Effect of Portland Cement versus Sulphoaluminate Cement on the Properties of Blended Lime-Based Mortars Prepared by Carbide Slag
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Design
2.3. Methods
2.3.1. Mechanical Properties
2.3.2. Porosity
2.3.3. Capillary Water Absorption
2.3.4. Dry Shrinkage and Weight Loss
2.3.5. X-ray Diffraction
2.3.6. Scanning Electron Microscopy
3. Results
3.1. Mechanical Properties
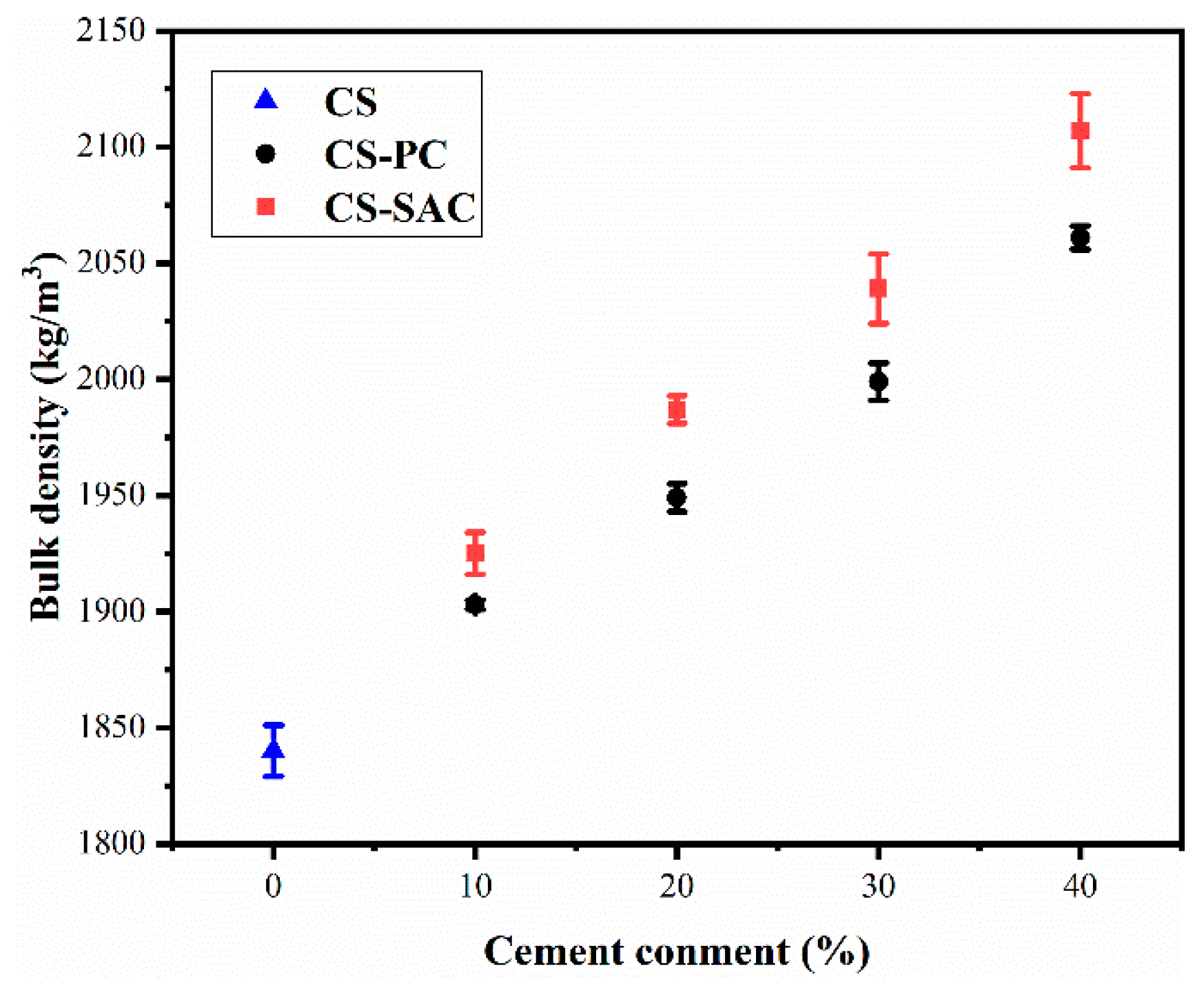
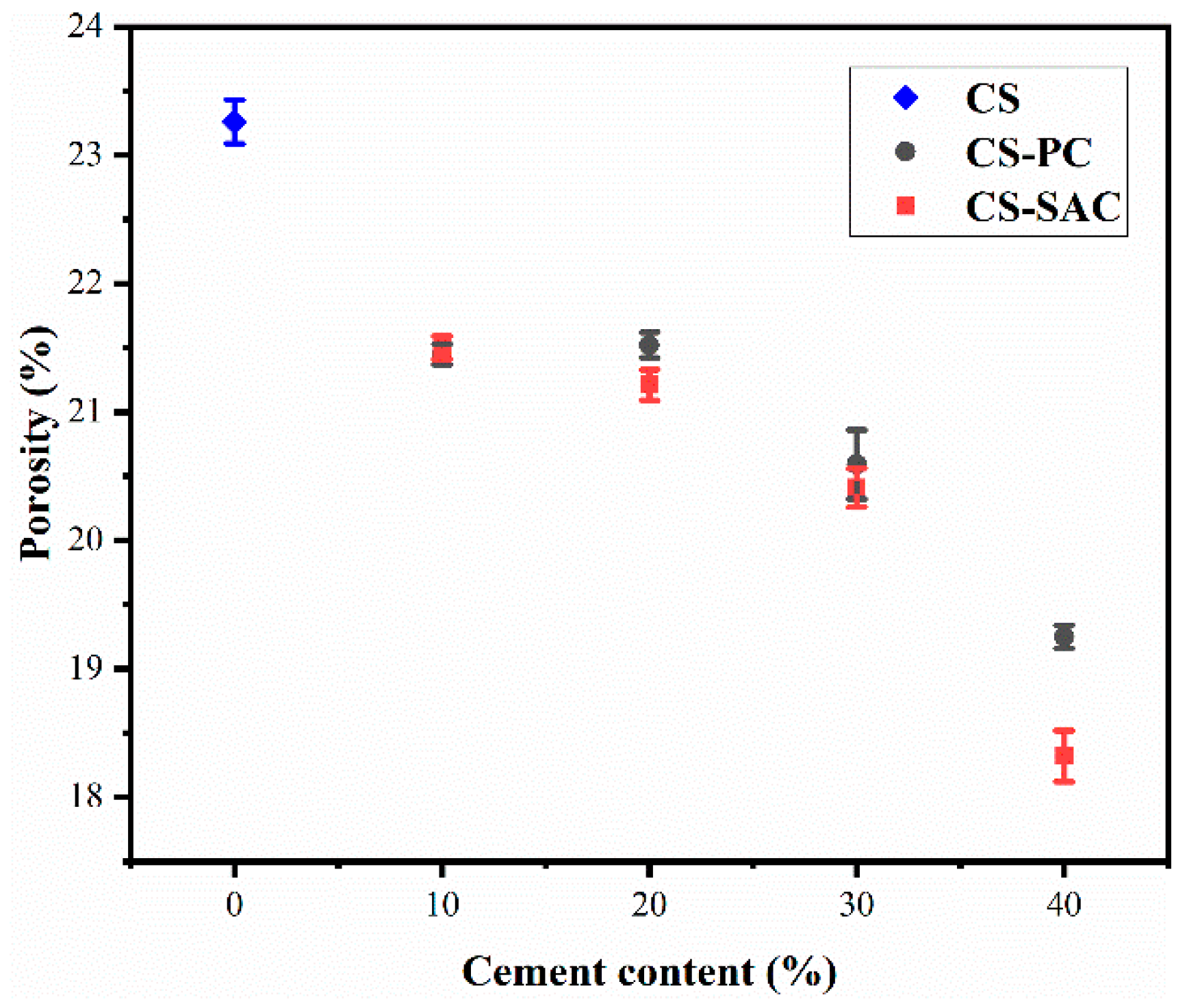
3.2. Bulk Density and Porosity
- (i)
- A decrease of mixing water and the formation of hydration products diminish the porosity of blended mortars due to the addition of cement.
- (ii)
- The sand volume increases with the content of cement in blended mortars (Table 3), which causes a rise of interfacial transition zone (ITZ). Therefore, the porosity in ITZ increases.
3.3. Capillary Water Absorption
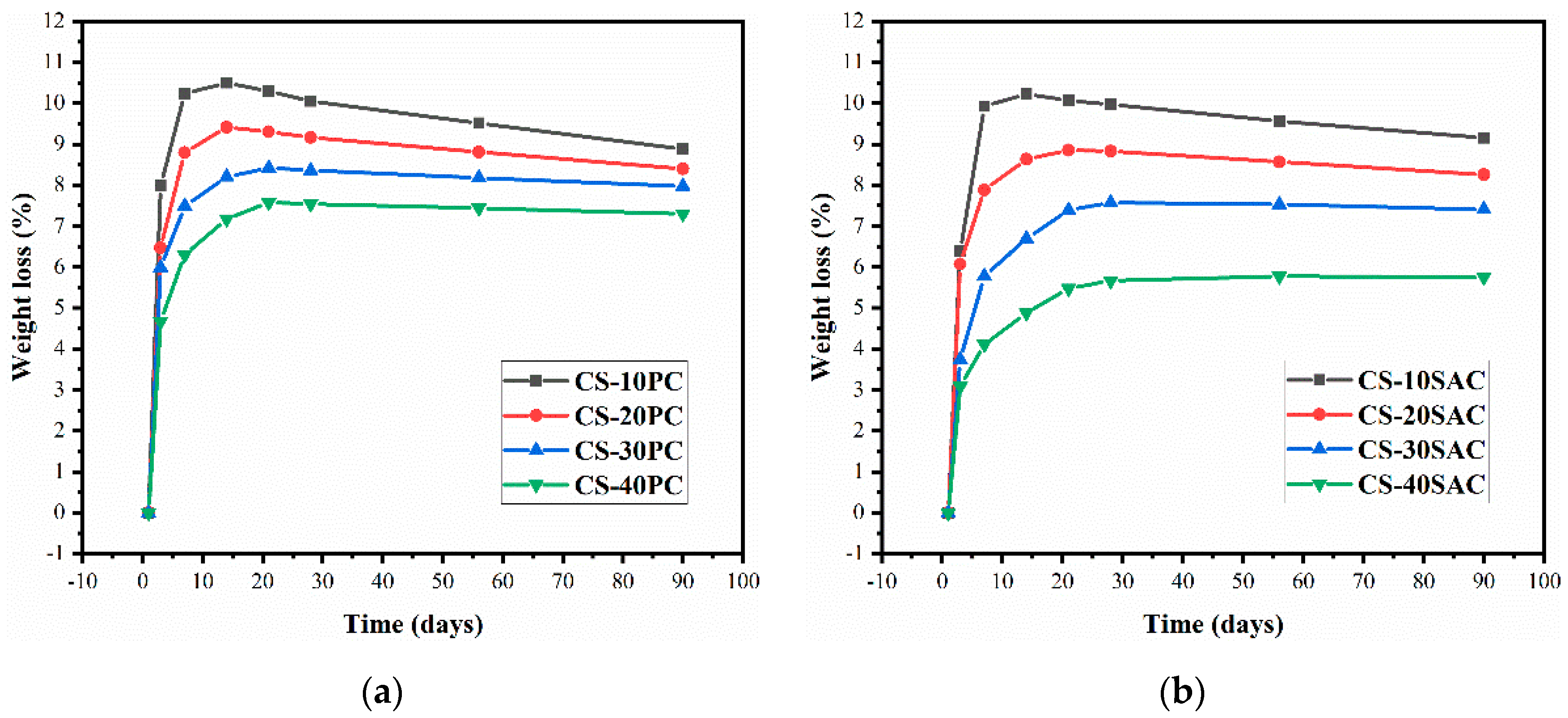
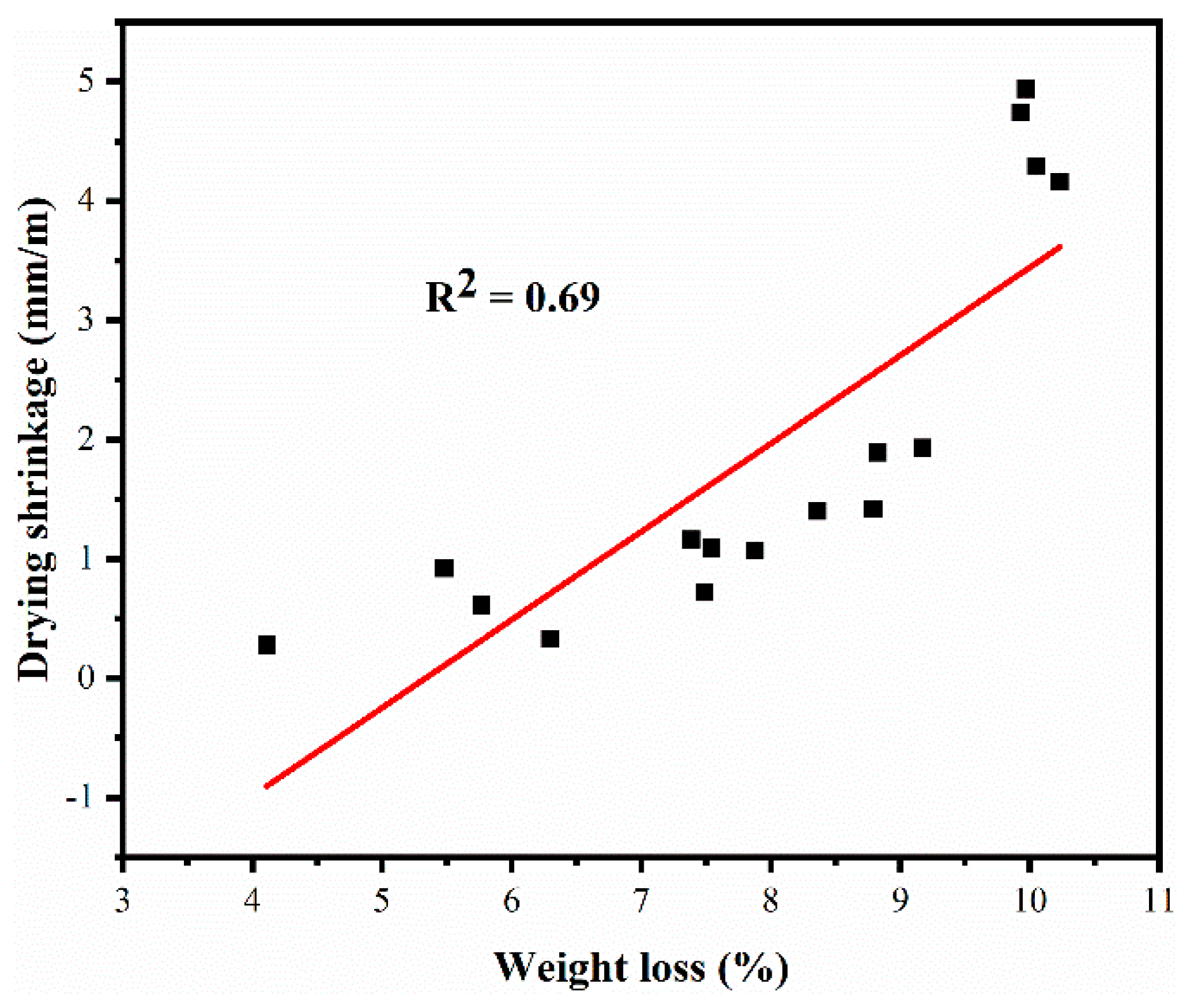
3.4. Weight Loss
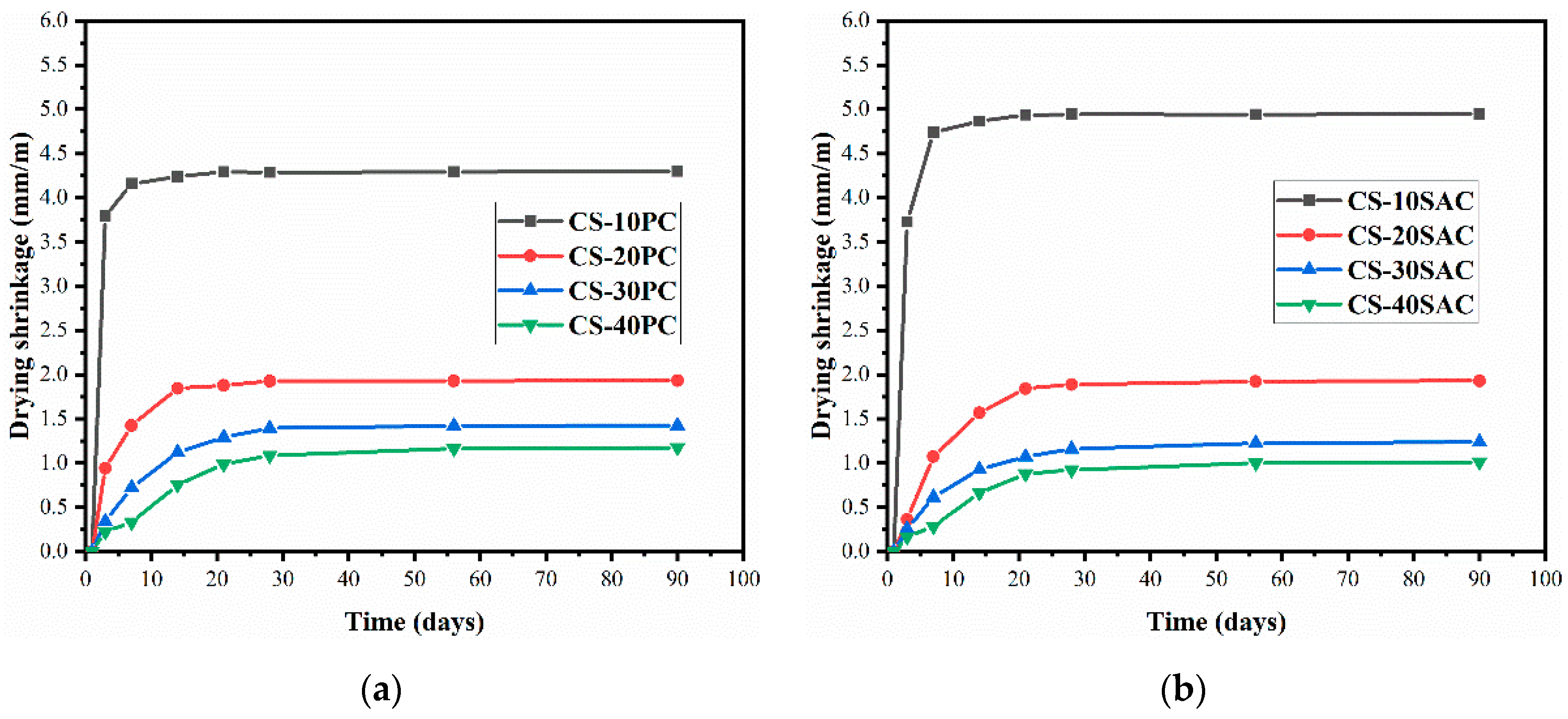
3.5. Drying Shrinkage
3.6. X-Ray Diffraction Analysis
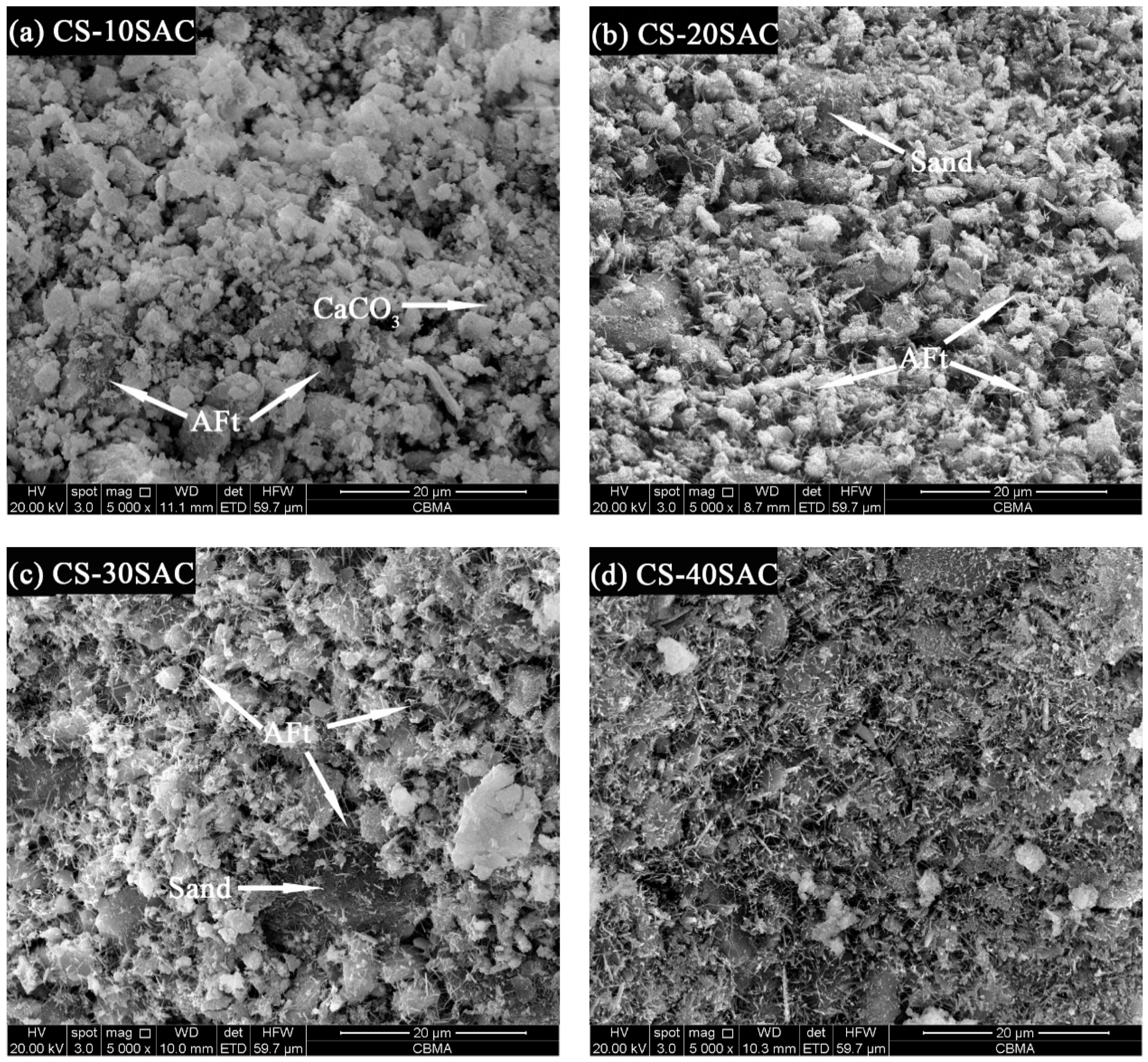
3.7. Scanning Electron Microscopy Analysis
4. Conclusions
- The addition of cement can significantly improve the compressive and flexural strength of blended mortars. For CS-PC blended mortars, the content of Portland cement should be more than 20% so than to enhance mechanical properties. Compared with Portland cement, sulphoaluminate cement contributes more to the strength, the compressive and flexural strength of CS-SAC blended mortars are almost 2 times stronger than that of CS-PC blended mortars.
- The addition of cement can diminish the porosity of blended mortars, which results in the increase of strength and the reduction of capillary water absorption. CS-SAC blended mortars have lower capillary coefficient than CS-PC blended mortars, but their maximum water absorption is nearly equal.
- The drying shrinkage of blended mortars effectively reduces due to the presence of cement, which is one of the reasons to improve mechanical behavior. The fast formation of AFt constrains a large amount of mixing water, which reduces the evaporation of water and enables CS-SAC blended mortars to have lower early shrinkage.
- The microstructure depends on cement type in blended mortars. C-S-H gel in CS-PC blended mortars mainly covers the surface of other phases, while fine-needle like AFt interlaces with other phases, forming a dense microstructure.
Author Contributions
Funding
Conflicts of Interest
References
- del Mar Barbero-Barrera, M.; Maldonado-Ramos, L.; Van Balen, K.; García-Santos, A.; Neila-González, F.J. Lime render layers: An overview of their properties. J. Cult. Herit. 2014, 15, 326–330. [Google Scholar] [CrossRef] [Green Version]
- Veiga, R. Air lime mortars: What else do we need to know to apply them in conservation and rehabilitation interventions? A review. Constr. Build. Mater. 2017, 157, 132–140. [Google Scholar] [CrossRef]
- Barbero-Barrera, M.M.; Flores Medina, N.; Guardia-Martín, C. Influence of the addition of waste graphite powder on the physical and microstructural performance of hydraulic lime pastes. Constr. Build. Mater. 2017, 149, 599–611. [Google Scholar] [CrossRef]
- Izaguirre, A.; Lanas, J.; Álvarez, J.I. Effect of water-repellent admixtures on the behaviour of aerial lime-based mortars. Cem. Concr. Res. 2009, 39, 1095–1104. [Google Scholar] [CrossRef] [Green Version]
- Izaguirre, A.; Lanas, J.; Álvarez, J.I. Ageing of lime mortars with admixtures: Durability and strength assessment. Cem. Concr. Res. 2010, 40, 1081–1095. [Google Scholar] [CrossRef] [Green Version]
- Gulbe, L.; Vitina, I.; Setina, J. The Influence of Cement on Properties of Lime Mortars. Procedia Eng. 2017, 172, 325–332. [Google Scholar] [CrossRef]
- Lanas, J.; Alvarez-Galindo, J.I. Masonry repair lime-based mortars: Factors affecting the mechanical behavior. Cem. Concr. Res. 2003, 33, 1867–1876. [Google Scholar] [CrossRef]
- Mosquera, M.J.; Silva, B.; Prieto, B.; Ruiz-Herrera, E. Addition of cement to lime-based mortars: Effect on pore structure and vapor transport. Cem. Concr. Res. 2006, 36, 1635–1642. [Google Scholar] [CrossRef]
- Arandigoyen, M.; Alvarez, J.I. Pore structure and mechanical properties of cement–lime mortars. Cem. Concr. Res. 2007, 37, 767–775. [Google Scholar] [CrossRef] [Green Version]
- Grilo, J.; Faria, P.; Veiga, R.; Santos Silva, A.; Silva, V.; Velosa, A. New natural hydraulic lime mortars—Physical and microstructural properties in different curing conditions. Constr. Build. Mater. 2014, 54, 378–384. [Google Scholar] [CrossRef]
- Fourmentin, M.; Faure, P.; Gauffinet, S.; Peter, U.; Lesueur, D.; Daviller, D.; Ovarlez, G.; Coussot, P. Porous structure and mechanical strength of cement-lime pastes during setting. Cem. Concr. Res. 2015, 77, 1–8. [Google Scholar] [CrossRef]
- Silva, B.A.; Ferreira Pinto, A.P.; Gomes, A. Natural hydraulic lime versus cement for blended lime mortars for restoration works. Constr. Build. Mater. 2015, 94, 346–360. [Google Scholar] [CrossRef]
- Sepulcre-Aguilar, A.; Hernández-Olivares, F. Assessment of phase formation in lime-based mortars with added metakaolin, Portland cement and sepiolite, for grouting of historic masonry. Cem. Concr. Res. 2010, 40, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Nunes, C.; Slížková, Z. Freezing and thawing resistance of aerial lime mortar with metakaolin and a traditional water-repellent admixture. Constr. Build. Mater. 2016, 114, 896–905. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Assessment of pozzolanic activity of different calcined clays. Cem. Concr. Compos. 2013, 37, 319–327. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Ma, Q.; Zhao, X.; Zhang, T. Study on the lightweight hydraulic mortars designed by the use of diatomite as partial replacement of natural hydraulic lime and masonry waste as aggregate. Constr. Build. Mater. 2014, 73, 33–40. [Google Scholar] [CrossRef]
- Veiga, M.R.; Velosa, A.; Magalhães, A. Experimental applications of mortars with pozzolanic additions: Characterization and performance evaluation. Constr. Build. Mater. 2009, 23, 318–327. [Google Scholar] [CrossRef]
- Grist, E.R.; Paine, K.A.; Heath, A.; Norman, J.; Pinder, H. Compressive strength development of binary and ternary lime–pozzolan mortars. Mater. Des. 2013, 52, 514–523. [Google Scholar] [CrossRef]
- Pavía, S.; Regan, D. Influence of cement kiln dust on the physical properties of calcium lime mortars. Mater. Struct. 2010, 43, 381–391. [Google Scholar] [CrossRef]
- Matias, G.; Faria, P.; Torres, I. Lime mortars with ceramic wastes: Characterization of components and their influence on the mechanical behaviour. Constr. Build. Mater. 2014, 73, 523–534. [Google Scholar] [CrossRef] [Green Version]
- Iucolano, F.; Liguori, B.; Colella, C. Fibre-reinforced lime-based mortars: A possible resource for ancient masonry restoration. Constr. Build. Mater. 2013, 38, 785–789. [Google Scholar] [CrossRef]
- Wang, Y.; He, H.; He, F. Effect of slaked lime and aluminum sulfate on the properties of dry-mixed masonry mortar. Constr. Build. Mater. 2018, 180, 117–123. [Google Scholar] [CrossRef]
- Yang, H.; Cao, J.; Wang, Z.; Chen, H.; Gong, X. Discovery of impurities existing state in carbide slag by chemical dissociation. Int. J. Miner. Process. 2014, 130, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Li, X.; Ma, B.; Lv, Y. Effect of binding materials on carbide slag based high utilization solid-wastes autoclaved aerated concrete (HUS-AAC): Slurry, physic-mechanical property and hydration products. Constr. Build. Mater. 2018, 188, 221–236. [Google Scholar] [CrossRef]
- Sun, R.; Li, Y.; Zhao, J.; Liu, C.; Lu, C. CO2 capture using carbide slag modified by propionic acid in calcium looping process for hydrogen production. Int. J. Hydrogen Energy 2013, 38, 13655–13663. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhan, J.; Liu, G.; Zheng, M.; Jin, R.; Yang, L.; Hao, L.; Wu, X.; Zhang, X.; Wang, P. Evaluation of dioxins and dioxin-like compounds from a cement plant using carbide slag from chlor-alkali industry as the major raw material. J. Hazard. Mater. 2017, 330, 135–141. [Google Scholar] [CrossRef]
- Krammart, P.; Tangtermsirikul, S. Properties of cement made by partially replacing cement raw materials with municipal solid waste ashes and calcium carbide waste. Constr. Build. Mater. 2004, 18, 579–583. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, P.; Wang, S.; Chen, Y. Effects of Calcium Carbide Residue and High-Silicon Limestone on Synthesis of Belite-Barium Calcium Sulphoaluminate Cement. J. Inorg. Organomet. P. 2011, 21, 900–905. [Google Scholar] [CrossRef]
- Wang, Y.L.; Dong, S.J.; Liu, L.L.; Cui, S.P. Using Calcium Carbide Slag as One of Calcium-Containing Raw Materials to Produce Cement Clinker. Mater. Sci. Forum 2013, 743–744, 171–174. [Google Scholar] [CrossRef]
- Wang, Y.L.; Dong, S.J.; Liu, L.L.; Cui, S.P.; Xu, H.B. Study Formation Process of Cement Clinker Minerals by Using Calcium Carbide Slag as Raw Material. Appl. Mech. Mater. 2013, 389, 341–345. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, X.; Wang, Z.; Cao, J.; Guo, Z. Preparation of block CaO from carbide slag and its compressive strength improved by H3PO4. Int. J. Miner. Process. 2014, 129, 6–11. [Google Scholar] [CrossRef]
- Guo, B.; Zhao, T.; Sha, F.; Zhang, F.; Li, Q.; Zhao, J.; Zhang, J. Synthesis of vaterite CaCO3 micro-spheres by carbide slag and a novel CO2-storage material. J. CO2 Util. 2017, 18, 23–29. [Google Scholar] [CrossRef]
- Li, Y.; Sun, R.; Liu, C.; Liu, H.; Lu, C. CO2 capture by carbide slag from chlor-alkali plant in calcination/carbonation cycles. Int. J. Greenh. Gas Con. 2012, 9, 117–123. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Phetchuay, C.; Chinkulkijniwat, A.; Cholaphatsorn, A. Strength development in silty clay stabilized with calcium carbide residue and fly ash. Soils Found. 2013, 53, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Namarak, C.; Satching, P.; Tangchirapat, W.; Jaturapitakkul, C. Improving the compressive strength of mortar from a binder of fly ash-calcium carbide residue. Constr. Build. Mater. 2017, 147, 713–719. [Google Scholar] [CrossRef]
- Namarak, C.; Tangchirapat, W.; Jaturapitakkul, C. Bar-concrete bond in mixes containing calcium carbide residue, fly ash and recycled concrete aggregate. Cem. Concr. Compos. 2018, 89, 31–40. [Google Scholar] [CrossRef]
- GB 175. Common Portland Cement; China Standards Press: Beijing, China, 2007. [Google Scholar]
- JC/T 2282. Quick Setting and Rapid Hardening Sulphoaluminate Cement; China Building Materials Industry Press: Beijing, China, 2014. [Google Scholar]
- GB/T 2419. Test Method for Fluidity of Cement Mortar; China Standards Press: Beijing, China, 2005. [Google Scholar]
- GB/T 17671. Method of Testing Cements-Determination of Strength; China Standards Press: Beijing, China, 1999. [Google Scholar]
- BS EN 1936. Natural Stone Test Methods-Determination of Real Density and Apparent Density, and of Total and Open Porosity; British Standards Institution: London, Britain, 2006. [Google Scholar]
- BS EN 1015-18. Determination of Water Absorption Coefficient Due to Capillary Action of Hardened Mortar; British Standards Institution: London, Britain, 2002. [Google Scholar]
- JGJ/T 70. Standard for Test Method of Performance on Building Mortar; China Building Industry Press: Beijing, China, 2009. [Google Scholar]
- Li, H.; Yang, X.; Xu, W.; Wu, J.; Xu, J.; Zhang, G.; Xia, Y. Application of dry composite electroplating sludge into preparation of cement-based decorative mortar as green pigment. J. Clean. Prod. 2014, 66, 101–106. [Google Scholar] [CrossRef]
- Arandigoyen, M.; Bernal, J.L.P.; López, M.A.B.; Alvarez, J.I. Lime-pastes with different kneading water: Pore structure and capillary porosity. Appl. Surf. Sci. 2005, 252, 1449–1459. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Babafemi, A.J. Performance of strain hardening cement-based composite (SHCC) under various exposure conditions. Cogent Eng. 2017, 4, 1345608. [Google Scholar] [CrossRef]
- Arandigoyen, M.; Alvarez, J.I. Blended pastes of cement and lime: Pore structure and capillary porosity. Appl. Surf. Sci. 2006, 252, 8077–8085. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.J.; Foley, E.M.; Reda Taha, M.M. Nano-mechanical characterization of synthetic calcium–silicate–hydrate (C–S–H) with varying CaO/SiO2 mixture ratios. Cem. Concr. Compos. 2013, 36, 65–70. [Google Scholar] [CrossRef]
- Naber, C.; Bellmann, F.; Sowoidnich, T.; Goetz-Neunhoeffer, F.; Neubauer, J. Alite dissolution and C-S-H precipitation rates during hydration. Cem. Concr. Res. 2019, 115, 283–293. [Google Scholar] [CrossRef]
- Nežerka, V.; Slížková, Z.; Tesárek, P.; Plachý, T.; Frankeová, D.; Petráňová, V. Comprehensive study on mechanical properties of lime-based pastes with additions of metakaolin and brick dust. Cem. Concr. Res. 2014, 64, 17–29. [Google Scholar] [CrossRef]
- Hargis, C.W.; Kirchheim, A.P.; Monteiro, P.J.M.; Gartner, E.M. Early age hydration of calcium sulfoaluminate (synthetic ye’elimite, C4A3S¯) in the presence of gypsum and varying amounts of calcium hydroxide. Cem. Concr. Res. 2013, 48, 105–115. [Google Scholar] [CrossRef]
- García-Maté, M.; De la Torre, A.G.; León-Reina, L.; Losilla, E.R.; Aranda, M.A.G.; Santacruz, I. Effect of calcium sulfate source on the hydration of calcium sulfoaluminate eco-cement. Cem. Concr. Compos. 2015, 55, 53–61. [Google Scholar] [CrossRef] [Green Version]






| Contents | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | TiO2 | Cl | LOI |
|---|---|---|---|---|---|---|---|---|---|
| CS | 7.82 | 2.61 | 0.62 | 58.50 | 0.84 | 1.09 | 0.00 | 0.15 | 28.20 |
| PC | 20.12 | 5.27 | 2.95 | 64.90 | 2.51 | 1.22 | 0.40 | 0.00 | 1.13 |
| SAC | 16.83 | 17.41 | 1.38 | 44.68 | 4.47 | 12.58 | 0.88 | 0.00 | 0.98 |
| Samples | CS | PC | SAC | Sand | Water/Binder Ratio | Slump (mm) |
|---|---|---|---|---|---|---|
| CS | 450 | 0 | 0 | 1350 | 0.65 | 168 |
| CS-10PC | 415 | 45 | 0 | 1350 | 0.62 | 167 |
| CS-20PC | 360 | 90 | 0 | 1350 | 0.59 | 170 |
| CS-30PC | 315 | 135 | 0 | 1350 | 0.57 | 169 |
| CS-40PC | 270 | 180 | 0 | 1350 | 0.55 | 168 |
| CS-10SAC | 415 | 0 | 45 | 1350 | 0.62 | 167 |
| CS-20SAC | 360 | 0 | 90 | 1350 | 0.59 | 163 |
| CS-30SAC | 315 | 0 | 135 | 1350 | 0.57 | 169 |
| CS-40SAC | 270 | 0 | 180 | 1350 | 0.54 | 165 |
| Mortars | Binder/Sand Ratio by Mass | Binder/Sand Ratio by Volume | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CS | PC | SAC | Sand | CS | PC | SAC | Sand | ||
| CS | 1.0 | - | - | 3.0 | 1.00 | - | - | 2.61 | |
| CS-10PC | 0.9 | 0.1 | - | 3.0 | 0.92 | 0.08 | - | 2.68 | |
| CS-20PC | 0.8 | 0.2 | - | 3.0 | 0.84 | 0.16 | - | 2.76 | |
| CS-30PC | 0.7 | 0.3 | - | 3.0 | 0.76 | 0.24 | - | 2.83 | |
| CS-40PC | 0.6 | 0.4 | - | 3.0 | 0.67 | 0.33 | - | 2.92 | |
| CS-10SAC | 0.9 | - | 0.1 | 3.0 | 0.92 | - | 0.08 | 2.67 | |
| CS-20SAC | 0.8 | - | 0.2 | 3.0 | 0.83 | - | 0.17 | 2.72 | |
| CS-30SAC | 0.7 | - | 0.3 | 3.0 | 0.75 | - | 0.25 | 2.78 | |
| CS-40SAC | 0.6 | - | 0.4 | 3.0 | 0.65 | - | 0.35 | 2.85 | |
| Samples | Capillarity Coefficient (kg·m−2·min−1/2) | Asymptotic Value (kg/m2) |
|---|---|---|
| CS | 1.71 | 19.0 |
| CS-10PC | 1.70 | 18.1 |
| CS-20PC | 1.69 | 17.5 |
| CS-30PC | 1.16 | 16.0 |
| CS-40PC | 0.68 | 13.8 |
| CS-10SAC | 1.67 | 18.3 |
| CS-20SAC | 1.29 | 17.0 |
| CS-30SAC | 0.68 | 15.9 |
| CS-40SAC | 0.52 | 13.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, S.; Wang, J.; Lan, M.; Wang, Y.; Zhang, Q. Effect of Portland Cement versus Sulphoaluminate Cement on the Properties of Blended Lime-Based Mortars Prepared by Carbide Slag. Materials 2019, 12, 1012. https://doi.org/10.3390/ma12071012
Nie S, Wang J, Lan M, Wang Y, Zhang Q. Effect of Portland Cement versus Sulphoaluminate Cement on the Properties of Blended Lime-Based Mortars Prepared by Carbide Slag. Materials. 2019; 12(7):1012. https://doi.org/10.3390/ma12071012
Chicago/Turabian StyleNie, Song, Jianfeng Wang, Mingzhang Lan, Yali Wang, and Qiaowei Zhang. 2019. "Effect of Portland Cement versus Sulphoaluminate Cement on the Properties of Blended Lime-Based Mortars Prepared by Carbide Slag" Materials 12, no. 7: 1012. https://doi.org/10.3390/ma12071012





