical stability and cytotoxicity of CuInS2 and CuInS2/ZnS core/shell quantum dots. J. Phys. Chem. C 2015, 119, 2852–2860. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Liao, Q.; Xu, Z.; Li, H.; Zheng, L.; Fu, H. Embedding perovskite nanocrystals into a polymer matrix for tunable luminescence probes in cell imaging. Adv. Funct. Mater. 2017, 27, 1604382. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiencies, National Renewable Energy Laboratory. Available online: https://www.nrel.gov/pv/assets/pdfs/pv-efficiency-chart.20190103.pdf (accessed on 3 January 2019).
- Conings, B.; Drijkoningen, J.; Gauquelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic thermal instability of methylammonium lead trihalide perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Leijtens, T.; Eperon, G.E.; Noel, N.K.; Habisreutinger, S.N.; Petrozza, A.; Snaith, H.J. Stability of metal halide perovskite solar cells. Adv. Energy Mater. 2015, 5, 1500963. [Google Scholar] [CrossRef]
- Niu, G.D.; Guo, X.D.; Wang, L.D. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.M.; Song, J.Z.; Xiao, L.; Zeng, H.B.; Sun, H.D. All-inorganic colloidal perovskite quantum dots: a new class of lasing materials with favorable characteristics. Adv. Mater. 2015, 27, 7101–7108. [Google Scholar] [CrossRef]
- Yakunin, S.; Protesescu, L.; Krieg, F.; Bodnarchuk, M.I.; Nedelcu, G.; Humer, M.; De Luca, G.; Fiebig, M.; Heiss, W.; Kovalenko, M.V. Low-threshold amplified spontaneous emission and lasing from colloidal nanocrystals of caesium lead halide perovskites. Nat. Commun. 2015, 6, 8056. [Google Scholar] [CrossRef] [Green Version]
- Soosaimanickam, A.; Jeyagopal, R.K.; Sridharan, M.B. Cesium lead halide (CsPbX3, X = Cl, Br, I) perovskite quantum dots-synthesis, properties, and applications: a review of their present status. J. Photon. Energy 2016, 6, 042001. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 quantum dots for lighting and displays: room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Kumar, S.; Bar, J.; Bertolotti, F.; Masciocchi, N.; Guagliardi, A.; Grotevent, M.; Shorubalko, I.; Bodnarchuk, M.I.; et al. Dismantling the “red wall” of colloidal perovskites: highly luminescent formamidinium and formamidinium–cesium lead iodide nanocrystals. ACS nano 2017, 11, 3119–3134. [Google Scholar] [CrossRef]
- Meyns, M.; Perálvarez, M.; Heuer-Jungemann, A.; Hertog, W.; Ibáñez, M.; Nafria, R.; Genç, A.; Arbiol, J.; Kovalenko, M.V.; Carreras, J.; et al. Polymer-enhanced stability of inorganic perovskite nanocrystals and their application in color conversion LEDs. ACS Appl. Mater. Interfaces 2016, 8, 19579–19586. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Ruan, C.; Yin, C.; Wang, X.; Wang, Y.; Yu, W.W. Efficient and stable white LEDs with silica-coated inorganic perovskite quantum dots. Adv. Mater 2016, 28, 10088–10094. [Google Scholar] [CrossRef]
- Dirin, D.N.; Protesescu, L.; Trummer, D.; Kochetygov, I.V.; Yakunin, S.; Krumeich, F.; Stadie, N.P.; Kovalenko, M.V. Harnessing defect-tolerance at the nanoscale: highly luminescent lead halide perovskite nanocrystals in mesoporous silica matrixes. Nano Lett. 2016, 16, 5866–5874. [Google Scholar] [CrossRef]
- Wang, H.C.; Lin, S.Y.; Tang, A.C.; Singh, B.P.; Tong, H.C.; Chen, C.Y.; Lee, Y.C.; Tsai, T.L.; Liu, R.S. Mesoporous silica particles integrated with all-inorganic CsPbBr3 perovskite quantum-dot nanocomposites (MP-PQDs) with high stability and wide color gamut used for backlight display. Angew. Chem.-Int. Edit. 2016, 55, 7924–7929. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Liu, Q.; Xia, Z. Synergetic effect of postsynthetic water treatment on the enhanced photoluminescence and stability of CsPbX3 (X = Cl, Br, I) Perovskite nanocrystals. Chem. Mater. 2018, 30, 6922–6929. [Google Scholar] [CrossRef]
- Li, Z.C.; Kong, L.; Huang, S.Q.; Li, L. Highly luminescent and ultrastable CsPbBr3 perovskite quantum dots incorporated into a silica/alumina monolith. Angew. Chem.-Int. Edit. 2017, 56, 8134–8138. [Google Scholar] [CrossRef]
- Li, Z.J.; Hofman, E.; Li, J.; Davis, A.H.; Tung, C.H.; Wu, L.Z.; Zheng, W.W. Photoelectrochemically active and environmentally stable CsPbBr3/TiO2 core/shell nanocrystals. Adv. Funct. Mater. 2018, 28, 1704288. [Google Scholar] [CrossRef]
- Li, X.; Xue, Z.; Luo, D.; Huang, C.H.; Liu, L.Z.; Qiao, X.Z.; Liu, C.; Song, Q.; Yan, C.; Li, Y.C.; et al. A stable lead halide perovskite nanocrystals protected by PMMA. Sci. China Mater. 2018, 61, 363. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Xie, Z.F.; Sun, X.K.; Zhang, S.Y.; Pan, G.C.; Zhu, Y.S.; Dong, B.; Bai, X.; Zhang, H.Z.; Song, H.W. Highly Efficient and Stable Inorganic Perovskite Quantum Dots by Embedding into a Polymer Matrix. ChemNanoMat 2019, 5, 346–351. [Google Scholar] [CrossRef]
- Dirin, D.N.; Cherniukh, I.; Yakunin, S.; Shynkarenko, Y.; Kovalenko, M.V. Solution-Grown CsPbBr3 Perovskite Single Crystals for Photon Detection. Chem. Mater. 2016, 28, 8470–8474. [Google Scholar] [CrossRef]
- Bondarenko, S.; Villringer, C.; Steglich, P. Comparative Study of Nano-Slot Silicon Waveguides Covered by Dye Doped and Undoped Polymer Cladding. Appl. Sci. 2019, 9, 89. [Google Scholar] [CrossRef]
- Schubert, E.F. Light-Emitting Diodes, 2nd ed.; Cambridge University Press: New York, NY, USA, 2012; p. 89. [Google Scholar]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-C.; Tien, C.-H.; Tseng, Z.-L.; Dong, Y.-S.; Yang, S. Influence of PMMA on All-Inorganic Halide Perovskite CsPbBr3 Quantum Dots Combined with Polymer Matrix. Materials 2019, 12, 985. https://doi.org/10.3390/ma12060985
Chen L-C, Tien C-H, Tseng Z-L, Dong Y-S, Yang S. Influence of PMMA on All-Inorganic Halide Perovskite CsPbBr3 Quantum Dots Combined with Polymer Matrix. Materials. 2019; 12(6):985. https://doi.org/10.3390/ma12060985
Chicago/Turabian StyleChen, Lung-Chien, Ching-Ho Tien, Zong-Liang Tseng, Yu-Shen Dong, and Shengyi Yang. 2019. "Influence of PMMA on All-Inorganic Halide Perovskite CsPbBr3 Quantum Dots Combined with Polymer Matrix" Materials 12, no. 6: 985. https://doi.org/10.3390/ma12060985




